Introduction
Rationale
Escherichia coli (E. coli) are a diverse group of bacteria that are a normal part of poultry microflora. E. coli are found throughout the intestinal and upper respiratory tracts, as well as on the skin and feathers of healthy birds (Nolan et al., Reference Nolan, Barnes, Vaillancourt, Abdul-Aziz, Logue, Swayne, Glisson, McDougald, Nolan, Suarez and Nair2013). Although most strains of E. coli are not detrimental to bird health, some are capable of causing disease outside of the intestinal tract. Those that are capable of causing disease in birds, or cause disease when host defenses have been impaired, are referred to as avian pathogenic E. coli (APEC) (Dziva and Stevens, Reference Dziva and Stevens2008). Colibacillosis refers specifically to a localized or systemic infection caused by an APEC and is a leading cause of morbidity and mortality in the global poultry industry (Guabiraba and Schouler, Reference Guabiraba and Schouler2015). Syndromes of APEC-associated disease include colisepticemia, hemorrhagic septicemia, coligranuloma (Hjarre's disease), airsacculitis (chronic respiratory disease, CRD), swollen head syndrome, polyserositis, enteritis, venereal colibacillosis, coliform cellulitis (inflammatory or infectious process, IP), peritonitis, salpingitis, orchitis, osteomyelitis/synovitis (including turkey osteomyelitis complex), panophthalmitis, and omphalitis/yolk sac infection (Barnes et al., Reference Barnes, Nolan, Vaillancourt, Saif, Fadly, Glisson, McDougald, Nolan and Swayne2008; Nolan et al., Reference Nolan, Barnes, Vaillancourt, Abdul-Aziz, Logue, Swayne, Glisson, McDougald, Nolan, Suarez and Nair2013; Guabiraba and Schouler, Reference Guabiraba and Schouler2015). Colibacillosis can develop as a primary infection, or as a secondary infection alongside other viral or bacterial pathogens (Nolan et al., Reference Nolan, Barnes, Vaillancourt, Abdul-Aziz, Logue, Swayne, Glisson, McDougald, Nolan, Suarez and Nair2013).
Prevention and control of colibacillosis can be challenging, as E. coli are part of the normal intestinal flora of birds, and approaches that focus on management strategies have limited success. Approaches to prevention of colibacillosis include biosecurity to manage access of personnel and the movement of birds to limit the introduction of pathogenic E. coli and reduce exposure of the flock. Ensuring adequate environmental sanitation and optimal climate conditions, such as humidity, ventilation, and temperature can also help minimize pathogen growth in the flock and reduce the numbers of E. coli in the water and feed. In addition, protecting flocks from other bacterial or viral infections that can decrease host resistance can reduce the risk of colibacillosis (Nolan et al., Reference Nolan, Barnes, Vaillancourt, Abdul-Aziz, Logue, Swayne, Glisson, McDougald, Nolan, Suarez and Nair2013). Antibiotics are also used for APEC, either in flocks where the birds are not diseased but may at risk of illness in order to prevent illness (prophylaxis) or in flocks where some birds are already ill with the intention to prevent further illness or mortality (metaphylaxis) (Singer and Hofacre, Reference Singer and Hofacre2006). Current challenges in the prevention and control of colibacillosis include the limited availability of drugs and the emergence of strains that are highly virulent and resistant due to virulence and resistance plasmids (Johnson et al., Reference Johnson, Siek, Johnson and Nolan2005, Reference Johnson, Wannemeuhler, Scaccianoce, Johnson and Nolan2006).
There is a global consensus that antibiotics should be used prudently in humans and animals to reduce the risk of antimicrobial resistance; both the World Health Organization (WHO) and the World Organization for Animal Health (OIE) have published recommendations on the judicious use of antimicrobials in response to the threat of antimicrobial resistance (WHO, 2015; OIE, 2018). In order to reduce antibiotic use, veterinarians and poultry specialists need access to unbiased, and accurate evidence regarding the efficiency of antimicrobials for the prevention and control of colibacillosis in broilers. Such information enables informed comparisons between the benefits and the harms associated with various antibiotics, which in turn allows practitioners to select the most appropriate and effective preventive applications or treatments.
Systematic reviews provide a rigorous and transparent method of identifying and summarizing the available literature to address a specific question related to the efficacy of an intervention (European Food Safety Authority, 2010; Higgins and Green, Reference Higgins and Green2011; O'Connor and Sargeant, Reference O'Connor and Sargeant2014; Sargeant and O'Connor, Reference Sargeant and O'Connor2014). Systematic reviews follow defined steps and require the involvement of multiple reviewers at each stage to reduce the potential for bias. When sufficient data exist, the results from multiple studies can be combined in a statistical meta-analysis to provide a summary measure of the effect size of an intervention across studies (Higgins and Green, Reference Higgins and Green2011; O'Connor et al., Reference O'Connor, Sargeant and Wang2014). Where there are multiple treatment options for a specific disease or condition, a network meta-analysis (NMA) provides a method for evaluating the comparative efficacy of the treatment choices (Salanti, Reference Salanti2012). Research synthesis methods such as systematic reviews, meta-analyses, and NMA are therefore powerful tools that can provide scientifically valid information about the scope and conclusions of the existing literature on preventive approaches to colibacillosis in broiler poultry; these syntheses can in turn support evidence-based decision-making by managers and practitioners.
Objectives
Our objective was to conduct a systematic review and a network meta-analysis, if supported by the data, to address the following review question: ‘What is the efficacy of antibiotics to prevent or control colibacillosis in broiler chickens?’
Methods
Protocol and registration
An a priori protocol for this review was prepared and is archived in the University of Guelph's institutional repository (The Atrium; https://atrium.lib.uoguelph.ca/xmlui/handle/10214/14349). The protocol was also published online on the Systematic Reviews for Animals and Food (SYREAF) website (available at http://www.syreaf.org/). The review protocol was reported in accordance with PRISMA-P guidelines (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015), and this systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009; Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009).
Eligibility criteria
Primary research studies available in English were eligible for inclusion in the systematic review. In addition, studies must have been conducted in broiler chickens (the target population) and must have evaluated an antibiotic regime licensed for use in broilers in ovo, by injection, in feed, or in drinking water at doses consistent with therapeutic or prophylactic use (target intervention). Studies must have compared the antibiotic intervention to a placebo, an untreated control group, a non-antibiotic intervention, or a different antibiotic treatment. In the protocol, the eligible antibiotic regimes included any antibiotic used in treating or preventing colibacillosis in poultry that is included in the OIE list of approved antimicrobial agents of veterinary importance (OIE, 2015), regardless of their importance to human medicine. However, this was later modified to include any antibiotic regime included in a published study, due to the difference in approved antibiotic regimes over time and among countries. Eligible studies must have examined at least one of the following outcomes: mortality, feed conversion ratio (FCR), condemnations at slaughter due to colibacillosis, or total antibiotic use. Although FCR is a performance measure, it was included as an eligible outcome because it is likely to reflect the clinical and subclinical disease experience of a flock. Only controlled trials with natural disease exposure were eligible for inclusion, although we documented the number of controlled trials with deliberate disease challenge and the number of analytical observational studies evaluating eligible interventions and outcomes that were captured during the full-text screening stage.
Information sources
The databases searched were MEDLINE (via PubMed; 1946 to date of search), CAB Abstracts (via the University of Guelph CAB interface; 1900 to date of search), Science Citation Index and Conference Proceedings Citation Index – Science (via Web of Science; 1900 to date of search), and AGRICOLA (via ProQuest; 1970 to date of search). Additionally, a single reviewer hand-searched the proceedings of the Western Poultry Disease Conference (1980–2018) and the section of the United States Food and Drug Administration website dedicated to recent animal drug approvals for relevant data.
Search
Initially, the search strategy was designed around the concepts of poultry, antibiotic interventions, colibacillosis, and antibiotics to exclusively capture primary studies that examined prophylactic uses of antibiotics to prevent colibacillosis in poultry. However, during preliminary screening, articles were identified in which antibiotic interventions were applied as metaphylaxis to control colibacillosis (i.e. to prevent further illness or death in flocks where colibacillosis infections were present in some birds). In these instances, the authors referred to the interventions as ‘treatment for disease,’ The American Veterinary Medical Association defines ‘disease control’ at the population level as the use of antimicrobials to reduce the incidence of illness in groups of animals where some are already showing signs of disease or infection and ‘disease treatment’ at the population level as administration of an antimicrobial to those animals within the group with evidence of disease (https://www.avma.org/KB/Policies/Pages/AVMA-Definitions-of-Antimicrobial-Use-for-Treatment-Control-and-Prevention.aspx). The terminology used in the colibacillosis literature is not entirely consistent with the AVMA definitions, while noting that these concepts may not have been as explicitly defined at the time of publication of all of the relevant articles. We therefore deviated from the review protocol and modified the search to include search terms related to antibiotic use for prevention, control, or treatment, regardless of the specific terminology used by the authors. The updated search was conducted on 28 October 2018 through the University of Guelph, Canada. The searches were not limited by date, language, or publication type. Table 1 shows the modified search strategy as it was applied in the Science Citation Index database (via the Web of Science platform). The search string formatting was modified as needed to reflect differences in database interfaces for each of the remaining databases.
Table 1. Full electronic search string used to identify studies examining the prevention or treatment of colibacillosis in broilers as applied in the Science Citation Index (via Web of Science) on 28 October 2018
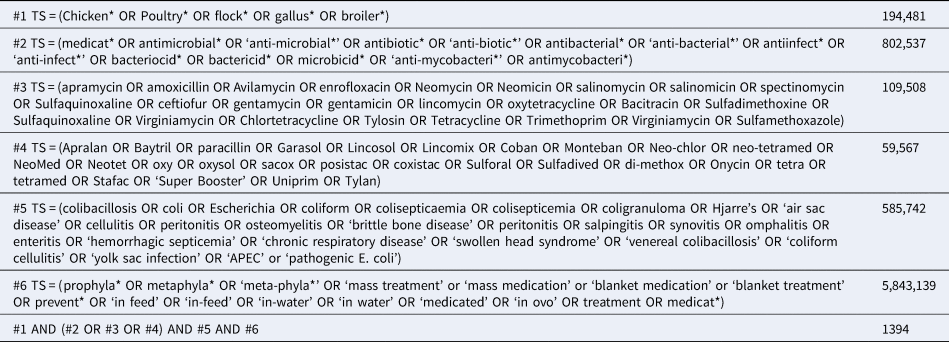
Search results were uploaded to EndNoteX7 (Clarivate Analytics, Philadelphia, PA) and duplicate citations were removed. Citations were then uploaded to the systematic review management software DistillerSR (Evidence Partners Inc., Ottawa, ON) and additional duplicates were removed. When the same data were presented in both a conference proceeding and a journal article, the conference proceeding was removed.
Study selection
DistillerSR was used to manage the screening, data extraction, and risk of bias assessment stages of the review. Initially, titles and abstracts of all citations identified in the search were screened for eligibility. All reviewers had training in epidemiology and systematic review methods, and all reviewers participated in a pre-test of the first 250 titles and abstracts to resolve any uncertainties about the wording of the screening questions. Thereafter, two reviewers independently evaluated each citation. The following questions were used to assess relevance:
(1) Is this a primary study evaluating the use of one or more antibiotics to prevent or treat* colibacillosis in broilers? [* question wording differs from the protocol, based on a protocol deviation to allow metaphylactic use in infected flocks to be included as eligible]
YES, NO (EXCLUDE), UNCLEAR
(2) Is there a concurrent comparison group? (i.e. controlled trial with natural or deliberate disease exposure or analytical observational study)
YES, NO (EXCLUDE), UNCLEAR
(3) Is the full text available in English?
YES (include for full-text screening), NO (EXCLUDE), UNCLEAR (include for full-text screening)
Citations were excluded if both reviewers responded ‘no’ to any of the screening questions. Disagreements whether to include or exclude were resolved by consensus. If consensus could not be reached, the article was marked as ‘unclear’ and advanced to full-text screening.
Following the title and abstract screening, full-text articles were retrieved and were subject to additional eligibility screening. Two reviewers independently evaluated the full-text articles, with agreement required at the question level. Any disagreements were resolved by consensus, or if consensus could not be reached, a third reviewer arbitrated the decision. All reviewers conducted a pre-test on the first ten full-texts to ensure that all eligibility questions were clear. The same three questions that were applied at the title and abstract screening level were applied again in the full-text screening, but reviewers could select only ‘yes’ (neutral) or ‘no’ (EXCLUDE). The full-text screening form also included the following questions:
(1) Is the full text available with >500 words? YES, NO (EXCLUDE)
(2) Does the study assess the use of any antibiotic intervention for the prevention of colibacillosis or pathogenic E. coli, either as prophylaxis in healthy birds or as metaphylaxis to prevent further illness/death when colibacillosis is present in the flock? YES, NO (EXCLUDE) [Italics represent deviations from the original protocol]
(3) Are at least one of the following outcomes described: mortality, FCR, condemnations due to colibacillosis, or total antibiotic use? YES, NO (EXCLUDE)
(4) Eligible study design: Is the study a controlled trial with natural disease exposure? YES (moves to data extraction stage), NO, the study is a controlled trial with deliberate disease induction (indicate the antibiotic(s) evaluated, but exclude from data extraction) NO, the study is an observational study (indicate the antibiotic(s) evaluated, but exclude from data extraction)
Data collection process
Two reviewers used a standardized form to extract data from all citations that met the full-text screening criteria. Nested forms were created in DistillerSR to facilitate data extraction for multiple intervention comparisons or outcomes within a trial. All reviewers were trained in the use of nested forms, and all reviewers piloted the forms on the first five articles to ensure consistency. Discrepancies in data extraction were resolved by consensus, or if consensus could not be reached, by a discussion with CBW or JMS.
Data items
Study characteristics
Study-level data extracted included year and country of conduct, months of data collection, setting (research or commercial flock(s)), strain of birds, sex of birds, number of flocks/farms enrolled, inclusion criteria at the flock level, rearing conditions (conventional, organic, antibiotic-free) and whether the treatment was given as prophylaxis (all birds free of colibacillosis at the start of treatment) or as metaphylaxis (some birds ill at the time of treatment initiation). These definitions are consistent with the American Veterinary Medical Association (AVMA) definitions of antimicrobial use for prevention and treatment (AVMA, 2019). Data on study characteristics were extracted for all studies included after the full-text screening. Further data on the effect sizes of the interventions were only collected, and risk of bias assessment was only undertaken if sufficient data were presented for one or more of the eligible outcomes.
Intervention details
Details on the interventions evaluated in each study were recorded, including a description of the intervention (antibiotic name, dose, route, and frequency of administration), a description of the comparison group(s), the number of birds, and flocks enrolled, the length of follow-up, any losses to follow-up, and descriptions of concurrent treatments.
Eligible outcomes
Outcomes eligible for data extraction were mortality, FCR, condemnations at slaughter due to colibacillosis, and total antibiotic use. For each outcome reported in a study, if an adjusted summary effect was presented (adjusted odds ratio (OR) or risk ratio (RR) if the outcome was binary, or least square mean differences if the outcome was continuous), these data were extracted. Variables included in the adjustment and the corresponding precision estimates were recorded. If an adjusted measure was not reported, unadjusted summary effect size (second priority) or arm-level data (third priority) were recorded along with applicable variance components. Data were not extracted if they were presented without variance measures and if a measure of variance could not be calculated.
Risk of bias in individual studies
The Cochrane Risk of Bias tool for Randomized Trials (RoB 2.0, 2016 version) was used to assess the risk of bias at the outcome level for all outcomes with extracted data (Higgins et al., Reference Higgins, Sterne, Savović, Page, Hróbjartsson, Boutron, Reeves, Eldridge, Chandler, McKenzie, Boutron and Welch2016). Signaling questions were modified for the use in livestock and poultry trials. The following domains of bias were assessed: bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of reported results. In the Cochrane risk of bias instrument, a single question in the ‘bias due to randomization’ domain asks whether the authors described the method for generating the random sequence. We modified this question to include a response category for studies in which the authors reported that allocation to the intervention groups was ‘random,’ but did not provide details on the actual method for generating the random sequence. Under the risk of bias domain related to deviations from the intended intervention, there is a question on whether the participants were aware of their assigned interventions; in the present review, the ‘participants’ in all applicable trials were broiler chickens, and so this question was always answered as ‘no’. Another question under this domain asks whether study personnel were blinded; for the purposes of this review, the animal caregivers were considered to be the relevant study personnel.
The overall risk of bias within each domain was calculated as per Higgins et al. (Reference Higgins, Sterne, Savović, Page, Hróbjartsson, Boutron, Reeves, Eldridge, Chandler, McKenzie, Boutron and Welch2016), with one exception: for bias due to the randomization process domain, we did not include allocation concealment in the algorithm because all animals within a flock are included in the type of trial involved in this review. Further, it is unlikely that a producer or investigator would have any treatment preference for a given flock, as the differential economic value of a flock would not be known at the time of allocation. This approach has been used in a previous synthesis study evaluating the risk of bias in livestock trials (Moura et al., Reference Moura, Totton, Sargeant, O'Sullivan, Linhares and O'Connor2019).
Summary measures
An effect size (OR or mean difference) was calculated for the results from individual studies where the data were presented at the arm level (i.e. raw data on the number of events and the total number of observations for each intervention group were reported). For binary data reported at the arm level, the OR and 95% confidence intervals were calculated using Epi Tools Epidemiological Calculators, available at: http://epitools.ausvet.com.au/content.php?page=2by2Table. For continuous data presented at the arm level, the mean difference and confidence intervals were calculated using OpenEpi, available at: https://www.openepi.com/Mean/t_testMean.htm.
Synthesis of results
As described in the protocol, the intention of this review was to conduct a network meta-analysis. However, due to the heterogeneity of the interventions and outcomes in the eligible studies that were captured in the search, no quantitative synthesis was performed. Trial results were presented in a forest plot for purposes of visualization, but no summary measure was calculated and heterogeneity was not formally assessed.
Risk of bias across studies
Risk of bias across studies (‘publication bias’) is usually evaluated by examining a funnel plot examination for small-study effects using pairwise comparisons. Two steps generally are recommended: a visual evaluation of the symmetry in the funnel plots, and a formal statistical test for symmetry, if sufficient data are available (>10 studies) (Higgins and Green, Reference Higgins and Green2011). In this dataset, too few observations were available for each intervention, so any assessments of symmetry could not be reliable. Therefore, an evaluation of the risk of bias across studies was not conducted.
Additional analyses
No additional analyses were conducted.
Results
Study selection
Of the 3425 unique citations identified by the search, 301 were advanced to the full-text screening (Fig. 1). There were 73 articles at the full-text screening stage that evaluated antibiotics in broilers and included at least one eligible outcome, but ultimately were excluded because they involved a deliberate disease exposure (i.e. challenge trials). No observational studies with relevant exposures and outcomes were identified. Nine controlled trials with natural disease exposure were included in the review.

Fig. 1. Flow diagram illustrating the selection of eligible studies for a systematic review of the efficacy of antibiotics for the prevention and treatment of colibacillosis in broilers.
Study characteristics
The study characteristics for the eligible trials are shown in Table 2. Reporting of the characteristics of interest was not complete for some trials, particularly concerning the months and years during which some trials were conducted. The included trials were conducted in several countries in both commercial and research flocks. The majority of studies (n = 8/9) were conducted in a single flock. Seven trials reported FCR (Jamroz et al., Reference Jamroz, Orda, Kamel, Wiliczkiewicz, Wertelecki and Skorupinska2003; Olnood et al., Reference Olnood, Mikkelsen, Choct and Iji2007; Baurhoo et al., Reference Baurhoo, Ferket and Zhao2009; Viveros et al., Reference Viveros, Chamorro, Pizarro, Arija, Centeno and Brenes2011; Amerah et al., Reference Amerah, van Rensburg, Plumstead, Kromm and Dunham2012; Bostami et al., Reference Bostami, Ahmed, Mun, Hong and Yang2016; Vineetha et al., Reference Vineetha, Tomar, Saxena, Kapgate, Suvarna and Adil2017), and one trial also reported mortality (Amerah et al., Reference Amerah, van Rensburg, Plumstead, Kromm and Dunham2012). Two trials reported assessment of one or more relevant outcomes, but the data were not presented in a form that could be extracted (Cracknell et al., Reference Cracknell, Andreotis, Facibeni, Owais and Pradella1986; Huff et al., Reference Huff, Huff, Rath, Balog and Donoghue2004). In all of the studies with extractable data, antibiotics were used prophylactically (i.e. for the prevention of colibacillosis).
Table 2. Characteristics of eligible trials investigating the efficacy of antibiotics for the prevention and treatment of colibacillosis in broilers

Risk of bias within studies
Six of the seven trials with one or more outcomes assessed for bias reported the use of random allocation to treatment group (Jamroz et al., Reference Jamroz, Orda, Kamel, Wiliczkiewicz, Wertelecki and Skorupinska2003; Olnood et al., Reference Olnood, Mikkelsen, Choct and Iji2007; Baurhoo et al., Reference Baurhoo, Ferket and Zhao2009; Amerah et al., Reference Amerah, van Rensburg, Plumstead, Kromm and Dunham2012; Bostami et al., Reference Bostami, Ahmed, Mun, Hong and Yang2016; Vineetha et al., Reference Vineetha, Tomar, Saxena, Kapgate, Suvarna and Adil2017), although none provided information on the method used to generate the random sequence. For the remaining domains of bias, none of the trials provided the information necessary to evaluate the potential risks of bias, and therefore there were ‘some concerns’ about the potential risk of bias for all bias domains for all outcomes.
Results of individual studies
The most commonly reported outcome was FCR, which was evaluated in seven trials with a total 25 treatment comparisons with at least one eligible antibiotic (Jamroz et al., Reference Jamroz, Orda, Kamel, Wiliczkiewicz, Wertelecki and Skorupinska2003; Olnood et al., Reference Olnood, Mikkelsen, Choct and Iji2007; Baurhoo et al., Reference Baurhoo, Ferket and Zhao2009; Viveros et al., Reference Viveros, Chamorro, Pizarro, Arija, Centeno and Brenes2011; Amerah et al., Reference Amerah, van Rensburg, Plumstead, Kromm and Dunham2012; Bostami et al., Reference Bostami, Ahmed, Mun, Hong and Yang2016; Vineetha et al., Reference Vineetha, Tomar, Saxena, Kapgate, Suvarna and Adil2017) (Table 3). Results were presented at the arm level for all of the trials and thus the effect sizes shown in Table 3 were calculated post hoc. The antibiotics evaluated in one or more treatment comparisons were bacitracin (four studies, 14 comparisons), virginiamycin (one study, three comparisons), avoparacin (one study, three comparisons), chlortetracycline (one study, two comparisons), and avilamycin (two studies, two comparisons). Comparison groups varied but included groups receiving no intervention, various probiotic products, mannan-oligosaccharides, herbal mixtures, grape derivatives, and one direct comparison of different antibiotics. All interventions in all trials were applied at the group (flock) level. Comparisons for FCR are shown in Fig. 2. None of the comparisons between an antibiotic intervention and an alternative intervention showed a benefit of one intervention over another. The comparisons between an antibiotic and a no-treatment control group showed numeric benefits of the antibiotic treatment, although many of the confidence intervals were wide and generally included the null value. The heterogeneity among specific interventions and comparators precluded a quantitative summary of antibiotic efficacy, and thus no summary effect or evaluation of heterogeneity is included in Fig. 2.

Fig. 2. Forest plot to illustrate the efficacy of antibiotics compared to alternative treatments or to non-treated controlled from a systematic review on the efficacy of antibiotics to prevent or treat colibacillosis in broiler chickens.
Table 3. Intervention arms and results for studies included in a systematic review of the efficacy of antibiotics for the prevention and treatment of colibacillosis in broilers
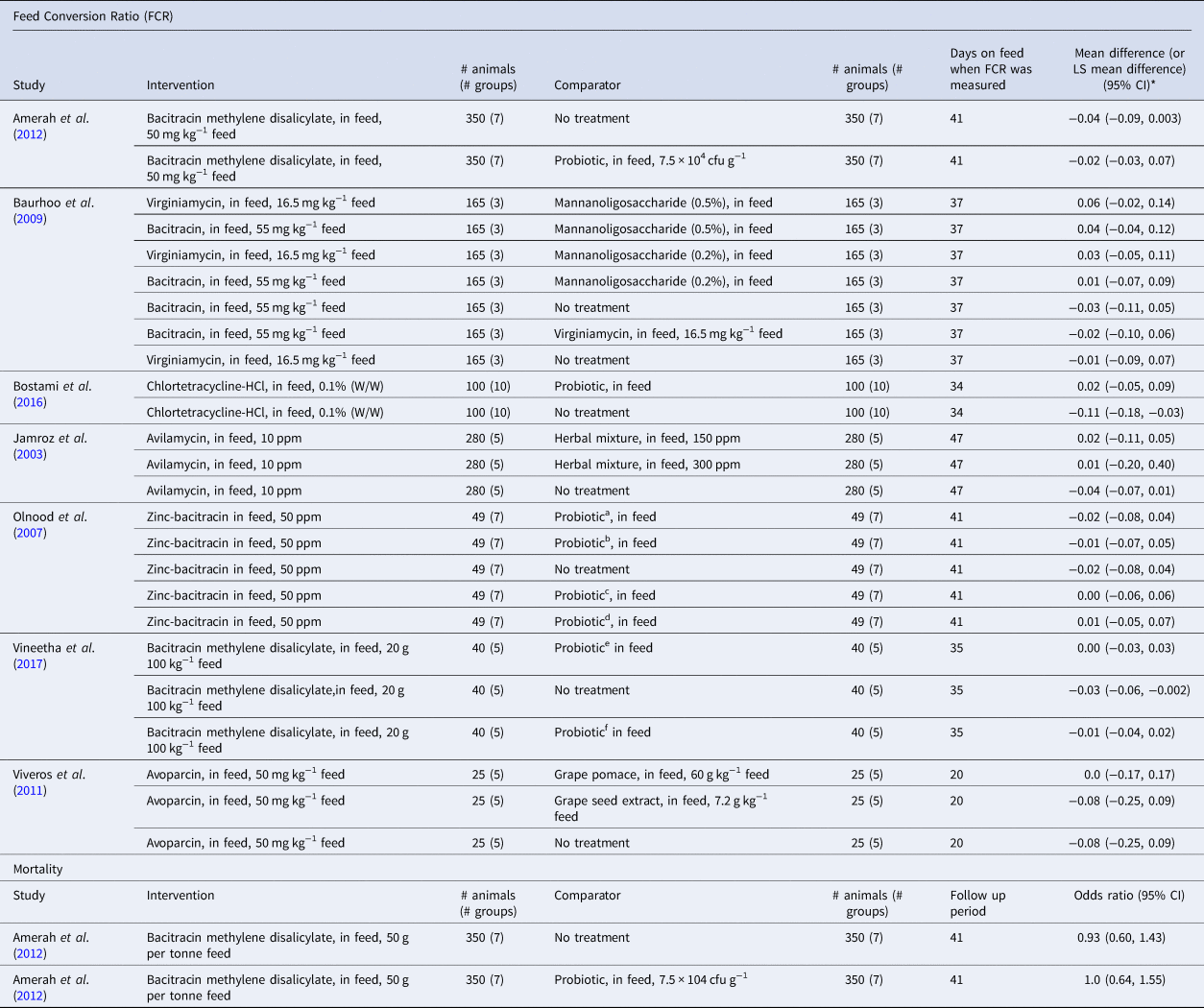
a No. 1286 L. johnsonii.
b No. 709 L. crispatus.
c L. salivarius.
d No 461 unidentified lactobacilli sp.
e 1 × 108 cfu of L. plantarum LGFCP4 g−1 feed, mannanoligosaccharide 1 g kg−1 feed.
f 1 × 108 cfu of L. acidophilus g−1 feed, mannanoligosaccharide 1 g kg−1 feed.
*Effect sizes calculated post hoc from raw data provided in publications.
None of the included trials reported results for condemnations at slaughter due to colibacillosis, and none of the trials reported total antibiotic use by the group. A single trial reported mortality outcomes for two comparisons: one between a group receiving bacitracin methylene disalicylate and a no-treatment control group, and another between the antibiotic group and a probiotic control group (Amerah et al., Reference Amerah, van Rensburg, Plumstead, Kromm and Dunham2012) (Table 3). The results of both comparisons had corresponding confidence intervals that included the null value.
Synthesis of results
The research synthesis approach proposed in the protocol was not conducted due to the sparsity of data available to address the review question.
Risk of bias across studies
Not conducted due to insufficient data.
Additional analysis
None conducted.
Discussion
Summary of evidence
Although antibiotic use in the poultry industry has decreased significantly as a result of the Veterinary Feed Directive (VFD; https://www.fda.gov/animalveterinary/developmentapprovalprocess/ucm071807.htm), and consumer pressure for antibiotic-free products, antibiotics are still used to manage some diseases. Given the societal imperative to use antibiotics judiciously, it is important to consider the scientific efficacy of specific antibiotics for specific diseases as a part of the treatment decision-making process. Based on the available scientific literature, there is no strong scientific evidence for the efficacy of antibiotics to prevent or treat colibacillosis in broilers. However, it is important to consider that only a small volume of literature exists, and existing trials examined heterogeneous interventions and comparison groups. In addition, most trials had poor reporting of key trial design features that are necessary to assess the validity of the research. As a result, there may be some uncertainty about the true efficacy of antibiotics for the prevention or control of colibacillosis in broilers. Although there is no compelling evidence that antibiotics are effective, there is also no compelling evidence that they are not.
We only included controlled trials with natural disease exposure in this review. For interventions where it is feasible to allocate individuals to treatment groups, results from controlled trials provide higher evidentiary value compared to studies using an observational design (Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a; Roudebush et al., Reference Roudebush, Allen, Dodd and Novotny2004). Challenge trials may be a useful component of the development and validation process for an intervention, as they may provide proof of concept for efficacy. However, the conditions of the disease challenge may not be representative of natural disease exposure, and in addition, challenge trials tend to be conducted in more controlled settings than what is typical of commercial operations (Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a, Reference Sargeant, Kelton and O'Connor2014b). Published challenge trials also tend to result in exaggerated treatment effects compared to natural disease exposure trials evaluating the same intervention and outcome (Egger et al., Reference Egger, Smith, Schneider and Minder1997; Wisener et al., Reference Wisener, Sargeant, O'Connor, Faires and Glass-Kaastra2014). This is partially related to publication bias; challenge trials often involve smaller numbers of animals than natural disease exposure trials, and small studies are more likely to be published if they show statistically significant results (Egger and Smith, Reference Egger and Smith1998). Thus, small challenge trials that show a statistically significant intervention benefit are more likely to be published compared to small challenge trials that show no effect of the intervention. Therefore, although challenge trials represented a larger body of literature (73 challenge trials versus 9 trials with natural disease exposure met the eligibility criteria for population, intervention, and outcome) we chose not to include challenge studies in this review.
The eligible outcomes used in this review were selected based on their importance for decision-making concerning the use of antibiotics. The most consistently reported outcome was FCR measured across the entire growing period. Feed conversion ratio provides a measure of the amount of weight each bird gains per unit of food consumed and is thus a measure of bird performance. The antibiotic interventions examined in the trials included in this review were intended to prevent or treat illness, rather than for growth promotion purposes. However, FCR was a commonly reported measure in the literature and is of importance to poultry producers.
Meta-analysis can be conducted when there is a minimum of two studies reporting the same outcome. In this review, six trials measured FCR, but there was essentially no replication of interventions or comparison groups within the trials measuring this outcome. The results of a single study represent one observation within a distribution of possible study results, which may vary due to nuanced differences in the populations, interventions, outcome measurements, and disease exposures between trials. Therefore, without replication, it is not possible to evaluate whether the results from a single study represent the true efficacy of an intervention or whether the results represent an outlier (e.g. a type I where the results suggest a statistical difference when none is present, or a type II error result where there is an actual difference but it is not identified in the sample population).
Comparison groups in the captured trials included treatments with other antibiotics, non-treated controls, and alternative (non-antibiotic) treatments. Given the importance of identifying effective alternatives to antibiotics, alternative products may be the most appropriate control group. In this review, eligible studies needed to include at least one antibiotic treatment arm. Since the goal of this review was not to assess the efficacy of non-antibiotic interventions, our search was not designed to identify all trials evaluating the efficacy of non-antibiotic interventions. However, future reviews could investigate the number and quality of trials examining alternative treatments. Such reviews could provide valuable syntheses of the existing evidence for the efficacy of non-antibiotic interventions, which would further inform treatment decision-making.
The results of this review highlight a number of issues related to the completeness of reporting primary studies. These issues included reporting of the characteristics of the trials, most notably related to reporting of the country where the trial was conducted, the month(s) and year of the trial, and whether the trial was conducted in a commercial setting. Information on the study setting and population(s) is necessary for the reader to make judgements about the external validity of trial results in their own context. Without knowledge of the specific context of a trial, a reader cannot evaluate the various context-specific factors that might impact the application of the research findings to other contexts.
Information on key trial design features is necessary to allow readers to evaluate the potential for bias in the results of the trial. Additionally, poor reporting of design features is associated with exaggerated treatment effects (Schulz et al., Reference Schulz, Chalmers, Hayes and Altman1995; Moher et al., Reference Moher, Pham, Jones, Cook, Jadad, Moher, Tugwell and Klassen1998; Burns and O'Connor, Reference Burns and O'Connor2008; Sargeant et al., Reference Sargeant, Elgie, Valcour, Saint-Onge, Thompson, Marcynuk and Snedeker2009a, Reference Sargeant, Saint-Onge, Valcour, Thompson, Elgie, Snedeker and Marcynuk2009b). The information needed to access the risk of bias in the domains of bias included in the Cochrane Risk of Bias instrument (Higgins et al., Reference Higgins, Sterne, Savović, Page, Hróbjartsson, Boutron, Reeves, Eldridge, Chandler, McKenzie, Boutron and Welch2016) was not provided in any of the trials included in this review. The Cochrane risk of bias tool was developed for evaluating randomized controlled trials in human healthcare. However, the general tenets of good trial design do not vary between human and veterinary medical research. It is possible that study authors did not conduct the trials using accepted methods for high-quality trial design, or they may have conducted the trials with a high degree of methodological rigor, but did not report information on key design features. Nonetheless, a reader has only the information provided in the publication by which to access the methodological rigor, and if that information is not available, then the reader cannot judge the appropriateness of the approach, methods, and ultimately the results of the study.
Deficiencies in reporting have been documented in numerous publications examining reporting quality in food animal studies (Wellman and O'Connor, Reference Wellman and O'Connor2007; Burns and O'Connor, Reference Burns and O'Connor2008; Sargeant et al., Reference Sargeant, Elgie, Valcour, Saint-Onge, Thompson, Marcynuk and Snedeker2009a, Reference Sargeant, Saint-Onge, Valcour, Thompson, Elgie, Snedeker and Marcynuk2009b; Brace et al., Reference Brace, Taylor and O'Connor2010; Winder et al., Reference Winder, Churchill, Sargeant, LeBlanc, O'Connor and Renaud2019). The REFLECT statement was developed by an expert consensus process in response to concerns about the quality of reporting in clinical trials in livestock (O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010a). The REFLECT statement consists of a 22-item checklist to provide guidance on what should be reported in livestock trials, as well as an explanation and elaboration document that provides additional details for each item on the checklist. The REFLECT statement methods and elaboration documents were co-published in multiple journals (O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010a, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010b, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010c, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010d, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010e; Sargeant et al., Reference Sargeant, O'Connor, Gardner, Dickson, Torrence, Dohoo, Lefebvre, Morley, Ramirez and Snedeker2010a, Reference Sargeant, O'Connor, Gardner, Dickson, Torrence, Dohoo, Lefebvre, Morley, Ramirez and Snedeker2010b), and are also available online (http://www.reflect-statement.org/; https://meridian.cvm.iastate.edu/). Improved reporting of trials will allow readers to make a clearer, more accurate judgement about the validity of study results.
Limitations
In searching for relevant literature, we used multiple electronic databases, as well as some gray literature sources. However, it is known (although difficult to document), that there is considerable research conducted in-house within the vertically integrated poultry industry, and the results of this research may not be publicly available. Thus, it is possible that our results do not reflect the body of research that has been conducted to evaluate the efficacy of antibiotics for preventing or treating colibacillosis. However, our results do reflect the publicly available literature for decision-making by those outside of poultry groups who conduct their own research.
Conclusions
In conclusion, based on a small volume of heterogeneous studies, there is no strong evidence for or against the efficacy of antibiotics to prevent or treat colibacillosis in broilers. Reporting of study characteristics and key trial design features was generally poor and could be improved by following recommended reporting guidelines for controlled trials.
Acknowledgments
The authors thank Rachael Vriezen for editorial support.
Author contributions
JMS developed the review protocol, coordinated the project team, assisted with data analysis, interpreting the results, and wrote the manuscript drafts.
MB, KC, KD, BD, JD, MR conducted relevance screening, extracted data, conducted risk of bias assessments, commented on manuscript drafts and approved the final manuscript version.
AMOC assisted with the development of the review protocol, provided guidance on the interpretation of the results, commented on manuscript drafts and approved the final manuscript draft.
CML and AV assisted with the development of the review protocol, provided guidance on the interpretation of the results, commented on manuscript drafts, and approved the final manuscript.
CBW assisted with the development of the review protocol, assisted with data screening, data extraction and risk of bias assessment, conducted the analysis, provided guidance on the interpretation of the results, commented on manuscript drafts, and approved the final manuscript draft.
Financial support
Support for this project was provided by The Pew Charitable Trusts.
Conflicts of interest
None of the authors has conflicts to declare.







