INTRODUCTION
West Nile virus (WNV) is an emerging pathogen of the Flaviviridae family. Its ecology and epidemiology are complex and involve arthropod vectors, wildlife, domestic animals and humans. From Central Africa, where it was originally detected about 60 years ago, WNV has expanded its range to tropical and temperate areas of Africa and Eurasia, with frequent incursions into southern and eastern Europe and Russia; since 1999 it has become endemic in the Western hemisphere, including most of North America, Central America and the Caribbean as well as substantial areas in South America [Reference Gubler1].
For many years WNV was not regarded as a significant human pathogen because most human cases had been associated with asymptomatic infection or mild febrile illness [Reference Hayes2]. However, the virus subtype that emerged in North Africa in the early 1990s showed greater epidemic potential and virulence [Reference Gubler1]. Since 1999, WNV infections involving severe or fatal disease have been documented in a broad range of vertebrate taxa, including birds, humans and numerous other species of mammals, reptiles and amphibians [Reference Klenk and Komar3]. However, in order to predict the putative risk of the spread of WNV in Europe in forthcoming years, more information is needed on the ecology of the virus, of the vectors and reservoirs and their complex and dynamic interactions, and of the prevalence of WNV-specific immunity in human and animal populations. Based on the currently available information, this putative risk can only be predicted in part, relying on surveillance data collected in areas at risk of WNV introduction or areas that have already been exposed to the virus.
Italy has been considered to be at risk of WNV since 1998, when an epidemic of WNV encephalitis occurred in horses in a wetland in Tuscany region [Reference Autorino4]. Although no human cases were recorded during the epidemic, Italy's veterinary authorities, in light of the risk of further introductions, established a national WNV surveillance programme. Since 2001, this programme has been implemented in 15 Italian wetland areas considered to be at high risk for the introduction and spread of WNV. The programme was mainly based on the surveillance of birds (dead wild birds and sentinel chickens) and sentinel horses for the early detection of WNV circulation. On a few occasions, the surveillance activity detected WNV in sentinel chickens in some areas of northern Italy [Reference Rizzoli5]. In August 2008, WNV infection was diagnosed in a horse that showed neurological symptoms and in a magpie (Pica pica), both detected in the province of Ferrara, Emilia-Romagna region [Reference Macini6].
From September 2008 to November 2009, routine surveillance was complemented with a more intensive WNV surveillance programme, that was implemented in the regions of Veneto, Lombardy (southernmost part of the Mantua province) and Emilia-Romagna, all of which are located in northern Italy. During this period, WNV infections in humans, horses and wild birds were detected [Reference Macini6–Reference Barzon8].
In the current study we describe the results of the intensive surveillance programme for Veneto region performed from September 2008 to November 2009 in horses, wild birds, mosquitoes, dogs, cattle, and humans. The surveillance was implemented with the aim of defining the area of WNV circulation and following the possible spread of the virus to neighbouring areas.
MATERIALS AND METHODS
Study area
The study area consisted of the provinces Rovigo, Padua and Venice in Veneto region in 2008, and in 2009 the provinces of Vicenza, Verona and Treviso were added to these. This area extends from the Padana Plain, with the Po River at the southern border with Emilia-Romagna, to the mountainous areas at the northern border (Dolomite mountains); the area also includes marine/coastal habitats of the Adriatic Sea. The province of Venice contains one of the 15 areas at high risk of WNV introduction, identified according to the criteria specified in the National Surveillance Plan for WNV, and routinely monitored by testing sentinel chickens and horses in the premises selected around the high-risk area.
The study mainly focused on the area located in Veneto of the Padana Plain. In this area, the means of the minimum, average and maximum daily temperatures remained constant during the period 1 April–31 August for the years 2006, 2007 and 2008, while rainfall and humidity increased from about 1·50 mm/day and 72%, respectively, in 2006 and 2007 to about 2·50 mm/day and 76%, respectively, in 2008.
Monitoring of horse stables
In September 2008, a representative sample of the 1348 horse stables in the southern part of Veneto region (Rovigo province and part of the provinces of Venice and Padua) was defined, taking into account an expected prevalence of infected stables of 10±3% (90% CI) and at least one stable per municipality. The sample included a total of 227 horse stables, randomly selected in 107 municipalities. In the selected stables, at least ten horses (all horses if <10 were housed at the stable) were tested for WNV antibodies using a commercial ELISA WNV assay (ID screen, ID-VET; Pourquier, France). Horses were selected for testing if in the 3 months prior to sampling: (i) they had not travelled outside Veneto region; (ii) they showed clinical signs compatible with WNV infection (ataxia, recumbency, paralysis or paresis of limbs).
Facilities with racing horses were excluded because these animals frequently travel and because the health controls routinely conducted for these horses are considered sufficiently sensitive for detecting clinical signs of WNV infection. In addition to the horses included in the surveillance programme, horses sampled for other surveillance programmes in the area were also tested (i.e. surveillance of equine infectious anaemia and equine viral arteritis).
In September 2009, because of the high prevalence of horses that were positive for WNV antibodies, the monitoring activity was modified as follows: the monitoring area was extended 20 km northwards and westwards from the location of the most outlying positive stables, including new municipalities in the provinces of Padua, Venice, Verona and Vicenza. The new area was defined as the ‘surveillance area’ (SA), whereas the area monitored in 2008 was defined as the ‘area with WNV circulation’ (AWC).
In the SA, 174 stables and 659 horses were selected according to the previously described criteria.
In the AWC, the stables to be monitored were selected from the whole area (56 stables) taking into consideration those with horses that were negative in 2008 (164 horses). Serological tests performed on equine samples collected in 2009 also included the detection of IgM antibodies by commercial ELISA kits (Promevet, Italy) to identify recent infections.
Monitoring of wild birds
In February 2009, testing of wild birds found dead began in the AWC. At the same time, healthy birds of the Corvidae family which had been caught for ringing or for campaigns for reducing populations of noxious birds were also virologically and serologically tested.
Entomological surveillance
Entomological surveillance began in October 2008. CO2 mosquito traps were placed in proximity to five stables located in the province of Rovigo in which infected horses had been found. In May 2009, 19 new CO2 traps were added to the five traps used in 2008. Fifteen traps were placed in the SA and nine in the AWC [provinces of Rovigo (5 traps), Padua (8 traps), Venice (7 traps) and Verona (4 traps)] (Fig. 1). Two traps were also placed in proximity to the confirmed human cases in Rovigo province, but they were not included in the results. The 24 traps (Fig. 1) were checked every 15 days from May to November 2009. The mosquitoes were identified, pooled according to species, and submitted for virus detection by real-time reverse transcriptase–polymerase chain reaction (RT–PCR) [Reference Scaramozzino9].
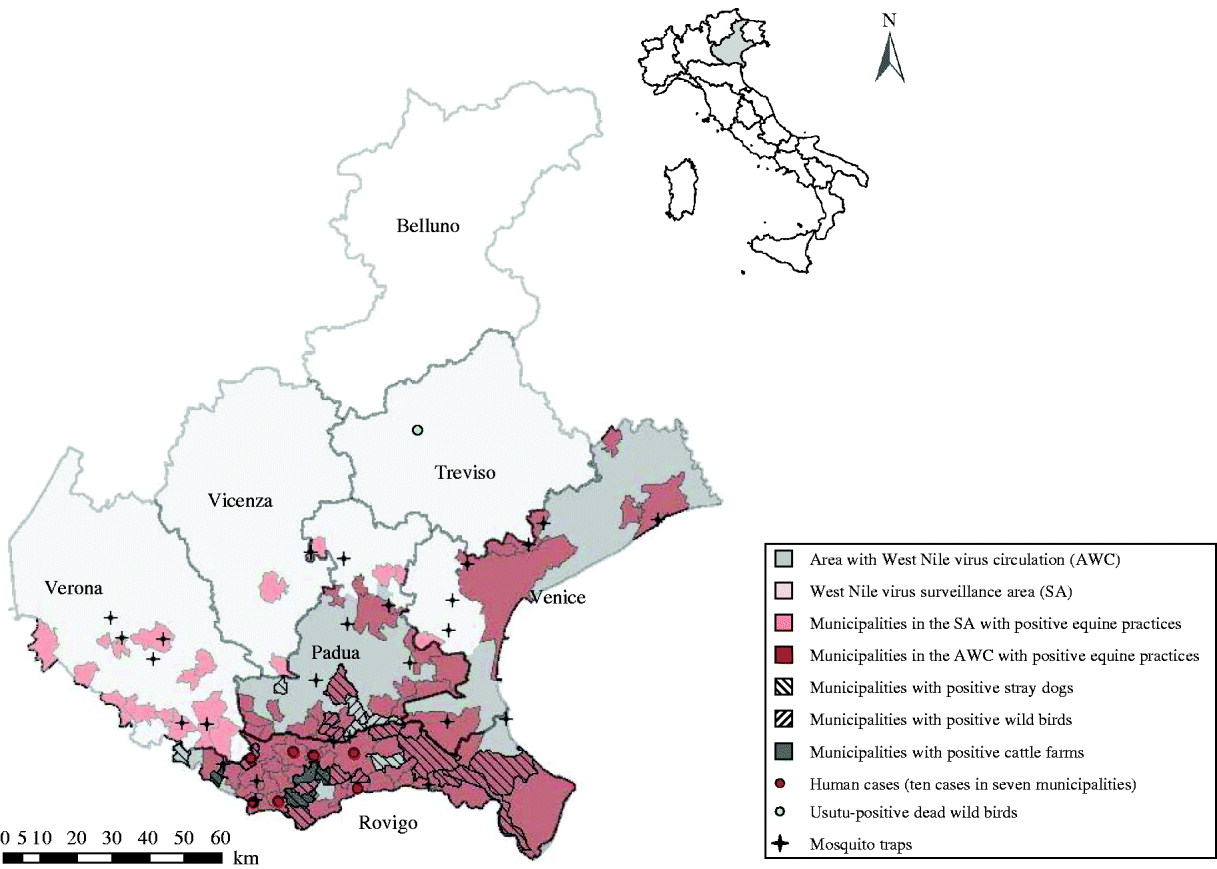
Fig. 1. Identification of the areas of West Nile virus (WNV) circulation and the surveillance area in Veneto during the 2008–2009 WNV epidemic. The municipalities with positivity to WNV are identified according to the animal species found positive. Location of human cases and mosquito-trapping sites are also shown.
Monitoring in other animal species
During September–December 2008, in a limited area of Rovigo and Padua provinces, serological screening for WNV infection was performed on sentinel cattle using serum samples collected in the framework of the Italian Bluetongue Surveillance Plan. Furthermore, in the same period, in order to identify the possible establishment of an urban cycle of WNV, stray dogs captured by the local veterinary services and dog wardens and housed in animal shelters in the cities of Rovigo and Padua were serologically tested for WNV using a commercial ELISA assay. For each dog tested, information was collected on the site and date of capture.
Laboratory diagnosis
To test blood and tissue samples, we used the methods described in a previous study by Monaco et al. [Reference Monaco7].
The ELISA assay used for the screening (ID screen, ID-VET; Pourquier, France) was previously tested at the National Reference Centre for Exotic Diseases (Istituto Zooprofilattico Sperimentale di Abruzzo e Molise, Teramo, Italy) to determine the sensitivity and specificity, and the results were 100% (95% CI 98·9–100) sensitivity and 69·4% (95% CI 62·1–75·8) specificity.
All the ELISA-positive samples were re-tested at the National Reference Centre for Exotic Diseases in Teramo (Italy) using the official methods provided by the World Organization for Animal Health (OIE), i.e. sero-neutralization (SN) assays based on IgG virus neutralization and plaque reduction neutralization, which are tests with a high specificity (about 100%).
To rule out possible cross-reactions, positive samples (a sample was considered positive with a titre ⩾1:10) were also tested for tick-borne encephalitis virus (TBEV) and Usutu virus (USUV) antibodies by plaque reduction neutralization [Reference Monaco7]. Only the ELISA-positive samples confirmed by SN assay were considered positive.
The virological tests were performed by RT–PCR for detection of flaviviruses, and the product of PCR amplifications was purified with the Qiaquick PCR Purification kit (Qiagen, Germany) and used for direct sequencing in both directions using the following primers: WN_E_484F: 5′-actcaggcagggagattca-3′; WN_E_622R: 5′-ttccgacagtcatcacgtagta-3′; WN_E_634F: 5′-ttggtccatcgtgagtggt-3′; WN_E_768R: 5′-gcccaatgctatcacagact-3′ [Reference Monaco7, Reference Scaramozzino9].
The location of exposure was established as that where the sample had been collected. Although it was not possible to determine the precise location of exposure for dogs and wild birds as the home range of these animals is limited, the identification of the area of WNV exposure was achieved with a good level of approximation.
All the analyses were performed with Stata version 10 (StataCorp, USA) and the confidence intervals of the proportions were calculated using the exact confidence interval (95% CI) of the binomial distribution.
RESULTS
The results of the monitoring activity performed in 2008 and 2009 in Veneto region revealed persistent circulation of WNV in different animal species and wild birds (Table 1, Fig. 1). In the same area, from August 2008 to November 2009, ten human cases were also observed (Fig. 1).
Table 1. Results of surveillance activity on horse stables carried out in Veneto region in 2008 and 2009. The surveillance activity in 2009 was divided in monitoring of the area with confirmed West Nile Virus circulation (AWC) and the surveillance area (SA)
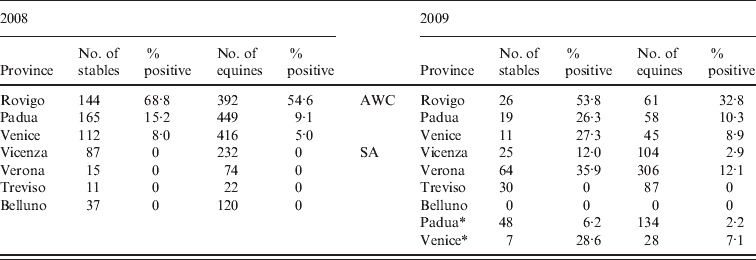
* Stables located in municipalities of the provinces of Padua and Venice not in the SA in 2008 but included in the SA in 2009.
Monitoring of horse stables
In 2008, 571 horse stables and 1705 horses were tested. In the provinces of Rovigo, Padua and Venice, the originally selected number of 227 was increased to 421 following the selection criteria presented in the Materials and Methods section. A total of 276 (16·2%) horses in 133 (23·3%) stables tested positive for WNV antibodies, and two of the positive animals also showed typical neurological symptoms of the disease. The proportion of positive stables was significantly higher in the province of Rovigo (99/144, 68·7%; 95% CI 60·5–76·2) than in the provinces of Venice (9/112, 8·0%; 95% CI 3·7–14·7) and Padua (25/165, 15·1%; 95% CI 10·0–21·5). With regard to WNV-infected horses in the positive stables, the proportion of horses positive by SN assay ranged from 35·6% (95% CI 23·4–47·8) in Venice province to 59·7% (95% CI 54·6–64·8) in Rovigo province.
Testing was also performed in an additional 150 stables (i.e. those included in surveillance activities for equine infectious anaemia and equine viral arteritis), located in the following provinces: Belluno (n=37), Vicenza (n=87), Treviso (n=11) and Verona (n=15); all were negative.
In 2009, 56 stables and 164 horses were tested in the AWC; 22 (39·3%, 95 CI% 26·5–53·2) stables and 30 (18·3%, 95 CI % 12·7–25·4) horses were positive. In the SA, 31 (17·8%) of the 174 (95 CI% 12·4–24·3) stables and 42 (6·4%) of the 659 (95 CI% 4·6–8·5) horses tested positive. Of the positive horses, two from Verona province (SA) tested positive for WNV IgM antibodies by commercial ELISA IgM assay.
Overall, WNV circulation was detected in 92 municipalities (75 in AWC, 17 in SA). The most heavily affected part of the study area was the province of Rovigo, at the border with Ferrara province (Emilia-Romagna region), which was also heavily involved in the WNV epidemic.
Other species and wild birds
Stray dogs with WNV antibodies were detected in 14 municipalities in Rovigo province and three municipalities in Padua province; the proportion of positive dogs was 47·2% (17/36) in Rovigo and 8·3% (3/36) in Padua.
Thirty cattle farms were tested in the study area, of which 29 were in Rovigo province. Four farms (all in Rovigo) were infected, with an overall prevalence of 2·8% seropositive animals.
From January to November 2009, 621 wild birds of the Corvidae family, mainly hooded crows (Corvus corone cornix) and magpies (Pica Pica), were captured and tested. Eleven birds (1·8%, 95% CI 0·9–3·1) were positive; ten were positive by serology (two hooded crows and eight magpies from Rovigo and Padua provinces) and one by PCR (a magpie in Padua province found in February 2009).
In September 2009, a number of blackbirds (Turdus merula) found dead in Treviso province (Fig. 1) and tested within the framework of the WNV passive surveillance were found positive for Usutu virus (USUV) by real-time PCR.
Entomological surveillance
In the five traps monitored in October and November 2008, 210 mosquitoes belonging to five species (Table 2) were captured. Mosquitoes belonging to the Culex genera represented 71·9% of captured mosquitoes. The samples were pooled into 57 pools and tested by PCR. Positivity to Flavivirus was detected in five pools, but none was confirmed as WNV.
Table 2. Results of the entomological surveillance performed in Veneto region in 2008–2009 by province and species of mosquito
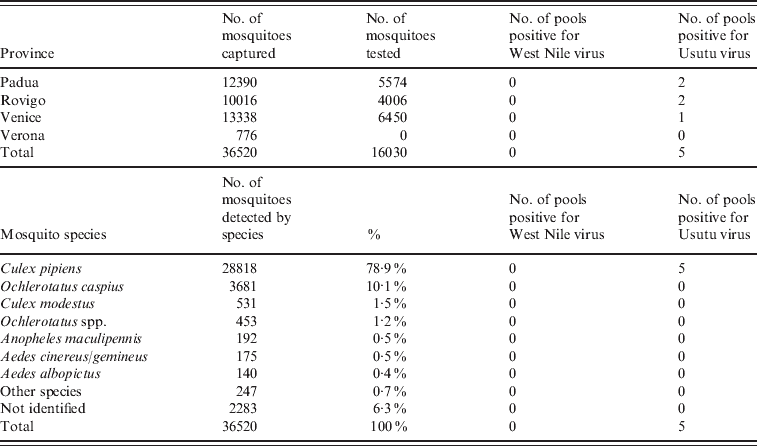
In the 24 traps monitored between May and November 2009, 36 520 mosquitoes belonging to 15 different species were identified; Culex pipiens represented 78·9% of the mosquitoes. Of these mosquitoes, 16 030 were pooled into 540 pools and tested by real-time PCR (Table 2). Six pools were positive for Flavivirus yet negative for WNV, and five pools, collected in the provinces of Padua (n=2), Venice (n=2) and Rovigo (n=1), were positive for USUV. The estimated minimum infection rate (MIR) in mosquitoes (no. of positive pools/no. of mosquitoes tested) that the surveillance activity performed in Veneto region in 2009 should have found was >0·23 (one-tailed 95% CI 0–0·23). According to the number of pools and mosquitoes tested, at the province level the detection capability was reduced to a MIR between 0·8 in Venezia and 1·0 in Padua (one-tailed 95% CI 0–0·8 and 0–1·0, respectively).
DISCUSSION
Serological and virological surveillance in different animal species in 2008 and 2009 allowed us to evaluate the extent of WNV circulation in Veneto region and to monitor the evolution of the epidemic in the area. The surveillance performed in 2008 mainly involved the provinces of Padua, Venice and Rovigo and focused on horses, cattle, birds and dogs. Horses and dogs showed the highest proportion of WNV positivity. Although previous WNV surveillance programmes have shown that horses are not effective predictors of the risk of infection for humans [Reference Corrigan10], cases of horse infection provide useful information on the geographic distribution of the virus. In the 2008–2009 WNV Italian epidemic, the AWC was mainly defined using data from the surveillance of horse stables.
In 2008, WNV circulated mainly in Rovigo province and to a lesser extent in the provinces of Venice and Padua. Serological results for stray dogs confirmed this finding. Although the number of samples was limited, positive samples were mainly from dogs captured in Rovigo province. The potential use of dogs as sentinel indicators for WNV circulation and for the risk of human exposure has been described previously [Reference Resnick11]. The testing of cattle showed a low rate of positivity, possibly because cattle are less susceptible to WNV infection compared to horses and dogs [Reference van der Meulen, Pensaert and Nauwynck12].
In 2008, surveillance began late in the year; for this reason only limited data on mosquitoes and wild birds were collected, thus limiting the understanding of WNV ecology in the study area during the first year of the epidemic. In 2009, WNV spread westwards and northwards, involving the provinces of Vicenza, Verona and Treviso, which had not been affected in 2008. This spread was mainly detected by the monitoring of horse stables and confirmed by the detection of recently infected horses (IgM positive). The westward spread was also confirmed by the detection of human cases of WNV neuroinvasive disease in the provinces of Mantua (Lombardy region) and Modena (Emilia-Romagna region) where surveillance of neuroinvasive diseases had been in place since 2008 [Reference Rizzo13].
In 2009, of the 11 WNV-positive wild birds (nine magpies, two hooded crows), only one was PCR positive. The detection of active WNV infection in a magpie in February 2009, a period of the year with very low mosquito activity, may suggest that the virus is transmitted among wild birds in other ways. One possible mode is direct bird-to-bird transmission, which has been documented in Corvidae under experimental conditions and also hypothesized to occur in nature [Reference Dawson14]. Although the proportion of positive wild birds was low (1·8%), it is comparable to the proportion found in southern France, an area in which WNV incursions have occurred since the 1960s [Reference Jourdain15, Reference Jourdain16]. Conversely, our proportion of positive wild birds was lower than the 8% reported by the limited surveillance activity in Romania in 1999–2000 [Reference Ceianu17]. Our data on wild birds seems to be consistent with the existence of a low but sustainable local WNV circulation in the study area. However, we do not have data for other bird species, in particular, long-distance migratory species, which in a survey performed in southern France showed a higher seropositivity [Reference Jourdain16].
The detection of USUV in wild birds in northern Italy is not a new finding; since 2001, USUV has been detected in Central Europe and Italy [Reference Rizzoli5, Reference Manarolla18, Reference Weissenböck19]. However, the detection of two human cases of USUV infection in Emilia-Romagna region in 2009 demonstrates the potential zoonotic impact of this virus [Reference Cavrini20, Reference Pecorari21], which, together with the virus's propensity to spread and establish itself in new areas, like the other members of the Flavivirus genus, needs to be seriously considered.
The entomological surveillance performed in 2008 and 2009 in the study area failed to detect WNV, although USUV was detected in five mosquito pools in 2009. The lack of WNV could be due to the low proportion of infected mosquitoes in this area. On the other hand, in Emilia-Romagna region, 27 WNV-positive mosquito pools were detected between June and September 2009, although in this region the intensity of the entomological surveillance was higher than that in Veneto region (92 traps were placed and about 190 000 mosquitoes in 1789 pools were tested [Reference Angelini22]). The capability of detecting infection in mosquitoes by surveillance is strictly related to the number of mosquitoes (and pools) tested and to the infection rate in mosquitoes. The infection rate in mosquitoes in Emilia-Romagna was probably higher than in Veneto, otherwise the surveillance performed in this region, although less intense, should have detected positive mosquitoes.
Of interest is the detection of a homogenous vector population (i.e. C. pipiens), which represented >70% of the population. In the years following the WNV outbreak in Tuscany in 1998, the virus did not reappear, probably due to the lack of adequate ecological conditions [Reference Autorino4]. Of interest is the finding that, in the involved area in Tuscany, entomological surveillance detected a more heterogeneous vector population, of which C. pipiens represented <40% [Reference Romi23]. C. pipiens is an important vector of WNV in continental Europe and North America, habitually biting both humans and birds, so that it serves as a bridge vector of infection from birds to humans [Reference Hubálek24, Reference Apperson25]. Moreover, WNV can persist in this mosquito species through midwinter [Reference Romi23], which may have played a role in the re-emergence of the virus in the Po valley in spring 2009, re-establishing the enzootic transmission cycle.
Although WNV circulates in the study area, as shown by high seropositivity in horses and stray dogs, the extent of human exposure, estimated in different population groups (workers on infected horse farms, blood and organ donors, results of a retrospective study on cases of aseptic meningoencephalitis), was comparably low, with only ten human cases detected [Reference Barzon8]. The low seroconversion rate found in sentinel and wild birds, together with the lack of detection of WNV in vectors, could be indicators of a low rate of transmission of the virus between ornithophilic mosquitoes and birds. Unfortunately, our data are mainly from late 2008 and 2009, and only limited information on the extent of WNV circulation is available for earlier periods [Reference Rizzoli5].
The 2008–2009 WNV epidemic in northeastern Italy differed to some extent from the outbreaks described in Romania and Russia in the 1990s, where hundreds of human cases and several deaths occurred [Reference Platonov27]. Nonetheless, taking into account the dynamics of WNV circulation in the study area and the limitation of the data collected from the surveillance activity, it is still difficult to estimate the risk of human exposure to the infection and to predict the possible evolution of the epidemiological situation. To do so, more intensive entomological and wild-bird surveillance is required, together with syndromic surveillance of neurological cases in horses and humans, which is already being done in areas involved in WNV circulation in Italy.
DECLARATION OF INTEREST
None.







