Introduction
In the mountain regions of France, most male calves produced from beef herds are sold lean at weaning, i.e. between 8 and 10 months of age (as ‘broutards’), to be finished elsewhere. In these areas, since the imposition of Common Agricultural Policy measures, dairy or mixed cow herds have frequently been replaced by suckler herds. In several areas of European Mediterranean countries, beef obtained from young bulls less than 1 year of age represents an important percentage of beef consumption (Chatellier et al., Reference Chatellier, Guyomard and Le Bris2003). Production of ‘finished broutards’ could thus represent an interesting possibility to enhance the profitability of mountain farms.
Salers is a dual-purpose breed used for beef production in mountainous areas of central France whose medium precocity characteristics and relatively high milking potential (D’Hour et al., Reference D’Hour, Petit, Pradel and Garel1995 and Reference D’Hour, Petit and Garel1996; Liénard et al., Reference Liénard, Lherm, Pizaine, Le Maréchal, Boussange, Barlet, Esteve and Bouchy2002) may be especially adequate for this type of production based on relatively young animals suckled until slaughter.
Many factors influence animal performance, carcass characteristics and meat quality. However, diet and, especially, forage type and concentrate level, are among the most important factors (Listrat et al., Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999; Geay et al., Reference Geay, Bauchart, Hocquette and Culioli2002; Nuernberg et al., Reference Nuernberg, Dannernberger, Nuernberg, Ender, Voigt, Scollan, Wood, Nute and Richardson2005). The effects of forage type and concentrate level used in different periods of production have previously been studied, but little information is available for young bulls (slaughtered between 10 and 12 months of age) suckled until slaughter. Therefore, the aims of this work were to examine the effect of diet (type of forage and level of concentrate) on performance, carcass and muscle characteristics as well as on meat quality of young suckled Salers bulls.
Material and methods
Animals and experimental design
This study was conducted at the experimental farm of INRA Marcenat (Cantal, France). Twenty-four winter-born (4 January ± 16.7 days) Salers male calves were used. All animals were reared indoors by the conventional winter calf-rearing procedure of French mountain zones until approximately 150 days of age: they were suckled twice a day by their dams, having free access to medium-quality cocksfoot hay. On turn-out to pasture (24 May) animals were randomly assigned to six groups according to diet and slaughter age: C0 (control) fed exclusively with winter diet and slaughtered at approximately 6 months of age (used as point 0 in carcass composition estimations explained below) and HH, HL, GH, GL and CP groups offered ad libitum cocksfoot hay plus concentrate and slaughtered at approximately 10 months of age (HH, hay – high concentrate) or in a quantity equivalent to half of ad libitum group (HL, hay – low concentrate); ad libitum cut grass plus concentrate (GH, grass – high concentrate) or in a quantity equivalent to half of ad libitum group (GL, grass – low concentrate); and ad libitum grazed highland grass and ad libitum concentrate (CP, control pasture) group.
HH, HL, GH and GL animals were housed in individual pens and feeds were offered once a day and individual intakes were recorded 5 days/week. They were suckled twice a day by their dams throughout the experimental period and the cows’ diet consisted of cocksfoot hay for dams of HH and HL animals and grazed grass for dams of GH and GL animals. Grass offered to GH and GL groups was green vegetative herbage (spring growths and summer regrowths) from a natural pasture cut every morning. The CP group remained on pasture (1100 m in altitude) with their mothers; they grazed in a continuous system of high diversity of natural pasture and were offered concentrate ad libitum as a group. Table 1 shows species composition (expressed as percentage) of parcel pastured by CP group and parcel used to obtain cut grass for GH and GL groups.
Table 1 Relative presence of species (%) of parcel pastured by group CP and parcel used to obtain cut grass for groups GH and GL†

†Abbreviations are: CP = control pasture; GH = cut grass – high concentrate; GL = cut grass – low concentrate.
All animals were individually weighed once a week before and after suckling in order to estimate milk ingestion and live weight (Le Neindre, Reference Le Neindre1973). Calves were weighed in the early morning before feed distribution. CP calves rested in the same pasture but separated from their mothers during the night before weighing. Average daily gain was calculated by linear regression. Concentrate composition and feed values are given in Tables 2 and 3, respectively. Water and salt blocks were always available to animals.
Table 2 Concentrate composition
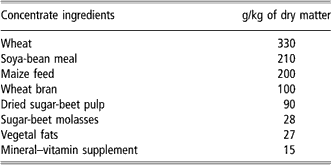
Table 3 Percentage of DM, chemical composition of feeds and feeding values (for grass, mean (range) of six analyses performed during all experimental period)†

†Abbreviations are: DM = dry matter; MM = mineral matter; TN = total nitrogen content; CF = crude fibre; UFL = net energy for maintenance and gain expressed in milk feed units (Unités Fourragères Lait); PDIN = proteins truly digestible in the small intestine allowed by the nitrogen content; PDIE = proteins truly digestible in the small intestine allowed by the energy content; BBU = bovine bulk unit.
Slaughter procedure
Animals were slaughtered at the experimental slaughterhouse at INRA, Centre of Clermont Ferrand-Theix (Puy de Dôme, France) in five batches: 1 July for C0 group animals and between 4 October and 8 November for the other groups. In each slaughter batch, the oldest animals in each group were slaughtered first. On the day of slaughter, animals were weighed in the early morning, transported 90 km to the slaughter facility and slaughtered at random within 4 h of removal from experimental farm. Carcasses were visually graded according to SEUROP beef carcass grading system for conformation (scale ranging from 18 (S+, very good, superior, conformation) to 1 (P−, very poor conformation)) and fatness (scale ranging from 5 (very high) to 1 (very low)) and placed at room temperature for 2 h, then in a refrigerated room set to 4°C until 24 h post mortem. After a 2 h chilling, subjective lean colour was judged by an experienced person using a scale used for veal carcass classification ranging from 1=white to 4=red. pH of semitendinosus (ST) and rectus abdominis (RA) muscles were measured 24 h post mortem. Colorimetric parameters of the CIE L* a* b* uniform colour space (Commission International de l’Eclairage, 1986) were measured on ST and RA muscles using a MINOLTA CM 2002 recording spectrocolorimeter (illuminant D65, observer angle 10°).
Body composition
The empty body weight, hot carcass weight, and weights of the metacarpus bones and the offal fatty tissues (kidney, heart and digestive tract) were measured. On the following day, the left half carcass of each animal was quartered between the 5th and 6th thoracic vertebrae and the 6th rib was extracted (as described by Robelin and Geay, Reference Robelin and Geay1975), weighed and dissected into lean tissue, fat and bone. Two days after slaughter, the right half carcasses of 14 animals (four C0 animals plus two animals of each of the other experimental groups) were dissected and the total weights of lean, bone and fat were recorded according to anatomical distribution.
Regression analysis was used to relate carcass composition traits (muscle, fatty tissues and bone weights) obtained from dissection of right half carcass of 14 animals with the predictors obtained from the dissection of 6th rib, metacarpus bone weight and carcass weight of these animals (Robelin and Geay, Reference Robelin and Geay1975). The regression analyses were performed using the GLM procedure of the Statistical Analysis Systems Institute (SAS, 1989) and independent variables were selected by stepwise method. Muscle, fat and bone carcass weights of all experimental animals were estimated using these equations. Total body fat was calculated as the sum of recorded kidney, heart and digestive tract fatty tissues and carcass fat estimated by linear regression.
Muscle characteristics
Two samples of ST and RA muscles of each animal were taken 1 h after slaughter for the determination of muscle fibre size and metabolic enzyme activities. Collagen and lipid contents were determined only in RA muscle samples. The epimysium was carefully dissected and about 300 g of muscle was taken. Part of each sample was frozen in isopentane, chilled in liquid nitrogen for fibre size determination and a further portion was directly frozen in liquid nitrogen and kept at −80°C until analysed for enzyme activities determination. In the case of RA muscle, the rest of the sample was cut in pieces (1 to 2 cm cross section), sealed under vacuum and stored at −20°C until analysed for collagen and lipid content.
Muscle fibre size
Serial sections of 10-μm thick RA and ST muscles were cut on a cryostat at −25°C, perpendicular to the muscle fibres. The cells were stained with azorubine to define their outline and their mean surface areas were measured in two randomly selected areas of serial sections with an image analysis software program (Visilog, INRA, France) (Jurie et al., Reference Jurie, Picard and Geay1998). An average of 100 fibres were analysed on each serial section.
Metabolic enzyme activities
RA and ST muscle samples were ground and homogenised in 140 mmol/l sucrose and 50 mmol/l triethanolamine buffer (pH 7.5) and centrifuged at 6000 × g for 15 min at 4°C. Enzyme activities were measured in the supernatant. Anaerobic glycolytic metabolism was assessed by lactate dehydrogenase (LDH) and phosphofructokinase (PFK) activities (Beutler, Reference Beutler and Beutler1971; Brandstetter et al., Reference Brandstetter, Picard and Geay1998). Aerobic oxidative metabolism was studied by measuring isocitrate dehydrogenase (ICDH), citrate synthase (CS) and cytochrome-c oxidase (COX) activities (Brandstetter et al., Reference Brandstetter, Picard and Geay1998; Piot et al., Reference Piot, Veerkamp, Bauchart and Hocquette1998). Enzyme activities were expressed in μmol/min per gram of wet muscle.
Collagen
Total hydroxyproline (OH-Prol) content and collagen in the insoluble part were measured as described by Listrat et al. (Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999 and Reference Listrat, Picard, Jailler, Collignon, Peccatte, Micol, Geay and Dozias2001). Data are presented as means of triplicate measurements and are expressed, for total and insoluble collagen, in μg OH-Prol per mg of dry matter (DM) and also as percentage of OH-Prol in the soluble part per total amount of OH-Prol.
Lipids
Total lipids of RA muscle were extracted according to the method of Folch et al. (Reference Folch, Lees and Stanley1957), vacuum dried and weighed. They were solubilised into 5 ml chloroform. Fatty acids from total lipids were esterified in boron-trifluoride/methanol as described by Glass (Reference Glass1971). The fatty acid composition was determined by gas–liquid chromatography, using a chromatograph DELSI DI 200, a flame ionisation detector, a 100-m long CP-Sil 88 (Varian) glass capillary column and H2 as the gas vector as described by Aurousseau et al. (Reference Aurousseau, Bauchart, Calichon, Micol and Priolo2004).
Sensory assessment
Seventh to 11th ribs were taken 24 h after slaughter from the left side of carcass. Samples were placed in sealed plastic bags under vacuum and kept at 4°C for 8 days for ageing and then frozen at −20°C until analysis. After thawing, ribs were cooked in a double sided grill (280°C) for 2 min and 30 s. Cooked longissimus thoracis muscle of each rib was isolated and cut in homogeneous portions and presented to 8 to 10 trained tasters isolated in booths. Each taster scored from 1 to 10 the intensity of the following characteristics: tenderness, juiciness and cardboard, metal, blood, milk, fat and grass flavours. Flavour variables used were selected by tasters in previous training sessions as the most important parameters to characterise young bull meat. Only a comparison of HH, GH and CP groups was conducted.
Statistical analysis
Statistical analyses were performed using the GLM procedure (SAS, 1989). Feed intake, energy utilisation and muscle characteristics data corresponding to HL, HH, GL and GH groups were subjected to analysis of variance to examine the effect of forage type (grass v. hay) and concentrate level (high v. low) using a two-factor model (2 × 2). Interaction between the two factors was included in the model when significant. In a second step, the same information corresponding to HL, HH, GL, GH and CP groups was compared using a one-factor (‘group’) model. Data corresponding to different muscles were analysed independently. Differences between means were separated by DUNCAN test. The results are presented as mean ± s.e.
For other production results and carcass characteristics, statistical analysis were also performed in two steps: in the first step (for HL, HH, GL and GH groups), model included forage type and concentrate level as independent variables, their interaction and weight at turn-out to pasture as covariate variable; in the second step (HL, HH, GL, GH and CP groups), model only included ‘group’ as independent variable and weight at turn-out to pasture as covariate. Forage type × concentrate level interaction was eliminated from the model when not significant (P > 0.1). Differences between means were separated by the PDIFF procedure of SAS. The results are presented as least-squared mean ± s.e.
Variance analysis of sensory results was performed taking the effects of group and taster into consideration.
Results
Intake
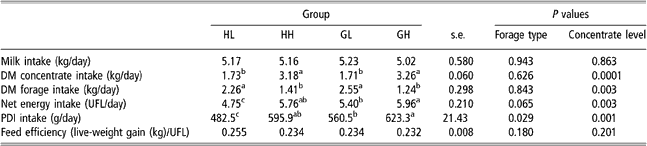
Table 4 shows average daily intake of milk, concentrate and forage for all the experimental period (151 ± 14 days, between turn-out to pasture and slaughter). Figure 1 represents weekly intake of milk, concentrate, hay and grass in HL and GL groups (Figure 1a – low concentrate) and in HH and GH groups (Figure 1b – high concentrate).
Table 4 Effect of forage type and concentrate level on voluntary intake and energy utilisation in HL, HH, GL and GH groups†
a,b,cMeans within a row with different superscripts differ significantly (P < 0.05).
†Abbreviations are: DM = dry matter; HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; UFL = net energy for maintenance and gain expressed in milk feed units (Unités Fourragères Lait); PDI = protein truly digestible in the small intestine.

Figure 1 Milk (



Total milk drunk was not different between HL, HH, GL and GH groups. DM concentrate intake in high-concentrate groups (HH and GH) increased from 0.5 kg/day to approximately 5.5 kg/day (Figure 1b) and was, as expected, almost twice as much of that of HL and GL groups. Average daily forage intake in low-concentrate groups was, approximately 1.8 times that of the high-concentrate groups (Table 4). Forage type had no effect on forage intake considering daily average values for the whole experimental period. But as illustrated in Figure 1b, concentrate substitution effect was higher in grass than in hay groups after the 13th experimental week.
Net energy intake tended to be affected by forage type (P = 0.06) and by concentrate intake level (P = 0.003). PDI (protein truly digestible in the small intestine) intake was affected by forage type (P = 0.03) and by concentrate intake level (P = 0.001). Values in HL group were lower than those in the other groups (P < 0.05) reflecting the lower feeding value of hay. Net energy and PDI intake values in GH group were higher than that in GL group (P < 0.05), and those of HH group were intermediate between those in GL and GH groups and not significantly different from these (P > 0.05).
Average total concentrate intake of CP group was 377 kg, approximately 17% and 23% lower than in HH and GH groups. Mean values for total and daily milk intake tended to be higher (P = 0.07 and P = 0.08, respectively) in CP group than in the others groups (942 ± 146 kg and 5.86 ± 0.73 kg/day).
Growth, carcass yield and carcass composition
Initial live weight (at turn-out to pasture, 141 ± 9 days of age) was on average 166 ± 9 kg. Age at slaughter was not different between groups (286, 290, 291, 296 and 293 ± 6 days for groups HL, HH, GL, GH and CP, respectively).
Average daily weight gain (ADWG) in CP group (1542 g/day) was significantly higher than that in the other four groups (P < 0.05). This group also had the highest live weight at slaughter (LWS), empty body weight (EBW) and carcass weight (395, 348 and 230 kg, respectively). However, significant differences were found only with HL group (P < 0.05) for EBW. LWS values of CP group tended to be higher than those of HL, HH and GL groups (P < 0.1). Carcass weight of CP group tended to be higher than those of HL and GL groups (P < 0.1). Dressing percentage in CP group was significantly higher (P < 0.05) than in the rest of experimental groups (66.2% and 64.2% for CP v. HL, HH, GL and GH groups, respectively).
ADWG tended to be higher (P = 0.09) in high-concentrate groups than in low-concentrate groups (1248 and 1354 g/day for HL and GL v. HH and GH groups, respectively). Forage type did not affect ADWG (Table 5).
Table 5 Average daily weight gain, weight at slaughter and carcass characteristics in five experimental groups (HL, HH, GL, GH, CP) and effect of forage type and concentrate level†

a,bMeans within a row with different superscripts differ significantly (P < 0.05).
c,dMeans within a row with different superscripts tended to differ significantly (P < 0.1).
† Abbreviations are: HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; CP = control pasture; F = forage type; C = concentrate level; EBW = empty body weight.
Concentrate level affected EBW (P = 0.05). Carcass weight tended to be higher (P = 0.07) in high-concentrate groups than in low-concentrate groups. After CP animals, GH presented the highest EBW and carcass weight values (334 and 216 kg, respectively) followed by HH (331 and 212 kg), GL (312 and 200 kg) and HL (308 and 198 kg) groups, in this order. Differences tended to be significant (P < 0.1) only for EBW between GH and HL groups.
Concentrate level significantly affected carcass muscle weight (P = 0.04). GH bulls were heavier than HL and GL bulls (155, 141 and 142 kg, respectively; P < 0.05). Carcass fatty tissue was not affected by either of the two experimental factors, forage type – concentrate level. Carcass muscle weight in CP group (average 165 kg) was higher (P < 0.05) than HL and GL groups. Offal fatty tissues weight, carcass fatness and conformation scores did not differ between groups (Table 5).
Muscle characteristics
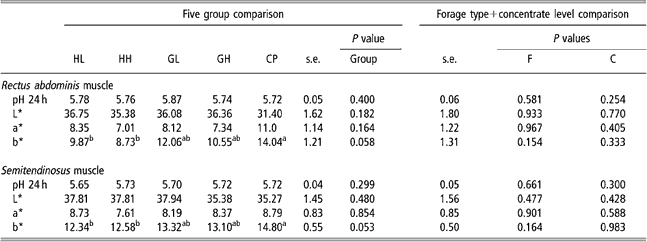
Colour and pH
Muscle pH (Table 6) was not affected by forage type or concentrate level. All animals obtained the maximal value (4=red) of carcass lean colour score. The CP group had higher b* values than HL and HH groups (P < 0.05).
Table 6 pH and colour parameters values in five experimental groups (HL, HH, GL, GH, CP) and effect of forage type and concentrate level in theses variables of rectus abdominis and semitendinosus muscles†
a,bMeans within a row with different superscripts differ significantly (P < 0.05).
†Abbreviations are: HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; CP = control pasture; F = forage type; C = concentrate level.
Histological and enzymatic characteristics
Table 7 presents muscle fibre areas and enzyme activities for RA and ST muscles. Whatever the muscle, neither the type of forage nor the concentrate level affected the cross-sectional areas or metabolic enzyme activities of muscle fibres. CP group exhibited the highest values of muscle fibre area for ST muscle but ‘group’ factor was not significant. When comparing the five experimental groups, ‘group’ factor tended to be significant for CS enzyme activity (P = 0.06) in ST muscle. The highest values corresponded to CP group but differences were significant (P < 0.05) only when comparing CP v. HH and GL groups.
Table 7 Fibre area and LDH, PFK, ICDH, CS and COX enzyme activities in five experimental groups (HL, HH, GL, GH, CP) and effect of forage type and concentrate level†

a,bMeans within a row with different superscripts significantly differ (P < 0.05).
† Abbreviations are: LDH = lactate dehydrogenase; PFK = phosphofructokinase; ICDH = isocitrate dehydrogenase; CS = citrate synthase; COX = cytochrome-c oxidase HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; CP = control pasture; F = forage type; C = concentrate level; LDH = lactate dehydrogenase; PFK = phosphofructokinase; ICDH = isocitrate dehydrogenase.
Total and heat-soluble collagen
Neither total collagen content nor insoluble collagen content were affected by forage type or concentrate level factors (Figure 2). Similarly, when comparing the five experimental groups, ‘group’ factor was not significant. However, quality of collagen estimated by soluble/total collagen ratio was significantly affected by forage type (P = 0.03). Collagen solubility was lower in HH group than the other three groups (P < 0.05). When comparing the five experimental groups, ‘group’ factor was significant (P = 0.01). The highest collagen solubility was found in CP group, in which it was significantly higher (P = 0.05) than in HL and HH groups.

Figure 2 Total and insoluble collagen content, and collagen solubility of rectus abdominis muscle in five experimental groups (a–c = means with different letters significantly differ at P < 0.05; OH-prol = hydroxy proline; DM = dry matter; HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; CP = control pasture).
Lipid content
Table 8 presents total lipid content and fatty acid composition of RA muscle. The lipid content of RA muscle was relatively low (on average 68.7 mg/g DM) and was not affected by feeding treatments (P > 0.05). Percentages of monounsaturated fatty acids were lower in hay-fed than in grass-fed animals (P = 0.05). Percentages of polyunsaturated fatty acids (PUFA) were higher in hay-fed animals but differences were not significant. The ratio between n-6/n-3 PUFA was lower (P = 0.01) in low-concentrate-fed groups than in high-concentrate-fed groups. Mean n-6/n-3 ratio in CP group (4.40) was intermediate between the highest values corresponding to HH and GH groups and the lowest values corresponding to HL and GL groups. Differences were significant (P < 0.05) only when GH v. HL and GL groups were compared.
Table 8 Effect of forage type and concentrate level in five experimental groups (HL, HH, GL, GH, CP) on lipid content and FA composition of rectus abdominis muscle†

a,bMeans within a row with different superscripts differ significantly (P < 0.05).
†Abbreviations are: F = forage type; C = concentrate level; HL = hay – low concentrate; HH = hay – high concentrate; GL = cut grass – low concentrate; GH = cut grass – high concentrate; CP = control pasture; FA = fatty acid; PUFA = polyunsaturated fatty acid.
¶Saturated FA = sum of total linear and total branched chain saturated FA. Monounsaturated FA = sum of total 16:1 cis and trans isomers, total 18:1 cis and trans isomers, C20:1 cis, C22:1 cis and C24:1 cis fatty acids. PUFA = sum of (n-6) PUFA and (n-3) PUFA.
‡(n-6) PUFA = sum of C18:2 n-6, C18:3 n-6, C20:2 n-6, C20:3 n-6, C20:4 n-6, C22:2 n-6, C22:4 n-6 fatty acids.
§(n-3) PUFA = sum of C18:3 n-3, C20:3 n-3, C18:4 n-3, C20:5 n-3, C22:5 n-3 and C22:6 n-3 fatty acids.
∥Linear saturated FA = sum of C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C21:0, C22:0, C23:0 and C24:0 fatty acids.
Muscle sensory traits
Table 9 presents the results for the taste panel scores in longissimus thoracis corresponding for groups CP, HH and GH. ‘Group’ factor was significant only for tenderness and juiciness variables (P = 0.01 and P = 0.003, respectively). CP group presented significantly lower tenderness and juiciness values (P < 0.05) than HH and GH groups.
Table 9 Meat sensory traits in longissimus thoracis muscle of HH, GH and CP groups†

a,bMeans within a row with different superscripts differ significantly (P < 0.05).
†Abbreviations are: HH = hay – high concentrate; GH = cut grass – high concentrate; CP = control pasture.
Discussion
The production system adapted resulted in the production of relatively lean carcasses averaging 210 kg. High-concentrate-fed groups presented slightly higher ADWG than low-concentrate-fed groups (approximately 100 g). High-concentrate feeding increased carcass weight by 15 kg compared with low-concentrate feeding. Differences in ADWG between high-concentrate and low-concentrate groups were expressed by differences in carcass weight associated with higher muscle weight but without differences in fatness. Carcass fatness is affected by several factors like sex, age, genotype and growth rate (Micol et al., Reference Micol, Robelin and Geay1993). Usually, higher growth rates are associated with higher carcass fatness, but many experiments that found this effect were performed with bulls slaughtered at higher ages than in the present work (Steen and Kilpatrick, Reference Steen and Kilpatrick1995; Listrat et al., Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999; Sami et al., Reference Sami, Augustini and Schwarz2004) or with animals differing in sex (heifers or steers) and age (French et al., Reference French, O’Riordan, Monahan, Caffrey, Mooney, Troy and Moloney2001). Sinclair et al. (Reference Sinclair, Cuthbertson, Rutter and Franklin1998) indeed observed significant differences in carcass weight but not in carcass fatness in young Charolais bulls slaughtered between 10 and 19 months, with daily gains differing on average by 600 g/day (1.41 v. 1.98 kg/day). In the same experiment, these authors obtained carcasses with the same weight but differing in fatness with bulls of a higher precocity genotype (Aberdeen Angus) differing by 300 g/day in growth rate (daily gains of 1.22 v. 1.55 kg/day).
Diet did not affect muscle pH, which agrees with the observations from numerous studies (French et al., Reference French, O’Riordan, Monahan, Caffrey, Vidal, Mooney, Troy and Moloney2000 and Reference French, O’Riordan, Monahan, Caffrey, Mooney, Troy and Moloney2001; Sami et al., Reference Sami, Augustini and Schwarz2004) and suggests that all diets supplied sufficient energy to allow a normal pH evolution. Muir et al. (Reference Muir, Deaker and Brown1998) indicated that grass-fed animals often presented higher pH values than grain-fed animals as a result of interaction of diet and their higher susceptibility to pre-slaughter stress because they are less used to handling. In our study, animals were slaughtered within 4 h after removal from experimental installations and all groups were used to handling, which may have minimised the impact of stress on the depletion of glycogen deposits.
Muscle colour is an important criterion by which many consumers evaluate meat quality and acceptability. The results concerning subjective classification of muscle colour showed that an important characteristic of the meat produced is that it was very red compared with traditional veal meat. Diet (forage type and concentrate level) had no effect on L* and a* colour parameters. In the two muscles that were considered, b* values were higher in grass-fed animals but differences were significant only when comparing pasture-fed v. hay-fed animals. Animals raised on pasture presented the lowest L* values compared with the other four groups of animals but differences were not significant. Meat from cattle raised on pasture is indeed known to be darker than meat from animals raised on concentrates (Priolo et al., Reference Priolo, Micol and Agabriel2001). But French et al. (Reference French, O’Riordan, Monahan, Caffrey, Vidal, Mooney, Troy and Moloney2000 and Reference French, O’Riordan, Monahan, Caffrey, Mooney, Troy and Moloney2001) did not observe any difference in colour parameters in steers finished (85 days) on diets constituting combinations of different forages (grass silage, grass or hay) and different concentrate levels allowing the same or different growth rates and slaughtered at the same age. Varela et al. (Reference Varela, Oliete, Moreno, Portela, Monserrat, Carballo and Sánchez2004) also failed to find differences in meat colour between steers finished (3 months) exclusively on pasture or with corn silage and concentrate, slaughtered at the same age and live weight. On the contrary, Nuernberg et al. (Reference Nuernberg, Dannernberger, Nuernberg, Ender, Voigt, Scollan, Wood, Nute and Richardson2005) reported lower L* values (a* and b* values were not measured) in bulls finished on grass-based v. concentrate-based diets. These authors hypothesised that these differences could be associated with higher physical activity of grass-fed animals compared with indoor kept concentrate-fed bulls. It is, however, necessary to point out that in this work the experimental period (between 11 and 19 months) was longer and started when animals were younger than in the experiments cited before and pasture-finished animals were slaughtered at higher age than concentrate-fed ones (18 v. 24 months). In agreement with our observations, Monserrat et al. (Reference Monserrat, Sánchez, Varela, Carballo and Oliete2001) also noted higher b* values in pasture-fed than in concentrate-hay-fed young bull meat.
Neither forage type nor concentrate level affected muscle fibre area. These results are in accordance with those of Listrat et al. (Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999) who did not find any difference in muscle fibre area between Salers bulls fed on isoenergetic and isoproteic diets based on grass silage or hay and concentrate. Cassar-Malek et al. (Reference Cassar-Malek, Hocquette, Jurie, Listrat, Jailler, Bauchart, Briand and Picard2004a), Maltin et al. (Reference Maltin, Lobley, Grant, Miller, Kyle, Horgan, Matthews and Sinclair2001) and Sami et al. (Reference Sami, Augustini and Schwarz2004) also failed to find differences in muscle fibre surface between animals finished on high- or low-energy diets and presenting moderate differences in growth rates (between 200 and 400 g/day). In contrast with these observations, Jurie et al. (Reference Jurie, Listrat, Giraud, Picard, Geay and Hocquette1999) showed that restricted growth rate was associated with lower fibre surface, but differences in growth rate observed in this latter experiment (approximately 640 g/day) were greater than differences observed in our study and in experiments cited before. It is interesting to note that in the ST muscle, the highest values of fibre size corresponded to pasture animals, although differences were not significant. In agreement with this observation, Jurie et al. (Reference Jurie, Picard and Geay1998) observed an increase in ST muscle fibre size in animals housed in loose housing compared with those housed in tying-type housing.
Several works have reported the effect of diet on muscle energy metabolism. The results obtained in several studies suggested an association between grass or grass silage feeding and an increase in oxidative activities of muscles (Jurie et al., Reference Jurie, Listrat, Giraud, Picard, Geay and Hocquette1999; Listrat et al., Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999). In the present work, diet did not affect enzyme activities. This result agrees with observations of Maltin et al. (Reference Maltin, Sinclair, Warris, Grant, Porter, Delday and Warkup1998) who did not find differences in these parameters in young bulls fed on grass-silage-based or barley-based diets. However, we found higher CS enzyme activities in pasture-fed animals. This observation agrees with the results of Jurie et al. (Reference Jurie, Ortigues-Marty, Micol, Cassar-Malek, Dozias, Picard and Hocquette2004) and Cassar-Malek et al. (Reference Cassar-Malek, Jurie, Bernard, Barnola, Gentes, Guivier, Dozias, Micol and Hocquette2004b) who observed that in the development of muscle metabolic characteristics mobility is a much more important factor than diet. In agreement with our results, Jurie et al. (Reference Jurie, Picard and Geay1998) observed that effects of physical activity on enzyme activities depended on type of muscle studied, or more precisely, the involvement of this muscle in movement (e.g. ST) or posture (e.g. RA).
Quantity and solubility of collagen may be influenced by several factors, including growth rate, nature of diet, physical activity and muscle analysed (Bailey and Light, Reference Bailey and Light1989). We did not observe significant differences between groups in total and insoluble collagen contents of RA muscle but soluble/insoluble collagen content ratio was higher in the three grass-fed groups. Contradictions exist in the literature about effects of growth rate and diet nature on collagen pattern. Sami et al. (Reference Sami, Augustini and Schwarz2004), comparing four diets based on different proportions of maize silage and concentrate formulated to allow different growth rates, did not find differences in collagen content but higher collagen solubility in animals with higher daily weight gain. Maltin et al. (Reference Maltin, Sinclair, Warris, Grant, Porter, Delday and Warkup1998) observed higher collagen content in longissimus lumborum muscle of animals finished on grass-silage-based diet having low growth rate than in animals finished on barley-based diet having high growth rate (approximate weight gain differences of 400 g/day) and no effect on collagen solubility. Listrat et al. (Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999), comparing two groups of Salers bulls fed on diets based on grass silage or hay, did not observe differences in ST muscle collagen content. In this experiment, hay-fed animals presented lower growth rates and higher collagen solubility than grass-silage-fed animals (−122 g/day).
Intramuscular fat content can be modified by several factors like age, genotype and growth rate. In general, intramuscular fat content increases with age and with growth rate and is higher in mixed breeds than in beef breeds (Nürnberg et al., Reference Nürnberg, Wegner and Ender1998). In the present experiment, intramuscular fat content was not affected by production strategies and values obtained (between 15.1 and 17.7 mg/g wet matter muscle and between 63.0 and 74.6 mg/g dry muscle) were very low compared with those obtained in other works for longissimus thoracis muscle, in general a muscle less fatty than RA muscle analysed in the present work. These results could be explained by the moderate growth rates obtained and by the young age at slaughter. For example, Serra et al. (Reference Serra, Gil, Gispert, Guerrero, Oliver, Sañudo, Campo, Panea, Olleta, Quintanilla and Piedrafita2004) reported mean values of intramuscular fat content of 24 mg/g wet muscle in Bruna dels Pirineus bulls slaughtered at 30 months. Varela et al. (Reference Varela, Oliete, Moreno, Portela, Monserrat, Carballo and Sánchez2004) did not observe differences in intramuscular fat content between 33 months Rubia Gallega steers finished on pasture or on maize-silage-based and concentrate-based diets. These authors also obtained, for longissimus thoracis muscle, mean values of 27 mg of total fat/g wet muscle. Maltin et al. (Reference Maltin, Sinclair, Warris, Grant, Porter, Delday and Warkup1998) observed an important effect of age on intramuscular fat content but did not find differences between animals with growth rates differing between 300 and 600 g/day. They reported intramuscular fat content of 171.1 mg/g DM in Aberdeen Angus and Charolais bulls slaughtered at 11.5 months of age.
United Kingdom Department of Health recommends a polyunsaturated:saturated (P/S) ratio ∼0.45 in the whole diet of man, and increasing intake of n-3 relative to n-6 PUFA and achieving a n-6/n-3 fatty acids ratio below 4.0 (Enser et al., Reference Enser, Hallett, Hewett, Fursey, Wood and Harrington1998). Ruminant meats are known to have low polyunsaturated/saturated fatty acid ratio because of rumen biohydrogenation of dietary unsaturated fat (Geay et al., Reference Geay, Bauchart, Hocquette and Culioli2002). In the present experiment, production strategies did not affect P/S ratio. Hornick et al. (Reference Hornick, Clinquart, Van Eenaeme, Diez and Istasse1996) showed that inclusion of milk in fattening diet of bulls increased saturated fatty acid content of intramuscular fat and decreased monounsaturated and polyunsaturated fatty acid contents. In our experiment, all animals remained unweaned until slaughter and, as in the experience of Hornick et al. (Reference Hornick, Clinquart, Van Eenaeme, Diez and Istasse1997), closure of oesophageal groove remained active. P/S ratios in the present work were similar to those obtained by Varela et al. (Reference Varela, Oliete, Moreno, Portela, Monserrat, Carballo and Sánchez2004) in Rubia Gallega steers, weaned at 9 months of age, maintained on pasture until 30 months of age and then finished for 3 months on concentrate or pasture systems (approximately 0.25 and 0.33, respectively). However, P/S ratios were higher than those reported by Nuernberg et al. (Reference Nuernberg, Dannernberger, Nuernberg, Ender, Voigt, Scollan, Wood, Nute and Richardson2005) in Holstein and Simmental bulls slaughtered between 17 and 25 months of age and assigned to concentrate-based or grass-based production systems from weaning, at 6 months, to slaughter (0.17 and 0.21 for Holstein bulls and 0.20 and 0.32 for Simmental bulls). These results may, in part, be explained by the fact that when intramuscular fat content is low, as occurred in the present work, the relative importance of membrane phospholipids, rich in unsaturated fatty acids, is higher (Bas and Sauvant, Reference Bas and Sauvant2001).
The n-6/n-3 PUFA ratio in muscle fatty acids of ruminants is often too high relative to the recommendation noted above for man (Enser et al., Reference Enser, Hallett, Hewett, Fursey, Wood and Harrington1998). Generally, muscles of grass-fed ruminants have lower n-6 PUFA and higher n-3 PUFA than concentrate-fed ruminants although adding sources of linoleic acid to concentrate (such as linseed) improves the equilibrium between these fatty acids in meat (Enser et al., Reference Enser, Hallett, Hewett, Fursey, Wood and Harrington1998; Geay et al., Reference Geay, Bauchart, Hocquette and Culioli2002). In the present work, in agreement with the observations of Enser et al. (Reference Enser, Hallett, Hewett, Fursey, Wood and Harrington1998), Aurousseau et al. (Reference Aurousseau, Bauchart, Calichon, Micol and Priolo2004) and Nuernberg et al. (Reference Nuernberg, Dannernberger, Nuernberg, Ender, Voigt, Scollan, Wood, Nute and Richardson2005) the more favourable values of n-6/n-3 PUFA ratio corresponded to meat from animals fed on low concentrate and higher forage.
Higher growth rates have been associated with the production of more tender meat (French et al., Reference French, O’Riordan, Monahan, Caffrey, Mooney, Troy and Moloney2001; Nuernberg et al., Reference Nuernberg, Dannernberger, Nuernberg, Ender, Voigt, Scollan, Wood, Nute and Richardson2005). In the present work, pasture-fed animals obtained significantly lower tenderness and juiciness scores than other groups, although they presented higher growth rates. Sami et al. (Reference Sami, Augustini and Schwarz2004) and Sinclair et al. (Reference Sinclair, Lobley, Horgan, Kyle, Porter, Matthews, Warkup and Maltin2001) have not observed differences in tenderness of meat from 15 months bulls or steers, respectively, differing in growth rate. Listrat et al. (Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999) obtained more tender meat in hay-fed Salers bulls than in grass-silage-fed animals having higher growth rates. Factors other than growth rate, such as diet composition (Listrat et al., Reference Listrat, Rakadjiyski, Jurie, Picard, Touraille and Geay1999) or physical activity (Jurie et al., Reference Jurie, Picard and Geay1998), can affect the metabolic and histological characteristics of the muscle and, as a result, the meat tenderness. The results referring to metabolic and histological characteristics of the muscle indicated that pasture animals could have more oxidative muscles and could be constituted of fibres with larger surface than other groups. Several authors have associated these characteristics with tougher meat (Jurie et al., Reference Jurie, Picard and Geay1998; Renand et al., Reference Renand, Picard, Touraille, Berge and Lepetit2001) but not with a lower juiciness, more associated with other characteristics such as fat content (Geay et al., Reference Geay, Bauchart, Hocquette and Culioli2002). Differences in juiciness could be explained considering correlation between tenderness and juiciness scores (r = 0.46; P < 0.0001), and that the pasture group presented the lowest mean value of fat content although values were not statistically different. Considering the importance of ageing process in quality of bovine meat coming from pasture systems (French et al., Reference French, O’Riordan, Monahan, Caffrey, Vidal, Mooney, Troy and Moloney2000; Renand et al., Reference Renand, Picard, Touraille, Berge and Lepetit2001), these results question whether the 8 days ageing period used was sufficient or not.
Conclusion
The results obtained suggest that the diets used have only a small effect on performance, offal fatty tissue weight, carcass and muscle characteristics and on meat eating quality traits. Meat produced was characterised by a very low fat content and a relatively high unsaturated fatty acid content, which could be favourable in a context of increased demand for leaner beef and consumer resistance to beef with a high fat content.
Acknowledgements
The authors wish, in particular, to thank I. Constant and C. Cirie for their help and support. The authors also thank Gloria López, INRA-Marcenat staff, M.C. Bayle, C2M and NEM staffs and the team of the experimental slaughterhouse in Theix.















