Recent meta-analysis of the productivity of livestock without enteric ciliate protozoa shows that the performance of protozoa-free animals exhibits an 11 % advantage in live-weight (LW) gain over normal (faunated) livestock(Reference Eugène, Archimède and Sauvant1). Additionally, enteric production of the greenhouse gas methane has been shown to be reduced by 13–30 % when the rumen is protozoa-free(Reference Kreuzer2, Reference Hegarty3), suggesting improved productivity can be achieved with reduced environmental impact through the control of protozoa.
Protozoa compete successfully with other rumen micro-organisms and can become established in the rumen from a small source of inoculum. Therefore, if the long-term effects of defaunation are to be studied, all protozoa must be completely removed from the rumen. Eradication of rumen protozoa has been achieved by feeding high-grain diets(Reference Whitelaw, Eadie, Mann and Reid4), by dietary additives as well as by oral drenches with surfactants and specific compounds toxic to protozoa(Reference Williams and Coleman5). However, these eradication methods also impact on the other populations of microbes in the rumen, so the changes associated with defaunation in these studies cannot be exclusively attributed to the absence of protozoa. An alternative means of obtaining protozoa-free livestock is to prevent stock from acquiring a protozoal population, so no chemical agents are used. This has been achieved by either separating the newborn animal at birth and rearing the young animal in isolation(Reference Pounden and Hibbs6) or by breeding from protozoa-free ewes(Reference Bird, Leng, Leng, Barker, Adams and Hutchinson7).
Both rearing livestock in isolation and protozoal eradication are possible commercial options to produce and maintain protozoa-free stock. A major study was conducted to determine the productivity and methane production characteristics of lambs with and without protozoa. Protozoa-free lambs were either reared protozoa-free, or chemically defaunated at weaning.
Materials and methods
Experimental animals
Merino ewes (n 108), 2·5 years of age, were selected from a flock of 573 ewes to have pre-mating LW between 37·5 and 46·0 kg and yearling greasy fleece weights between 2·5 and 3·5 kg with fibre diameters of 16–19 μm. Ewes were allocated using stratified randomisation on LW and greasy fleece weights to one of three treatment groups to provide flocks containing thirty-six ewes each. All ewes were inseminated using frozen semen from a single Poll Dorset ram. Treatment groups were as follows.
Ewe flock 1
Following artificial insemination, ewes were drenched with an anti-protozoal agent to remove protozoa from the rumen. Lambs were reared protozoa-free (BF) by their defaunated mothers.
Ewe flock 2
Ewes remained untreated (protozoa present in the rumen) after artificial insemination. Lambs were reared with protozoa until weaning and then drenched with an anti-protozoal agent to remove protozoa from the rumen (DF).
Ewe flock 3
Ewes remained untreated (protozoa present in the rumen) after artificial insemination. Lambs remained untreated with naturally established populations of protozoa in the rumen (F).
The two faunated flocks of ewes (flocks 2 and 3) were considered as a single treatment as there was no difference in management between the ewes and their lamb progeny in these flocks until lambs were weaned.
The mean LW of ewes in flocks 1, 2 and 3 at insemination was 41·5, 42·2 and 42·1 kg respectively. The mean greasy fleece weights and fibre diameters for flocks 1, 2 and 3 in the year preceding this experiment were 2·9, 3·0 and 2·9 kg, and 17·4, 17·7 and 17·5 μm respectively.
Experimental procedures
A schedule of experimental activities is provided in Table 1. In preparation for artificial insemination by a commercial operator, all ewes were treated using the following protocol. Intravaginal progesterone-releasing devices (Eazi-breed sheep and goat device CIDR; Pharmacia and Upjohn, Rydalmere, NSW, Australia) were inserted into ewes for 16 d then removed and each ewe injected with Folligon (1·5 ml/ewe; Intervet Australia Pty Ltd, Bendigo East, Vic, Australia). Approximately 25 h after CIDR removal, ewes were inseminated with semen from a single Poll Dorset sire. After insemination, the three flocks were grazed in separate paddocks in a rotational grazing system containing nine paddocks, with flocks being moved every 2–3 weeks. All ewes were ultrasonically scanned to determine their pregnancy status after 9 weeks. Thereafter, ewes in flock 1 were penned individually in an animal shed and fed a lucerne chaff diet (800 g/d) for 7 d. Commencing on day 8, each ewe was orally dosed with sodium 1-(2-sulfonatooxyethoxy) dodecane prepared as 100 ml 10 % solution from a stock of Empicol ESB/70AV (Allbright and Wilson Australia Ltd, Melbourne, Vic, Australia) to remove protozoa. Ewes were dosed on four consecutive days and feed was withheld during this period. Animals required 7 d to recover pre-drenching appetites after treatment was completed. The treatment dose was delivered with an oesophageal tube inserted into the rumen. After dosing, rumen fluid samples were collected (using an oesophageal tube) at weekly intervals from each ewe to check for the presence of protozoa. At 4 weeks after drenching, defaunated ewes were returned to the paddock. Flocks 2 and 3 remained in the rotational grazing system during this period. Protozoa status of the ewes was checked when lambs were weaned and again when ewes were shorn at the completion of the trial (Table 1). Defaunated ewes remained free of protozoa. Wool growth (greasy fleece weights) of the ewes was measured from September (year 1) to July (year 2), a period of 320 d.
Table 1 Schedule of activities in establishing flocks of faunated and defaunated ewes and of studies to measure methane production and growth of lambs in feeding studies with concentrate pellets (trial 1) and roughage only (trial 2)

VFA, volatile fatty acid.
Lambs were born within a 7 d period. All newborn lambs were identified to their dam, weighed and ear-tagged within 24 h of birth. At 4 weeks of age lambs were de-tailed, castrated, vaccinated for clostridial diseases (Tasvax; Schering-Plough Pty Ltd, Australia) and drenched with an anthelmintic (Cydectin; Fort Dodge Australia Pty Ltd, Baulkham Hills, NSW, Australia) to control internal parasites. Lambs received a second clostridial vaccination and anthelmintic drench at weaning (mean age 101 d). Ewes and lambs were weighed at 3-weekly intervals until weaning. The numbers of lambs weaned from flocks 1, 2 and 3 were thirty-eight, forty and forty respectively. Lambs were weaned into new paddocks for a period of 14 d before being individually penned in animal houses for controlled feeding and measurement of methane emissions.
After weaning, two controlled feeding trials were conducted. In trial 1, lambs were given a high-concentrate ration; in trial 2, lambs were given an all-roughage ration.
Trial 1
Protozoa-free lambs (BF; n 38 and DF; n 40) were housed in individual pens in the same building while faunated lambs (F; n 40) were housed in individual pens in a separate building. Lambs were initially offered lucerne chaff and then gradually adapted to include sorghum-based pellets (13·7 or 20·7 % crude protein) that also contained 20 % cotton hulls and contained a trace mineral supplement (Animal Nutrition Products Pty Ltd, Toowoomba, Qld, Australia). The mineral and vitamin composition (expressed as a % of DM) of the high- and low-protein pellet was similar and was as follows: Ca, 6·2 g/kg; P, 4·3 g/kg; K, 7·6 g/kg; Na, 4·3 g/kg; S, 2·7 g/kg; Mg, 2·8 g/kg; Co, 0·28 mg/kg; Cu, 6·73 mg/kg; I, 0·56 mg/kg; Mn, 30 mg/kg; Se, 0·22 mg/kg; Zn, 41·73 mg/kg; vitamin E, 8·97 mg/kg; vitamin A, 1346 μg/kg. After removal of lambs for inappetence or health problems the final number of lambs were thirty-four lambs in the BF group, thirty-three lambs in the DF group and thirty-two lambs in the F group. Protozoa were removed from the DF lambs using the same surfactant-dosing protocol that was used for the ewes with the exception that the dosing rate varied with body weight (35 ml dose solution per 15 kg body weight). This procedure was subsequently repeated for all DF lambs using an increased dose rate (50 ml dose solution per 15 kg body weight) because not all lambs were defaunated with the initial dose. All lambs remained on their allocated diet during this period and lambs in the BF and F groups were fed to appetite. At 2 weeks after the defaunation treatment of the DF lambs had been completed, the daily ration was fixed at 800 g pellets (as fed) and 200 g lucerne chaff (as fed) and 10 g calcium chloride (Table 2). The restricted ration was fed for a period of 2 weeks before trial 1 commenced. The average age of the lambs at the commencement of trial 1 was 195 d and the average LW of the lambs in the BF, DF and F groups was 32, 26 and 30 kg respectively. Feed refusals were minimal during trial 1, with all lambs consuming in excess of 98 % of feed offered.
Table 2 Composition (% in diet as fed) and nutrient profile of pellet and lucerne diets used in performance trial 1 and of mixed chaff ration used in performance trial 2
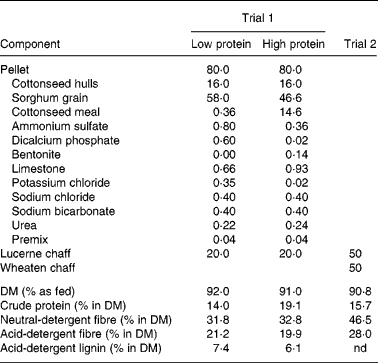
nd, Not determined.
Tissue depth at the GR site (110 mm from the midline over the 12th rib) of all lambs was assessed manually during weighing. An Aloka 500 ultrasound scanner with a 3·5 MHz probe (Aloka, Wallingford, CT, USA) was used to determine loin muscle depth at the 12th rib and fat depth at the C-site (45 mm from the midline over the 12th rib). Lambs were weighed and scanned for loin muscle depth on day 1, day 35 and day 70 of the 10-week experimental period(Reference Hegarty, Shands, Marchant, Hopkins, Ball and Harden8). Rumen fluid samples were collected (using an oesophageal tube) from each lamb in week 5 and week 10 of the study. Samples of the rumen fluid sample were analysed for volatile fatty acids (VFA)(Reference Erwin, Macro and Emery9), ammonia concentration and visual enumeration of small ( < 125 μm) and large (>125 μm) holotrich and entodiniomorph protozoa using a Hawksley Cristalite B.S. 748 counting chamber (Lancing, Sussex, UK). Wool growth was estimated using the dye-band method of Chapman & Wheeler(Reference Chapman and Wheeler10). Dye-bands were applied on day 9 and day 70 of the experimental period. Following the removal of the dye-bands all lambs were shorn and fleece weights recorded.
Methane emissions were measured from sixty lambs held in respiration chambers (n 4) during the final 3 weeks of the experimental period. Lambs were selected at random from those available to include five male and five female lambs from both the low- and high-protein dietary groups within each of the three treatment groups (BF, DF and F). Five lambs from each of the treatment groups were allocated to each of the four respiration chambers. The risk of chamber differences biasing treatment effects and the risk of transfer of protozoa from F to BF or DF lambs was minimised by F lambs being measured in chambers 3 and 4 on days 1–5 of measurement, and being measured in chambers 1 and 2 on days 11–15 of measurement. After day 5, chambers 3 and 4 were thoroughly cleaned to remove any residual protozoal cells that may have been present. At least one lamb from the high- and low-protein diet within each treatment group was allocated to each of the four chambers.
Methane production was measured over a 21 h period for each lamb, with the daily ration being provided as the lamb was placed in the chamber. If animals refused more than 100 g of feed while in the chamber, measurement of methane emissions was repeated at a later time. Chambers were 1·1–1·3 m3 in volume, of polycarbonate construction with approximately 150 litres/min of external air drawn through the chamber by a side channel blower (Uni-jet 40CE; ESAM, Parma, Italy) as measured by a dry gas meter (AL-800; American Meter Company, Horsham, PA, USA). Air within the chamber was continuously circulated by a 30 cm diameter fan. Methane concentration in the incoming and outgoing air stream was measured at approximately 10 min intervals using an Innova 1312 Photoacoustic multigas monitor with moisture compensation (Innova Airtech Instruments, Ballerup, Denmark). Recovery of a dose of pure methane introduced to each chamber was tested and found to be within 98–101 %. Methane production was calculated as air flow multiplied by methane concentration in the effluent air, adjusted for methane concentration of the incoming air and temperature and atmospheric pressure in the chamber.
Trial 2
Lambs were shorn at the conclusion of trial 1 and twelve female lambs were randomly selected to include six lambs from both the high- and low-protein dietary groups within each of the three treatment groups (BF, DF and F). The average age of the lambs at the commencement of trial 2 was 287 d and the average LW of the lambs in the BF, DF and F groups was 39, 36 and 37 kg respectively. Lambs were individually penned in the same animal house and protozoa-free lambs (BF and DF) were separated by at least 1·5 m from the F lambs to prevent protozoal contamination of the defaunated animals. Lambs were offered a ration (as fed) of lucerne chaff (400 g/d) and wheaten chaff (400 g/d) for a period of 21 d before measurement of methane emissions commenced. Rumen fluid samples were collected (using an oesophageal tube) from each lamb at the end of the study. Samples of the rumen fluid sample were analysed for VFA, ammonia concentration and enumeration of protozoa as for trial 1.
Statistical procedures
Allocation of ewes to flocks and lambs to treatments in trials 1 and 2 was made according to stratified randomisation. Analysis of protozoal effects on ewe LW and growth and compositional characteristics of the lambs was made using generalised linear models in Minitab (version 12; Minitab 1997). In all models, sex and litter size of the lambs were fitted as fixed effects. The diet × protozoa interaction for methane production was fitted and least square difference (LSD) value calculated using Genstat (version 9.1; VSN International Ltd, Hemel Hempstead, Herts, UK)(Reference Payne and Lane11).
Results
Grazing ewes
Ewes in the faunated flocks gained 1 kg LW in the period between insemination and peak lactation (considered to be 4 weeks after lambing) while the ewes in the protozoa-free flock lost 0·4 kg in the same period, during which they had been treated to achieve defaunation (Table 3). These changes in LW resulted in defaunated ewes being lighter than faunated ewes at peak lactation (P < 0·01). Despite the lower LW of defaunated ewes, their single lamb progeny were heavier at birth and both single and twin lambs grew faster in the period from birth to weaning than did lambs in the faunated ewe flocks (Table 3). The combined effects of higher birth weight and faster pre-weaning growth rate of lambs born to defaunated ewes led to these lambs being 11 % (2·9 kg) heavier than faunated lambs at weaning. Wool growth rate of the ewes tended to be greater in the defaunated flock (P = 0·10), while single-bearing ewes had faster fleece growth than did twin-bearing ewes (11·3 v. 10·6 g/d; P < 0·001).
Table 3 Live weights (LW) of faunated (+P) and protozoa-free (−P) ewes at insemination and at peak lactation and birth weights and growth rates of their lamb progeny before commencement of lamb performance trial 1
(Mean values with their standard errors)
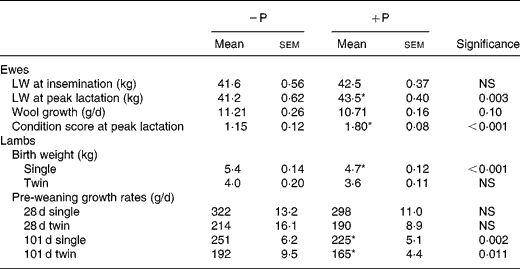
* Mean value was significantly different from that of the − P animals (P < 0·05).
Lamb performance trial 1
LW gain and body composition indices of lambs once penned and fed high-concentrate rations were not affected by protozoa treatments (Table 4). Wool growth rate of lambs reared free of protozoa (BF), however, was approximately 10 % higher during trial 1 than was wool growth rate of either chemically defaunated lambs (DF) or faunated lambs (F) which were similar (P < 0·05).
Table 4 Growth, methane production and ruminal characteristics of lambs either born and reared free of protozoa (BF), chemically defaunated post-weaning (DF) or faunated (F)*
(Mean values with their standard errors)

GR site, 110 mm from the midline over the 12th rib; C-site, 45 mm from the midline over the 12th rib; VFA, volatile fatty acid.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Data are averaged for low- and high-protein diets in performance trial 1.
The protozoal population in the rumen of faunated lambs (mean 7·5 × 105 cells/ml) consisted of small Entodinium spp. (97 %), and Epidinium spp. and large isotrichs (3 %). Populations of small holotrichs ( < 1 % total cells) were observed in some faunated lambs. Rumen fermentation pattern was influenced by the presence of protozoa, with BF and DF groups having a higher proportion of acetate (P < 0·001) while the proportion of propionate was lower compared with faunated lambs (P < 0·05; Table 4). The rumen concentrations of total VFA and of ammonia were higher in faunated lambs than in BF and DF lambs, respectively (P < 0·05). Methane production (litres/d) was not affected by protozoal treatments (Table 4) but was greater in single-born lambs than in twin lambs (19·7 v. 17·2 litres/d).
LW gain and indirect indicators of body composition of lambs were not affected by the level of protein in the diet (P>0·05; Table 5). When compared with the low-protein diet, however, wool growth rate was approximately 9 % higher for lambs fed the high-protein diet. High dietary protein was also associated with a significant increase in rumen VFA concentration and proportion of propionate (P < 0·05), suggesting increased rumen fermentation. The concentration of rumen ammonia in lambs fed the high-protein diet was more than double the concentration measured in lambs fed the low-protein diet (Table 5), while the density of protozoa (cells/ml) in rumen fluid collected from faunated lambs was not affected by the protein content of the diet. The effect of protozoa status on methane production was influenced by dietary protein level. BF lambs produced 18 % more methane from the low-protein diet when compared with the high-protein diet (Fig. 1). In contrast, methane production from the faunated lambs was higher (25 %) when fed the high-protein diet. Methane production from the DF lambs was not affected by the level of dietary protein (Fig. 1). BF lambs produced 30 % more methane than F lambs when fed the low-protein diet but 12 % less methane than faunated lambs when fed the high-protein diet (Fig. 1). DF lambs also produced more methane (18 %) than faunated lambs when fed the low-protein diet but methane production for these two groups did not differ when fed the high-protein diet.
Table 5 Growth, methane production and ruminal characteristics of lambs fed either a low- (14·0 % crude protein) or high-protein (19·1 % crude protein) ration†
(Mean values with their standard errors)

GR site, 110 mm from the midline over the 12th rib; C-site, 45 mm from the midline over the 12th rib; VFA, volatile fatty acid.
* Mean value was significantly different from that of the animals fed the ration containing 14·0 % crude protein (P < 0·05).
† Data are averaged over protozoa treatments for lamb performance trial 1.

Fig. 1 Mean methane production of lambs differing in protozoal status consuming diets of 14·0 % (![]() ) or 19·1 % (
) or 19·1 % (![]() ) crude protein. Lambs were either reared free of protozoa (BF), chemically treated at weaning (DF) or untreated and retaining a normal rumen protozoal population (F). Vertical bars represent maximum standard errors for comparison between means (1·55; least square difference = 3·12). a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
) crude protein. Lambs were either reared free of protozoa (BF), chemically treated at weaning (DF) or untreated and retaining a normal rumen protozoal population (F). Vertical bars represent maximum standard errors for comparison between means (1·55; least square difference = 3·12). a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
Feeding trial 2
This study was conducted specifically to determine the effects of defaunation on the production of methane from lambs fed a roughage diet. Results indicate that methane production was not affected by the defaunation treatments and, in contrast to trial 1, rumen fermentation characteristics did not differ among treatments (Table 6). Even at the lower feed intake of trial 2 (800 g/d) relative to trial 1 (1000 g/d), methane production from the lambs given the roughage diet in trial 2 was 23 % greater than from the lambs fed the concentrate diet in trial 1.
Table 6 Methane production and concentration of volatile fatty acids (VFA) and ammonia in rumen fluid collected from lambs that were either born free of protozoa (BF), chemically defaunated post-weaning (DF) or faunated (F)*
(Mean values with their standard errors)
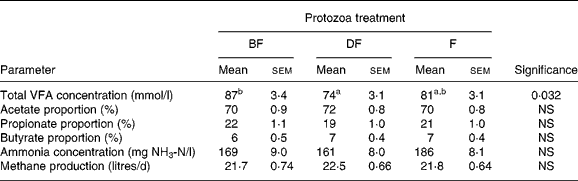
a,b Values within a row with unlike superscript letters were significantly different (P < 0·05).
* Lambs were fed 800 g of an all-roughage diet per d (lamb performance trial 2).
The protozoal population in the rumen of faunated lambs (mean 2·9 × 105 cells/ml) consisted of small Entodinium spp. (90 %) and large entodiniomorphs (10 %).
Discussion
The superior performance of the defaunated ewes, producing heavier lambs at birth, supporting faster lamb growth and tending (P = 0·1) to produce more wool, despite a lower body weight during lactation, is consistent with reported studies of wool and lamb growth(Reference Bird and Leng12, Reference Ivan, Dayrell, Mahadevan and Hidiroglou13). Since one of the prime nutritional changes in livestock after defaunation is an increased amino acid supply(Reference Eugène, Archimède and Sauvant1), the breeding ewe is the ideal beneficiary of defaunation, with both wool production(Reference Reis and Schinckel14) and pre-weaning growth(Reference Walker and Cook15) typically limited by amino acid supply. That amino acid supply was limiting productivity of the grazing ewes was apparent from the lower wool growth of twin-bearing, compared with single-bearing ewes. It is assumed that the superior early postnatal growth of lambs born to defaunated ewes resulted from increased milk production and/or milk protein content, since lamb growth is highly sensitive to protein supply(Reference Black and Griffiths16) and defaunation increases milk production(Reference Moate and Farrell17) while lambs consume little pasture in the early postnatal period(Reference Langlands18). The continued superior growth of protozoa-free lambs up until weaning may again have been a consequence of greater protein supply, arising not only from greater milk protein intake, but also from differences in flow of dietary and microbial amino acids from the rumen(Reference Ushida, Kayouli, De Smet and Jouany19).
Protein supply as manipulated by diet or protozoa did not affect indices of lamb body composition (loin muscle, tissue depth at GR site, C-fat depth), which is consistent with other studies of protein-supplemented weaned lambs(Reference Hegarty, Neutze and Oddy20). While body growth and compositional indices were similar for all combinations of dietary protein and protozoa status, growth of wool continued to respond to an increased protein supply produced by either dietary or protozoal treatments. The exception was wool production by DF lambs which did not differ from that of F lambs. The absence of a wool growth response in the DF lambs was probably due to low body protein reserves caused by the extended period of feed restriction and anti-protozoal drenching required to remove protozoa from these lambs before the commencement of performance trial 1.
Within the rumen, however, it was apparent that both diet and protozoa modified the fermentation, albeit in different manners. The absence of protozoa (BF) reduced total VFA concentration and decreased the acetate:propionate ratio, with increased acetate and reduced propionate proportions. The lower total VFA concentration may have resulted from decreased ruminal DM fermentation(Reference Veira21, Reference Ushida, Jouany, Demeyer, Tsuda, Sasaki and Kawashima22) as well as a greater percentage of fermented material being utilised in microbial cell synthesis rather than proceeding to VFA. A review of the literature(Reference Eugène, Archimède and Sauvant1) indicated that on average the molar proportion of propionate is lower in faunated than in protozoa-free ruminants (20·2 v. 23·1 molar %), but the changes in VFA following defaunation are not predictable(Reference Coleman23). The inconsistent effect of defaunation is exemplified in the studies of Christiansen et al. (Reference Christiansen, Kawashima and Burroughs24) and Males & Purser(Reference Males and Purser25) in which molar proportions of VFA were reported for faunated and defaunated lambs fed almost identical rations of lucerne and maize. In the former study the molar proportion of propionate in faunated lambs was 24·6 % and declined to 21·4 % in defaunated lambs, while in the latter study the molar proportion of propionate in faunated lambs was 23·3 % and increased to 30·3 % in defaunated lambs. The bacterial population is largely responsible for the fermentation of organic matter and the production of VFA and methane in the protozoa-free rumen. While it is known that the number of bacteria increase in the absence of protozoa(Reference Bryant and Small26) and the composition of the bacterial population changes(Reference Ozutsumi, Tajima, Takenaka and Itabashi27), the factors influencing the establishment and final composition of the bacterial population in the rumen following defaunation are not understood. Until more data become available on the composition of the bacterial population and corresponding measurement of VFA and methane in faunated and defaunated animals, our understanding of the relationship between defaunation and methane production will remain limited.
While fermentation results were consistent with the existing understanding of stoichiometric relationships between VFA and methane production, the expected differences between faunated and defaunated sheep with respect to VFA proportions and methane emissions were not observed in these studies. A review of the literature(Reference Hegarty3) indicated that in the absence of protozoa, methane emissions were reduced by an average of 13 %, with reductions in methane emissions of up to 25 % being reported(Reference Finlay, Esteban, Williams, Embley and Hirt28). Hegarty(Reference Hegarty3) suggested that lower methane emissions from protozoa-free sheep can be attributed to one or more of the following: reduced DM fermentation in the rumen; increased incorporation of fermented DM entering microbial cells; hydrogen production reduced commensurate with lower acetate production; hydrogen consumption increased commensurate with increased propionate production. It is assumed that concomitant with these changes in the protozoa-free rumen, the population of methanogens is also reduced. The greater wool production observed in protozoa-free stock in the present studies implies reduced DM available for total VFA production but there is no evidence that the presence of protozoa affecting the pattern of fermentation in a way that would reduce rumen hydrogen supply and methanogenesis; in fact the data are contrary. Given the lack of a methane response in protozoa-free animals in these studies, it is pertinent to examine studies that have reported reduced methane emissions in defaunated animals.
Most of the studies designed to measure methane production from faunated and protozoa-free animals have compared protozoa-free animals with animals that had either been initially defaunated and then refaunated or isolated at birth and then faunated. Results from these studies led to the conclusion that reduced methane emissions from the rumen could be achieved with the removal of protozoa, but little consideration was given to the fate of the rumen methanogen populations in the ciliate-free animals. It is known that some rumen methanogens have an endosymbiotic association with protozoa and it has been calculated that ciliate-associated methanogens may account for up to 37 % of the total methane production in the rumen(Reference Newbold, Lassalas and Jouany29). It was therefore not unreasonable to conclude that the reduced methane emissions from the ciliate-free animals were due to the loss of the ciliate-associated methanogens; however, the fate of the remaining population of methanogens needs to be considered. Newbold et al. (Reference Newbold, Lassalas and Jouany29) reported that the size of the methanogen population in rumen fluid collected from defaunated sheep and sheep that had been refaunated with mixed type B fauna(Reference Eadie30) was similar. In vitro production of methane (per unit of hexose fermented) was also similar for the type B fauna and ciliate-free incubations(Reference Newbold, Lassalas and Jouany29). Importantly, donor sheep used in the present study were given 6 months to adapt to the diet and fauna status before the study commenced, suggesting that the loss of the ciliate-associated methanogens in the defaunated animal has been compensated for by an increase in the population of the remaining methanogens. Therefore studies in which methane emissions have been measured in animals initially defaunated then measured again in the same animals when refaunated(Reference Whitelaw, Margaret Eadie, Bruce and Shand31–Reference Hegarty, Iqbal, Damry, Corbett, Choct, Nolan and Rowe33) may be a comparison of animals with temporarily low populations of methanogens (following defaunation treatment) and animals in which the methanogen populations have had time to recover and have been boosted with the refaunation inoculum (containing protozoa and bacteria), rather than a comparison of animals with and without protozoa. In the study of Kreuzer et al. (Reference Kreuzer, Kirchgessner and Muller34) methane emissions were initially measured in naturally faunated sheep and then repeated in the same animals following defaunation. Although Kreuzer et al. (Reference Kreuzer, Kirchgessner and Muller34) reported lower methane emissions in defaunated sheep, methane emissions were measured in the ciliate-free sheep just 8 d after the defaunation treatment so it was unlikely that methanogen populations had stabilised in this time. In a recent study, Yanez-Ruiz et al. (Reference Yanez-Ruiz, Hart, Belanche, Martin-Garcia and Newbold35) reported that at comparable daily feed intakes, ciliate-free lambs produced 26 % less methane than faunated lambs. In contrast to other studies, lambs were isolated from their mothers within 24 h of birth, reared in isolation and then inoculated at weaning with either fresh rumen fluid or centrifuged rumen fluid that had been frozen to produce faunated and ciliate-free animals respectively. Following inoculation, lambs were given 3 months to adapt to the diet and fauna status before methane emissions were measured. This is an interesting result because the 3-month period between inoculation and measurement should have been sufficient for the rumen methanogen population to stabilise. However, additional measurements are required to demonstrate that low methane emissions are maintained in the ciliate-free animals for an extended period of time. Further, it is not clear if the low methane emissions are due to the absence of protozoa or to the poor establishment of methanogens in animals that were isolated at birth from other ruminants.
Until it can be demonstrated that methane emissions are permanently lowered in ciliate-free animals relative to naturally faunated animals, defaunation cannot be confirmed as a strategy for reducing methane emissions from ruminants. The development of practical defaunation tools for the livestock industries remains desirable on the basis of enhanced livestock performance.
Acknowledgements
Financial support of the Australian Greenhouse Office and NSW Department of Primary Industries for this research is acknowledged. The assistance of Chris Shands in the ultrasonic scanning of lamb fat and muscle depths is acknowledged. The assistance of Stuart McClelland and Bill Johns in the management and sampling of sheep is also acknowledged with thanks. The experiments reported were designed and managed by S. H. B. and R. S. H.; B. A. V. managed animal health and nutrition in trial 1 and R. W. was responsible for all measures of methane production. None of the authors had personal, commercial, political, academic or financial conflicts of interests affecting the way the research was conducted or reported.











