Ductal-associated pulmonary artery coarctation is observed in univentricular heart physiology with pulmonary atresia. Reference Waldman, Karp, Gittenberger-de Groot, Agarwala and Glagov1,Reference Elzenga, von Suylen, Frohn-Mulder, Essed, Bos and Quaegebeur2 Complete occlusion of the pulmonary artery (PA) after juxtaductal coarctation may lead to inadequate growth and development of the pulmonary vasculature, and deficient lung maturation with subsequent pulmonary hypoplasia. Reference Waldman, Karp, Gittenberger-de Groot, Agarwala and Glagov1,Reference Masuda, Kado and Shiokawa3 Therefore, it is important to consider a surgical strategy that achieves well-balanced pulmonary blood flow, a key factor for a successful outcome after Fontan surgery. Previous studies reporting good outcomes suggest that Surgical PA-plasty for pulmonary artery coarctation should preferably be performed during the neonatal period or early infancy to prevent an unbalanced PA. Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4–Reference Kotani, Kobayashi and Kadowaki8 However, Surgical PA-plasty in neonates is always technically challenging. Reportedly, Surgical PA-plasty during the neonatal period does not contribute to catch-up growth of the ductal side of the PA. Reference Kotani, Kobayashi and Kadowaki8 Additionally, clinical judgement is often difficult before the first-stage palliative surgery because pulmonary artery coarctation may become evident only when the duct closes. Therefore, surgical indications and timing should be carefully considered. For patients diagnosed with pulmonary artery coarctation at neonatal period or early infancy prior to 2010, we did not proactively perform Surgical PA-plasty at first-stage palliation because its technical safety with cardiopulmonary bypass was not sufficiently established for the neonatal period or early infancy. For these cases, we constructed a unilateral systemic-pulmonary shunt, usually on the contralateral side of ductus arteriosus (DA) insertion. Subsequently, if pulmonary artery coarctation severity progressed, we performed an additional systemic-pulmonary shunt on the side with the hypoplastic PA. However, we found that the uneven pulmonary blood flow generated poor results in patients exhibiting pulmonary artery coarctation. Hence, we advocated concomitant systemic-pulmonary shunt and Surgical PA-plasty in patients diagnosed with pulmonary artery coarctation during the neonatal period or early infancy. The aim of this study was to analyse the long-term outcomes in patients with pulmonary artery coarctation who underwent Surgical PA-plasty with a specific focus on surgical indications and timing.
Materials and methods
Ethical statement
This study was approved by the Ethics Committee and Institutional Review Board of Osaka Women’s and Children’s Hospital (approval number: 1565, approved on 8th August 2022). The requirement for written informed consent was waived because of the retrospective design of the study.
Participants
Between January 1993 and June 2022, 170 patients with single ventricles underwent systemic-pulmonary shunts at the Osaka Women’s and Children’s Hospital, Osaka, Japan. Only 49 patients (29%) suffered from pulmonary artery coarctation during the interstage period (28 patients were diagnosed before the first palliation). Of the 142 patients who were not diagnosed with pulmonary artery coarctation before the first palliation, 21 (15%) developed pulmonary artery coarctation after the first palliation. This retrospective cohort study included all 49 patients with pulmonary artery coarctation in univentricular heart (Supplementary material Appendix 1).
Study design and population
We reviewed patient records, including cardiac catheterisation findings, echocardiographic findings, operative findings and techniques, and surgical outcomes. No patient was lost to follow-up or excluded. Twenty-eight patients were diagnosed during the neonatal period, and 14 underwent systemic-pulmonary shunt as first-stage palliation (Group 1), while the remaining 14 underwent systemic-pulmonary shunt with Surgical PA-plasty (Group 2). Twenty-one patients were diagnosed after first-stage palliation and underwent Surgical PA-plasty at the same time as a bidirectional Glenn procedure was performed (Group 3) (Supplementary material Appendix 1). Patient demographic characteristics are shown in Table 1.
Table 1. Demographic characteristics.

Data are expressed as n (%) or mean ± standard deviation. TAPVC = total anomalous pulmonary venous connection; AVVR = atrioventricular valve regurgitation.
Definition of pulmonary artery coarctation
Pulmonary artery coarctation was defined as a stenotic lesion measuring < 3.0 mm at the ductus arteriosus (DA) insertion site on CT. Pulmonary artery coarctation screening by echocardiography was first conducted and, if suspected, this was followed by CT to define pulmonary artery coarctation. Additionally, all patients who underwent bidirectional Glenn underwent CT pre-operatively. All of these patients were retrospectively reviewed, and pulmonary artery coarctation was identified.
Operative technique and management
Management of first palliation
For patients who underwent Surgical PA-plasty with systemic-pulmonary shunt, we selected patch augmentation at the ridge site, Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4 direct end-to-end repair, Reference Sakamoto, Ota and Fujimoto5 and posterior wall end-to-end repair with an anterior wall patch. Reference Brink, MacIver and Lee6 The material used as a patch was fresh autopericardium.
In patients who underwent Surgical PA-plasty with bidirectional Glenn, we selected patch augmentation at the ridge site, direct end-to-end repair, and posterior wall end-to-end repair with an anterior wall patch. The site of the Glenn anastomosis was placed on the Surgical PA-plasty site in seven patients (29%) and on the native PA, except for the angioplasty site, in the remaining patients.
The systemic-pulmonary shunt was constructed with a polytetrafluoroethylene graft. A 3.5–, 4.0–, or 5.0–mm graft was selected by the operating surgeons based on body weight and the size of the PA and subclavian arteries. Shunts were selected according to patient body weight, as follows: a 3.0-mm shunt for weight under 2.5 kg, 3.5-mm shunt for weight of 2.5–3.0 kg, 4.0-mm shunt for weight of 3.0–4.0 kg, and 5.0-mm shunt for weight over 4.0 kg. All anastomoses were performed with 7–0 or 6–0 synthetic monofilament suture material. In Group 2, the distal site of the systemic-pulmonary shunt anastomosis was placed on the native PA, except for the angioplasty site, in all patients. In Groups 1 and 3, the distal site of the systemic-pulmonary shunt anastomosis was placed on the contralateral side of DA insertion.
Regarding the management of DA pre- and post-first palliation, when ductus-dependent pulmonary artery circulation was identified, lipo-prostaglandin (PG) E1 was commenced at birth at a dose of 1.0 ng/kg/min. In this study, a dose of PGE1 was administered via injection to all patients. Lipo-PGE1 was then increased from 0.1 to 10.0 ng/kg/min. With symptoms of high-flow pulmonary hypertension, such as rapid respiratory rate and failure to thrive caused by congestive heart failure with excessive blood flow to the lungs, the dose of Lipo-PGE1 was decreased. With signs of narrowing DA and desaturation, the dose of Lipo- PGE1 was increased. If the DA could not be maintained open by Lipo-PGE1 over 10.0 ng/kg/min, Lipo-PGE1 was changed to PGE1-CD and a the first palliation was performed within 48 hours. PGE1 was discontinued immediately after first palliation.
According to the anticoagulation, all patients were administered aspirin (initial dose: 5 mg/kg/day) continuously since the first palliation.
Timing of surgery
Regarding the timing of first palliation, based on the evidence that the first month of life is a period of physiologic changes in pulmonary vascular resistance, Reference Lakshminrusimha9 the DA was maintained by PGE1 preparations during that period and then systemic-pulmonary shunt with/without Surgical PA-plasty was performed.
Regarding the timing of bidirectional Glenn, based on the evidence about early bidirectional Glenn strategy, Reference Petrucci, Khoury, Manning and Eghtesady10 pre-bidirectional Glenn cardiac catheterisation was commenced at approximately 2 months after first palliation to assess pulmonary artery growth and ventricular functions. If pulmonary growth was considered unsatisfactory, further intervention was performed before bidirectional Glenn, such as additional systemic-pulmonary shunt and/or percutaneous transcatheter pulmonary artery angioplasty. If ventricular function was not acceptable, for example in the case of atrioventricular valve regurgitation progression and/or ventricular enlargement, bidirectional Glenn was performed relatively early. Decision-making was based on the physician’s discretion.
Regarding the Fontan operation, extracardiac–total cavopulmonary connection was performed in all patients. A 16-, 18-, or 20-mm graft was selected by the operating surgeons based on body size. The Fontan procedure was performed when the following criteria applied: patients could stand up and walk, body length was > 70 cm, cardiac catheterisation showed PA index (PAI) > 100 mm2/m2, PA pressure was < 15 mmHg, pulmonary vascular resistance was < 2 woods, and ventricular function was preserved. We routinely created fenestration for high-risk Fontan procedures. High-risk patients included those with heterotaxy syndrome with high pulmonary vascular resistance following correction of total anomalous pulmonary venous connection, mean PA pressure > 15 mmHg, functional single-lung Fontan procedures, and hypoplastic PA. A hypoplastic PA is defined as a PA with a PAI < 150 mm/m2, severely unbalanced such that the diameter of the smaller side is < 50% of the diameter of the other side.
Pulmonary artery size measurement
PA size was measured via transthoracic echocardiography at birth and measured via angiography immediately before the bidirectional Glenn and Fontan operation. The size of the bilateral PAs before the upper branch bifurcation was measured to calculate the PAI. PA growth was then evaluated using the PAI measurements proposed by Nakata et al who calculated PAI using the sum of the cross-sectional area of the right and left PAs divided by the body surface area of the patient. Reference Nakata, Imai and Takanashi11 In this study, we assessed the right and left PAI to determine the extent of post-operative changes in PA size. The balance of bilateral PAs was evaluated using the “PA ratio (DA side PAI/contralateral side PAI)”. In patients with vertical DA, DA side PAI was defined as the smaller side PA at birth. We defined unbalanced bilateral PAs as those with a PA R/L ratio < 0.5.
Outcomes
The primary outcomes were Fontan complications, interstage mortality, and interstage reintervention (including catheterisation and surgical intervention; planned or staged intervention was excluded). The secondary outcomes were post-Fontan mortality, post-Fontan percutaneous reintervention, and post-Fontan revision/reoperation; Fontan complications include protein-losing enteropathy, plastic bronchitis, arrhythmia, bleeding complications, and thromboembolic complications. The tertiary outcomes included PA growth and the balance of the left and right PAs before the Fontan procedure; and cardiac catheterisation data pre-bidirectional Glenn, pre-Fontan, 1-year post-Fontan, and from the last cardiac catheterisation.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 18.0 (IBM Corp., Armonk, NY, USA). Frequencies are presented as absolute numbers and percentages. Continuous data are presented as mean ± standard deviation with ranges, as appropriate. Mean values were compared using Student’s t-test. Kaplan–Meier analysis was used to estimate the proportion of patients free of interstage mortality, interstage reintervention, Fontan complications, post-Fontan percutaneous reintervention, post-Fontan revision/reoperation, and the rate of successful Fontan procedure and compared using the log-rank test. P < 0.05 indicated statistical significance.
Results
No statistical differences in demographic characteristics were observed between the groups diagnosed with pulmonary artery coarctation during the neonatal period or early infancy (Groups 1 and 2) and the group diagnosed with pulmonary artery coarctation after first palliation (Group 3), except the rate of vertical DA (p = 0.004) There were no significant differences in the demographic characteristics between Group 1 and Group 2, although a higher rate of total anomalous pulmonary venous connection was observed in Group 2 (Table 1). There were no significant differences between Groups 1 and 2 when comparing pulmonary artery size or PA ratio (Table 2).
Table 2. Operative details.
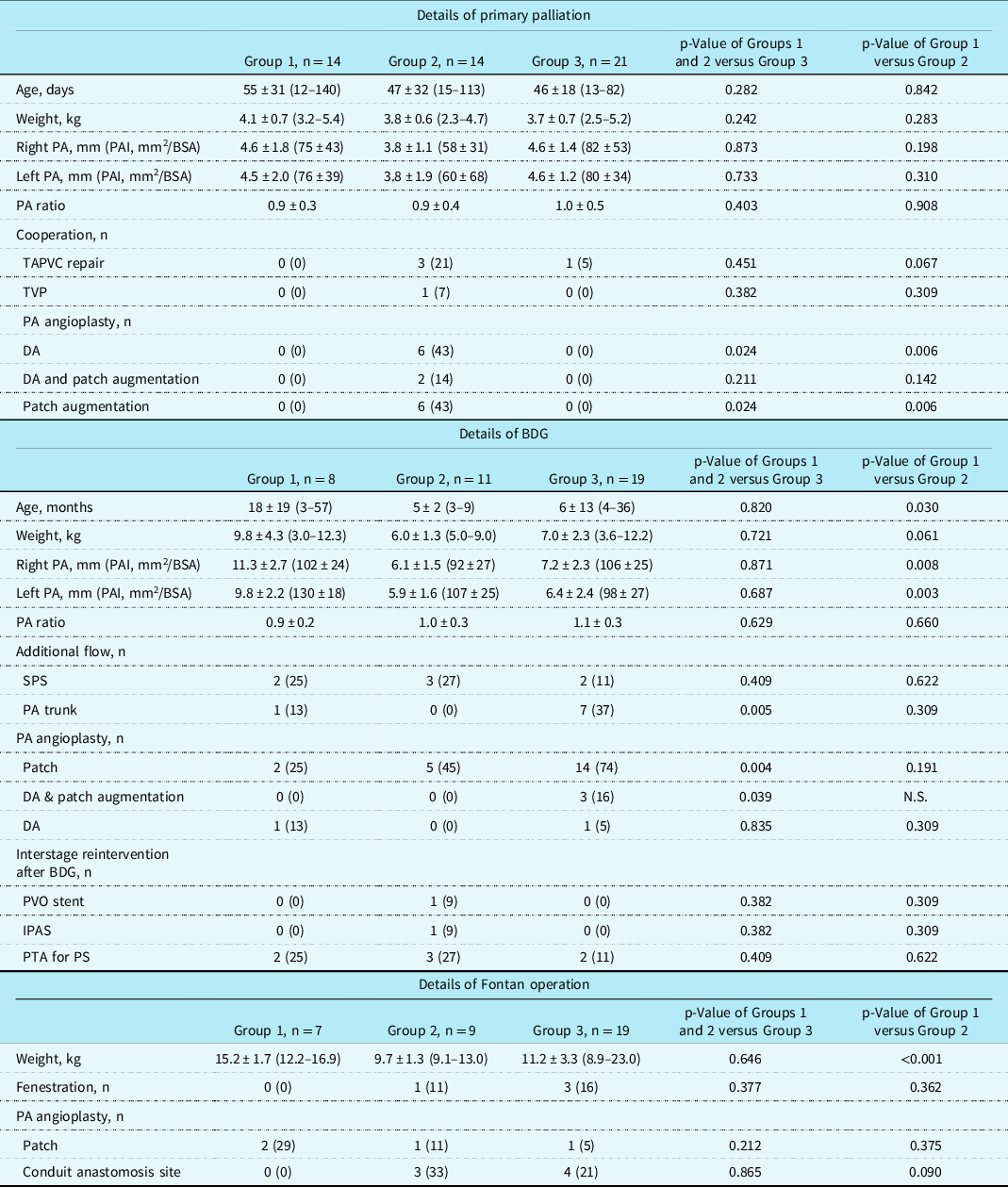
Data are expressed as n (%) or mean ± standard deviation (range). BDG = bidirectional Glenn procedure; BSA = body surface area; BW = body weight; DA = direct anastomosis; IPAS = intra-pulmonary artery septation; N.A = not available; PA = pulmonary artery; PAI = pulmonary artery index; PDA = patent ductus arteriosus; PTA = percutaneous transcatheter angioplasty; PS = pulmonary stenosis; PVO = pulmonary venous obstruction; SPS = systemic-pulmonary shunt.
Overall, the mean follow-up period after initial palliation was 6 ± 8 years (range, 17 days–26 years). The Fontan procedure was successful in 35 patients (71%) at the median age of 28 ± 26 months (range 18–139). Fontan procedure was performed significantly earlier in Group 2, followed by Group 3 and Group 1 (Group 1, 22 ± 13 months; Group 2, 14 ± 18 months; Group 3, 23 ± 12 months) (P = 0.032) (Fig. 1a). Ten patients (18%) died during follow-up after initial palliation: in Group 1, four patients (29%) died due to worsening pulmonary artery coarctation and desaturation, and two patients (14%) died due to worsening pulmonary venous obstruction. In Group 2, one patient (7%) died due to a hypoplastic PA and desaturation, and one (7%) due to worsening pulmonary venous obstruction. In Group 3, one patient (5%) died due to arrhythmia and another (5%) due to low cardiac output. The freedom from interstage death was very low in Group 1, followed by Group 2 and Group 3 (Group 1, 53%; Group 2, 85%; Group 3, 93%) (P = 0.010) (Fig. 1b). The freedom from interstage reintervention was also very low in Group 1, followed by Group 3 and Group 2 (Group 1, 50%; Group 2, 75%; Group 3, 73%) (P = 0.009) (Fig. 1c). Before bidirectional Glenn, five patients required additional systemic-pulmonary shunt and one patient required Surgical PA-plasty in Group 1, four patients required additional systemic-pulmonary shunt, and one patient required Surgical PA-plasty in Group 3, although no patients in Group 2 required reoperation. Table 2 shows details of the interstage operations.

Figure 1. Kaplan–Meier analysis estimations. Proportion of patients free from reinterventions ( a ) and death ( b ) during the interstage period. Cumulative curve showing the rate of successful Fontan surgery ( c ). Proportion of patients free from Fontan complications ( d ), catheter intervention ( e ), and Fontan revision/reoperation ( f ) after Fontan completion.
Follow-up after Fontan procedure
The mean follow-up period after the Fontan procedure was 7.8 ± 5.5 (range, 0.1–16.4) years. There were two deaths: one in Group 1 owing to arrhythmia 20 months post-operatively and one in Group 2 owing to pulmonary venous obstruction at 3.5 years. There was no difference in the freedom from Fontan complications, catheter intervention, and Fontan revision or reoperation between groups (Fig. 1d–f). The details of catheter reinterventions are summarised in Supplementary material Appendix 2. There were no between-group differences in haemodynamics after the Fontan procedure.
Pulmonary artery growth and balance
PAI increased significantly from 147 ± 57 (range, 44–280) mm2/m2 at birth to 213 ± 81 (range, 72–372) mm2/m2 before the Fontan procedure. The balance of the bilateral PAs (PA ratio) was 0.97 ± 0.37 (range, 0–1.91). In Group 1, one patient suffered from a non-confluent PA and achieved hemi-lung Fontan circulation. In Group 2, one patient suffered from a non-confluent PA and could not achieve Fontan circulation for 30 years, and another patient with pulmonary venous obstruction achieved hemi-lung Fontan circulation. No patients in Group 3 suffered from a non-confluent PA or hemi-lung Fontan circulation. (Fig. 2)
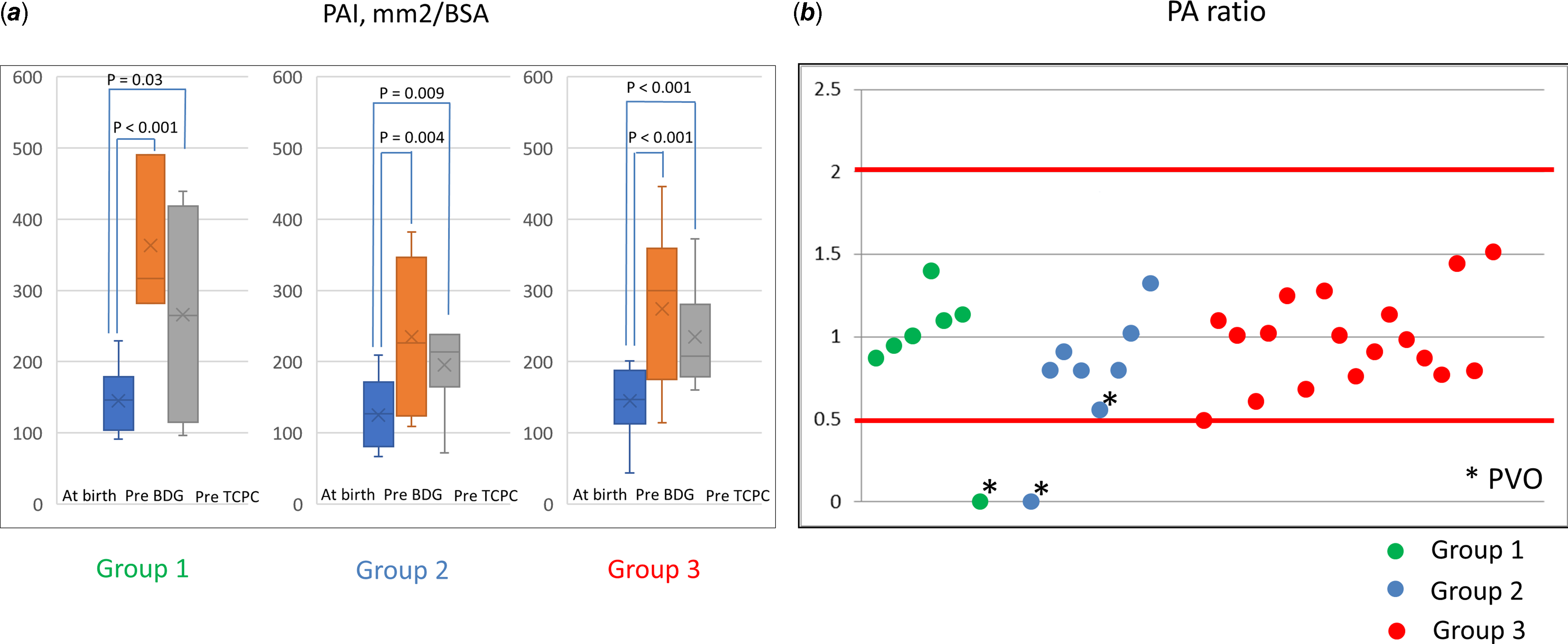
Figure 2. Pulmonary artery growth and balance. ( a ) Comparison of PAI at birth and pre-TCPC in each group. ( b ) Bilateral pulmonary artery balance of pre-TCPC calculated using the right PAI/left PAI. PAI = pulmonary artery index; TCPC = total cavopulmonary connection.
Single-ventricular function time course
There was no difference between groups in human atrial natriuretic peptide, cardio-thoracic ratio, degree of atrioventricular valve regurgitation, single-ventricle end-diastolic volume, cardiac index, or mean PA pressure. In all groups, human atrial natriuretic peptide and single-ventricle end-diastolic volume decreased with time. No patient experienced more than moderate atrioventricular valve regurgitation. The mean PA pressure was 5–14 mmHg post-bidirectional Glenn and post-Fontan surgery (Supplementary material Appendix 3).
Discussion
No significant differences in demographic characteristics were observed between the groups diagnosed with pulmonary artery coarctation during the neonatal period or early infancy (Groups 1 and 2) and the group diagnosed with pulmonary artery coarctation after first palliation (Group 3), except the rate of vertical DA. Compared to Group 2, Fontan completion occurred significantly later, and the interstage mortality and the rate of reintervention were highest in Group 1. There were significantly more patients with total anomalous pulmonary venous connection in Group 2 than in Group 1. Of the patients with unbalanced PA, even after multiple interventions, three patients presented with ipsilateral pulmonary venous obstruction with a hypoplastic PA. In Group 3, the surgical outcomes were good with a high rate of Fontan completion, low interstage mortality, and relatively balanced PA growth.
First-stage pulmonary artery coarctation treatment involves the construction of a unilateral systemic-pulmonary shunt, usually on the side with the hypoplastic PA. However, using only systemic-pulmonary shunt in patients with pulmonary artery coarctation can lead to exacerbation of unequal flow between the right and left PAs. Reference Elzenga, von Suylen, Frohn-Mulder, Essed, Bos and Quaegebeur2 In these patients, an additional systemic-pulmonary shunt on the contralateral side of the PA is required, which is also reportedly a risk for PA distortion. Reference Godart, Qureshi and Simha12 Additionally, some studies demonstrated that it is harder to restore continuity in older patients, particularly at the time of Fontan completion. Reference Zachary, Jacobs, Apostolopoulou and Fogel13,Reference Bacha, Lang, Mayer and McElhinney14 PA growth depends on latent growth ability, which appears to be more significant in patients less than 1 year of age. Reference Ishikawa, Takahashi and Sato15 Therefore, it is preferable to perform Surgical PA-plasty as first-stage palliation or at bidirectional Glenn in patients younger than 1 year to ensure sufficient and bilateral PA growth. Simultaneous Surgical PA-plasty with systemic-pulmonary shunt has been reported to yield highly satisfactory early post-operative results; in addition, patients appear to tolerate subsequent procedures as required. Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4–Reference Kim, Kim, Kim, Lim, Lee and Kim7 Over the last 20 years, there has been a shift in our practice, with the majority of pulmonary artery coarctation patients undergoing Surgical PA-plasty with systemic-pulmonary shunt as first-stage palliation, based on the expectation that it can better prevent unbalanced PA growth with unilateral PA hypoplasia and non-confluent PA. However, a recent report found that Surgical PA-plasty with systemic-pulmonary shunt did not contribute to the catch-up growth of the ductal side PA. Reference Kotani, Kobayashi and Kadowaki8 Therefore, the timing of the surgery should be carefully reconsidered.
No significant differences in the demographic characteristics were observed between the groups diagnosed with pulmonary artery coarctation during the neonatal period or early infancy and the group diagnosed with pulmonary artery coarctation after first palliation, except the rate of vertical DA. This result reconfirmed the challenges involved in making a clinical assessment before the first-stage palliative surgery; however, some anatomical characteristics such as vertical DA may be predictors of pulmonary artery coarctation. The “Vertical” DA (Supplementary material Appendix 4) is characterised by an origin from the transverse aortic arch with flexion and tortuousness and insertion of the PA close to the PA bifurcation. “Vertical” DA is commonly observed with pulmonary atresia. Reference Suzuki, Kim and Ishigaki16 In our study, of all 49 patients with pulmonary artery coarctation, vertical DA was observed in 21 (43%). Reportedly, in “vertical” DA, bifurcation of the PA often expresses EP4, a prostaglandin E-specific receptor that is rarely expressed in the normal PA but overexpressed at the aortic side in coarctation of the aorta. Reference Yokoyama, Minamisawa and Quan17,Reference Yokoyama18 Pulmonary artery coarctation also includes extensive infiltration of ductal tissue into the PA wall, possibly due to blood flow towards the pulmonary circulation with ductal tissue migration into the PA. Reference Wielenga and Dankmeijer19 In the flexion and tortuousness DA type, blood flow velocity may be faster on the outer side of the curvature, and DA tissue entrapment may likely occur at the PA where it meets the outer side of the DA curvature. This is supported by the report by Kotani et al. describing the characteristics of the DA and PA which progressed pulmonary artery coarctation post-systemic-pulmonary shunt as a flexion and tortuousness DA, similar to the “vertical” DA. Furthermore, many complications of stenting for this type of DA have been reported. Reference Bacha, Lang, Mayer and McElhinney14 Based on these reports, recently, we have performed Surgical PA-plasty for patients with “vertical” DA even if the pulmonary artery coarctation is absent to prevent future pulmonary artery coarctation progression.
In our study, the outcomes of systemic-pulmonary shunt only in patients who progressed to pulmonary artery coarctation before first palliation were not good, with significantly late Fontan completion, high interstage mortality, and high rate of reintervention. However, these improved by Surgical PA-plasty with systemic-pulmonary shunt as first palliation. Our result suggests that for patients progressing to pulmonary artery coarctation during the neonatal period or early infancy, Surgical PA-plasty should be performed at the first palliation. This suggestion is not consistent with the conclusions in the report by Kotani et al, Reference Kotani, Kobayashi and Kadowaki8 although our results are supported by other reports. Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4–Reference Kim, Kim, Kim, Lim, Lee and Kim7 We consider that these differences may be explained by the fact that various techniques have been reported for Surgical PA-plasty, and there is no report comparing the outcomes of each surgical techniques. Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4–Reference Kim, Kim, Kim, Lim, Lee and Kim7 In our study, there were three different techniques including patch augmentation at the ridge site, Reference Shinkawa, Yamagishi, Shuntoh, Miyazaki, Hisaoka and Yaku4 direct end-to-end repair, Reference Sakamoto, Ota and Fujimoto5 and posterior wall end-to-end repair with an anterior wall patch. Reference Brink, MacIver and Lee6 Without comparing these techniques, we could not definitively conclude which technique is more sufficient for Surgical PA-plasty. Further research is needed to reach a definitive conclusion on this point.
There were significantly more patients with total anomalous pulmonary venous connection in Group 1 than in Group 2. Of the patients with unbalanced PA, even after multiple interventions, three patients presented with ipsilateral pulmonary venous obstruction with a hypoplastic PA. Even in cases in which Surgical PA-plasty with total anomalous pulmonary venous connection repair is performed, if post-operative pulmonary venous obstruction develops, patients have a risk of unbalanced PA growth. Importantly, pulmonary artery coarctation often coexisted with asplenia and pulmonary atresia. The prognosis of patients with asplenia is very poor, owing to the combination of structural abnormalities in the systemic and pulmonary venous connections, significant arrhythmia, and atrioventricular valve regurgitation. Specifically, patients with asplenia with pulmonary atresia and pulmonary artery coarctation pose a clinical challenge with respect to establishing two-lung Fontan circulation. Reference Azakie, Merklinger, Williams, Van Arsdell, Coles and Adatia20 Our results suggest that aggressive intervention for pulmonary venous obstruction is required in addition to Surgical PA-plasty for pulmonary artery coarctation in order to gain balanced PA growth.
In Group 3, the surgical outcomes were good with a high rate of Fontan completion, low interstage mortality, and relatively balanced PA growth. These results suggest that performing Surgical PA-plasty at the timing of bidirectional Glenn is an acceptable timing for patients who progressed to pulmonary artery coarctation after first palliation. Conversely, Sakamoto et al. support Surgical PA-plasty with systemic-pulmonary shunt in cases, wherein pre-operative echocardiography and CT detected a ductal-associated pulmonary artery coarctation, but if not, it is very difficult to identify if the ductal-associated pulmonary artery coarctation progressed after stopping prostaglandin infusion. This is because they routinely performed Surgical PA-plasty with systemic-pulmonary shunt for all single-ventricle patients with pulmonary atresia who have ductal-dependent pulmonary blood flow. Reference Sakamoto, Ota and Fujimoto5 However, the benefits of Surgical PA-plasty with systemic-pulmonary shunt in patients who have not yet developed a clear coarctation remain controversial because this requires cardiopulmonary bypass, and a number of these patients will require repeated patching of the PAs. Reference Brink, MacIver and Lee6 Based on our results, we do not consider that prophylactic plasty, that is, Surgical PA-plasty for all the patients with pulmonary atresia in a single ventricle to prevent unbalanced PA growth, should be performed. However, our study did not include any patients who underwent PA-plasty in the absence of pulmonary artery coarctation (prophylactic plasty) and also did not include any patients in whom a pulmonary artery coarctation was identified, who were treated by shunt alone, followed by Surgical PA-plasty (rather than a second shunt). Additional research is imperative to explore our findings and gain a greater understanding of the most effective surgical approach for this disease.
Finally, based on our results, we modified our surgical indications for Surgical PA-plasty in patients with a single ventricle and pulmonary atresia/severe pulmonary stenosis but with/without pulmonary artery coarctation progression during the neonatal period or early infancy are as follows:
-
(1) For patients who progressed to pulmonary artery coarctation during the neonatal period or early infancy, Surgical PA-plasty should be performed at the first palliation.
-
(2) For patients with asplenia and total anomalous pulmonary venous connection, even in the absence of pulmonary artery coarctation in the neonatal period or early infancy, if vertical DA is present, Surgical PA-plasty should be performed at the first palliation and aggressive reinterventions for post-operative pulmonary venous obstruction should be performed to prevent unbalanced PA growth.
This study was a retrospective review based on data from a single centre. Because of the short-term follow-up and small sample size, we could not statistically conclude which technique was more sufficient. According to the timing of each procedure including first palliation, bidirectional Glenn, and Fontan operation, in Group 1, the timing of bidirectional Glenn and Fontan surgery is later than that in the other groups. We attributed this to the fact that in Group 1, patients needed longer and reinterventions to gain sufficient PA growth. However, since this study covers an extensive period, an era effect cannot be excluded.
Conclusion
Our results suggest that for patients who progressed to pulmonary artery coarctation during the neonatal period or early infancy, Surgical PA-plasty should be performed at the first palliation. Further research is imperative to investigate our findings in more detail and gain a deeper understanding of the most effective surgical approach for this disease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951123002676.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by Ethics Committee and Institutional Review Board of Osaka Women’s and Children’s Hospital (approval number: 1565).







