Introduction
The genus Nothacrobeles was described by Allen & Noffsinger (Reference Allen and Noffsinger1971) who found four new species from Israel, India and Australia, being the type species Nothacrobeles sheri Allen & Noffsinger, 1971. This genus is characterized by having lip region with labial probolae bifurcate with prongs having variable length, rarely absent, connecting basally by an expansion directed towards the secondary axil, and margins smooth or bordered by small membranous projections (pinnae). Later, Shahina & De Ley (Reference Shahina and De Ley1997) synonymized the genus Namibinema Rashid & Heyns, 1990 with it. Actually, the genus Nothacrobeles includes 21 valid species (Abolafia & Peña-Santiago, Reference Abolafia and Peña-Santiago2003; Ruiz-Cuenca & Abolafia, Reference Ruiz-Cuenca and Abolafia2020).
One of these species, Nothacrobeles lanceolatus, was described by Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2003) in xeric areas from Cabo de Gata-Níjar Natural Park (Spain). This species was considered as a possible endemism for this geographical area. In this study, new data about the distribution of this species, which can be considered now as Iberian littoral endemism, is provided. This new material is now characterized from the morphological, morphometric and molecular point of view.
Material and methods
Sampling and nematode extraction
The specimens of N. lanceolatus were extracted from the rhizosphere of xerophile plants from sand coastal dunes in the provinces of Huelva, Cádiz, Málaga, Almería, Murcia, Alicante, Valencia, Castellón, Tarragona and Barcelona (Spain) (fig. 1). The nematodes were extracted from soil samples using a modified Baermann's (Reference Baermann1917) funnel technique, killed by heat and fixed in a 4% formalin solution. The nematodes were processed to anhydrous glycerine according to Siddiqi's (Reference Siddiqi1964) method using lactophenol–glycerine solutions and were permanently mounted on glass microscope slides with the glycerine–paraffin method (de Maeseneer & d’Herde, Reference de Maeseneer and d'Herde1963) somewhat modified using hot liquid paraffin.
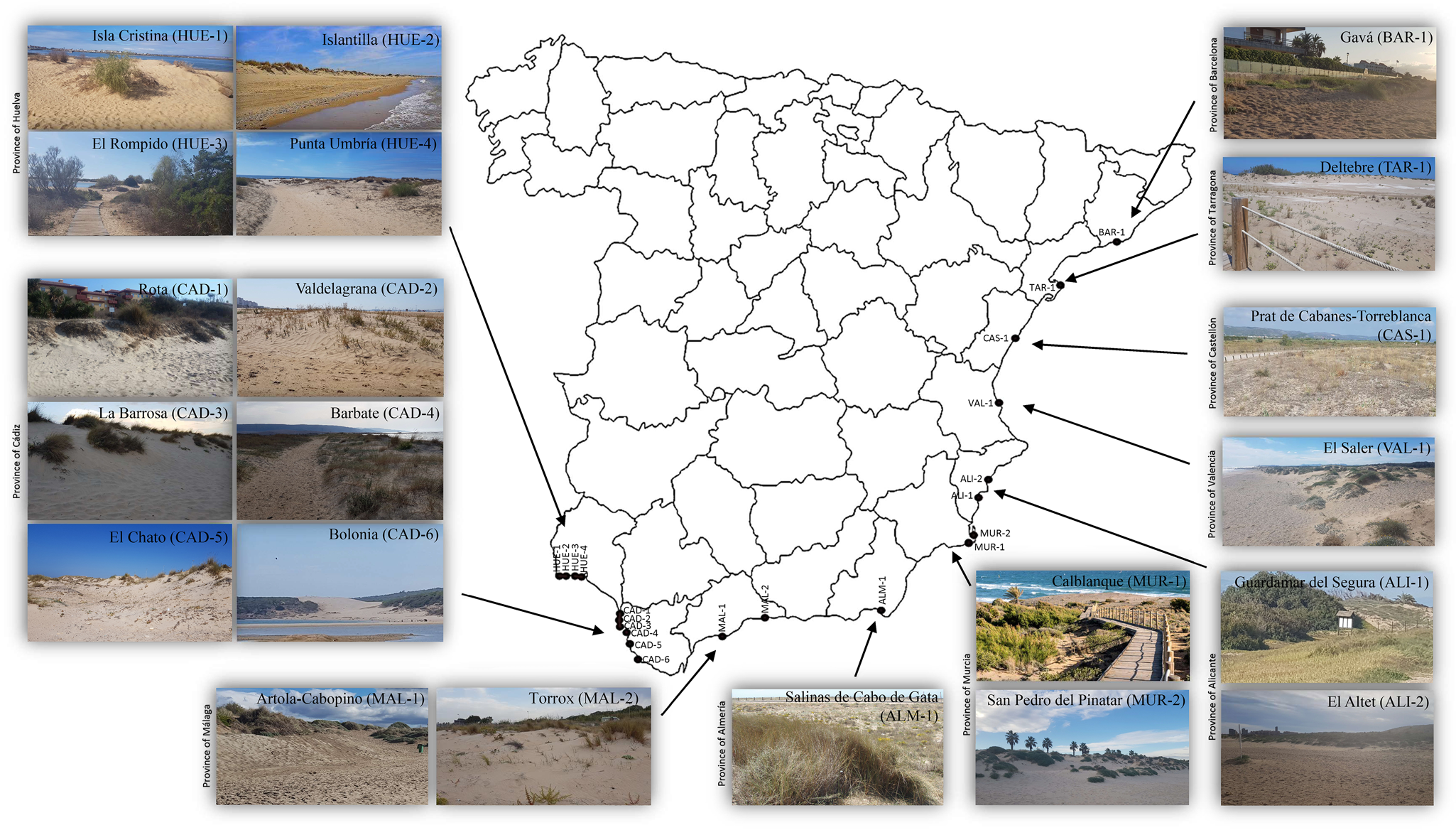
Fig. 1. Spanish localities with presence of Nothacrobeles lanceolatus Abolafia & Peña-Santiago, 2003.
Light microscopy (LM)
Observations were made and measurements were taken using a Nikon Eclipse 80i (Nikon, Tokyo, Japan) microscope with differential interference contrast optics. Photomicrographs were taken with a Nikon Digital Sight DS-U1 camera and processed with Adobe® Photoshop® CS (Adobe Inc., San José, California, USA) and figures mounted using Microsoft® PowerPoint® (Microsoft Corporation, Redmond, Washington, USA). Demanian indices (de Man, Reference de Man1881) and other ratios were calculated. The terminology used for the morphology of probolae, stoma and spicules follows Ruiz-Cuenca & Abolafia (Reference Ruiz-Cuenca and Abolafia2021), De Ley et al. (Reference De Ley, van de Velde, Mounport, Baujard and Coomans1995) and Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2017), respectively.
Scanning electron microscopy (SEM)
The specimens preserved in glycerine were selected for observation under SEM according to Abolafia (Reference Abolafia2015). The nematodes were hydrated in distilled water, dehydrated in a graded ethanol–acetone series, critical-point dried, coated with gold and observed with a Zeiss Merlin microscope (5 kV) (Zeiss, Oberkochen, Germany).
DNA extraction, polymerase chain reaction (PCR) and sequencing
Nematode DNA was extracted from single fresh individuals using the proteinase K protocol and PCR assays, as described by Castillo et al. (Reference Castillo, Vovlas, Subbotin and Troccoli2003), somewhat modified (Archidona-Yuste et al., Reference Archidona-Yuste, Navas-Cortés, Cantalapiedra-Navarrete, Palomares-Rius and Castillo2016). The specimens were cut into small pieces using a sterilized dental needle on a clean slide with 18 ml of Tris-EDTA (ethylene-diamine-tetraacetic acid) buffer (10 mm Tris-Cl (tris hydrochloride) + 0.5 mm EDTA; pH 9.0), transferred to a microtube, adding 2 μl proteinase K (700 μg/ml−1) (Roche, Basel, Switzerland) and stored to –80°C within 15 min (for several days). The microtubes were incubated at 65°C (1 h), then at 95°C (15 min). For DNA amplification, 3 μl of the extracted DNA was transferred to a microtube containing: 0.6 μl of each primer (10 mm), 3 μl Master Mix Taq DNA Polymerase (5× Hot FirePol Blend Master Mix, Solis BioDyne, Tartu, Estonia) and double distilled water (ddH2O) to a final volume of 20 μl. The primers used for amplification of the region of 18S ribosomal RNA (rRNA) gene were the forward primer 988 F (5′-CTCAAAGATTAAGCCATGC-3′) and the reverse primer 1912R (5′-TTTACGGTCAGAACTAGGG-3′) (Holterman et al., Reference Holterman, van der Wurff, van den Elsen, van Megen, Bongers, Holovachov, Bakker and Helder2006). The primers used for amplification of the D2-D3 region of 28S rRNA gene were the D2A (5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and the D3B (5′-TCGGAAGGAACCAGCTACTA-3′) primers (Nunn, Reference Nunn1992; De Ley et al., Reference De Ley, Felix, Frisse, Nadler, Sternberg and Thomas1999). PCR cycle conditions were as follows: one cycle of 94°C for 15 min, followed by 35 cycles of 94°C for 45 s + annealing temperature of 55°C for 45 s + 72°C for 45 s, and finally one cycle of 72°C for 5 min. After DNA amplification, 5 μl of product was loaded on a 1% agarose gel in 0.5% Tris-acetate-EDTA (40 mm Tris, 20 mm glacial acetic acid and 2 mm EDTA; pH = 8) to verify the amplification using an electrophoresis system (Labnet Gel XL Ultra V–2, Progen Scientific, London, UK). The bands were stained with RedSafe (20,000×) previously added to the agarose gel solution. The sequencing reactions of the PCR products were performed at Sistemas Genómicos (Paterna, Valencia, Spain) according the Sanger et al. (Reference Sanger, Nicklen and Coulson1977) method. The sequences obtained were submitted to the GenBank database.
Phylogenetic analyses
For phylogenetic relationships, analyses were based on 18S and 28S ribosomal DNA (rDNA) fragments. The newly obtained sequences were manually edited using BioEdit 7.2.6 (Hall, Reference Hall1999) and aligned with another 18S or 28S rDNA sequence available in GenBank using the ClustalW (Thompson et al., Reference Thompson, Higgins and Gibson1994) alignment tool implemented in MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Alignment ends were trimmed using MEGA7. The best-fit model of nucleotide substitution used for the phylogenetic analysis was statistically selected using jModelTest 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012). The phylogenetic trees were generated with the Bayesian inference method using MrBayes 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko and van der Mark2012). Drilocephalobus sp. (AY284680) for 18S rDNA and Teratolobus sp. (KJ652552) for 28S rDNA were chosen as outgroup. The analysis under the general time-reversible plus invariant sites plus gamma distribution (GTR + I + G) model (Tavaré, Reference Tavaré1986) was initiated with a random starting tree and run with the Markov Chain Monte Carlo (MCMC) (Larget & Simon, Reference Larget and Simon1999) for 1 × 106 generations. The trees were visualized and saved with FigTree 1.4.4 (Rambaut, Reference Rambaut2018).
Results
Nothacrobeles lanceolatus Abolafia & Peña-Santiago, 2003
Material examined
In this study, 20 females and 20 males from several Iberian coastal dunes were examined.
Measurements
For measurements, see table 1.
Table 1. Morphometrics of Nothacrobeles lanceolatus Abolafia & Peña-Santiago, 2003. All measurements in μm.
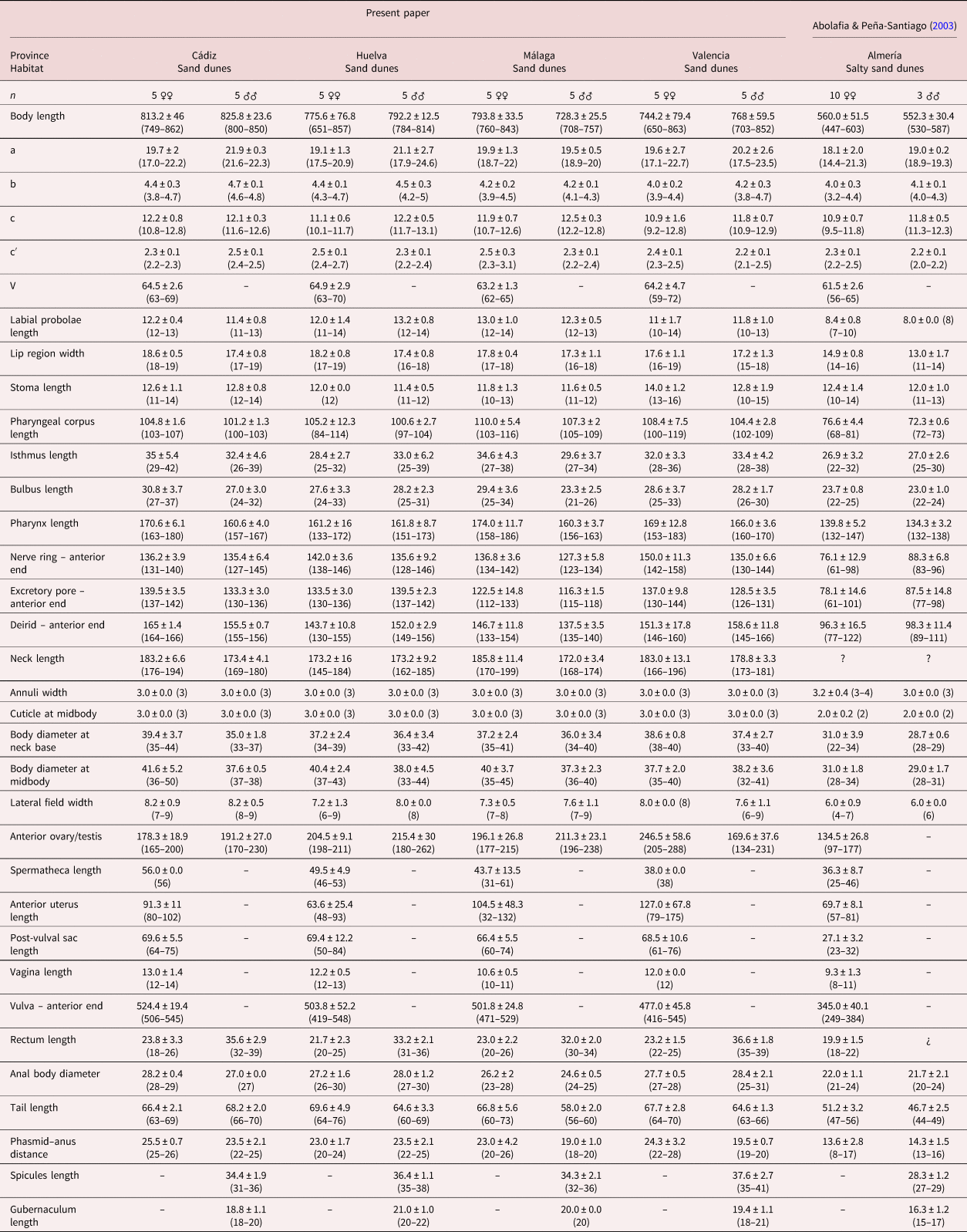
Measurements in μm and in the form: mean ± standard deviation (range) where appropriate. Demanian indices (de Man, Reference de Man1881): a, body length/body diameter; b, body length/pharynx length; c, body length/tail length; c′, tail length/anal body diameter; V, distance from anterior region to vulva/body length ×100.
Description
Adult (figs 2–5). Body cylindrical, with 0.6–0.8 mm long. Cuticle with tessellated annuli; annuli 3 μm wide at midbody. Lateral fields with three longitudinal incisures (or two longitudinal alae) occupying 16–25% of the body diameter at midbody. Lip region with dentate rectangular lips, grouped in pairs. Primary axils deep with two elongate guard processes and secondary axils shallow lacking guard processes. Oral opening surrounded by three labial probolae with dentate margin bearing rounded to conoid pinnae, 10–14 μm long, bifurcated, with a prominent basal ridge protruding toward the lips; each probolae with furca elongate. Amphid openings ovoid. Stoma cephaloboid, robust, with short cheilostom with large and rounded cheilorhabdia, very reduced gymnostom with minute gymnorhabdia, and well developed stegostom with minute stegorhabdia and large dorsal tooth. Pharynx also cephaloboid, with subcylindrical pharyngeal corpus having procorpus and metacorpus well differentiated, both with similar length; pharyngeal corpus–isthmus junction well demarcated and isthmus narrower than corpus; basal bulb spheroid with valvular apparatus (grinder) well developed. Nerve ring at 70–81% of neck length, surrounding the isthmus. Excretory pore at 66–78% of neck length, at isthmus level. Deirid at 77–93% of neck length, at isthmus or basal bulb level. Intestine without distinct specializations but having narrower walls at anterior part.
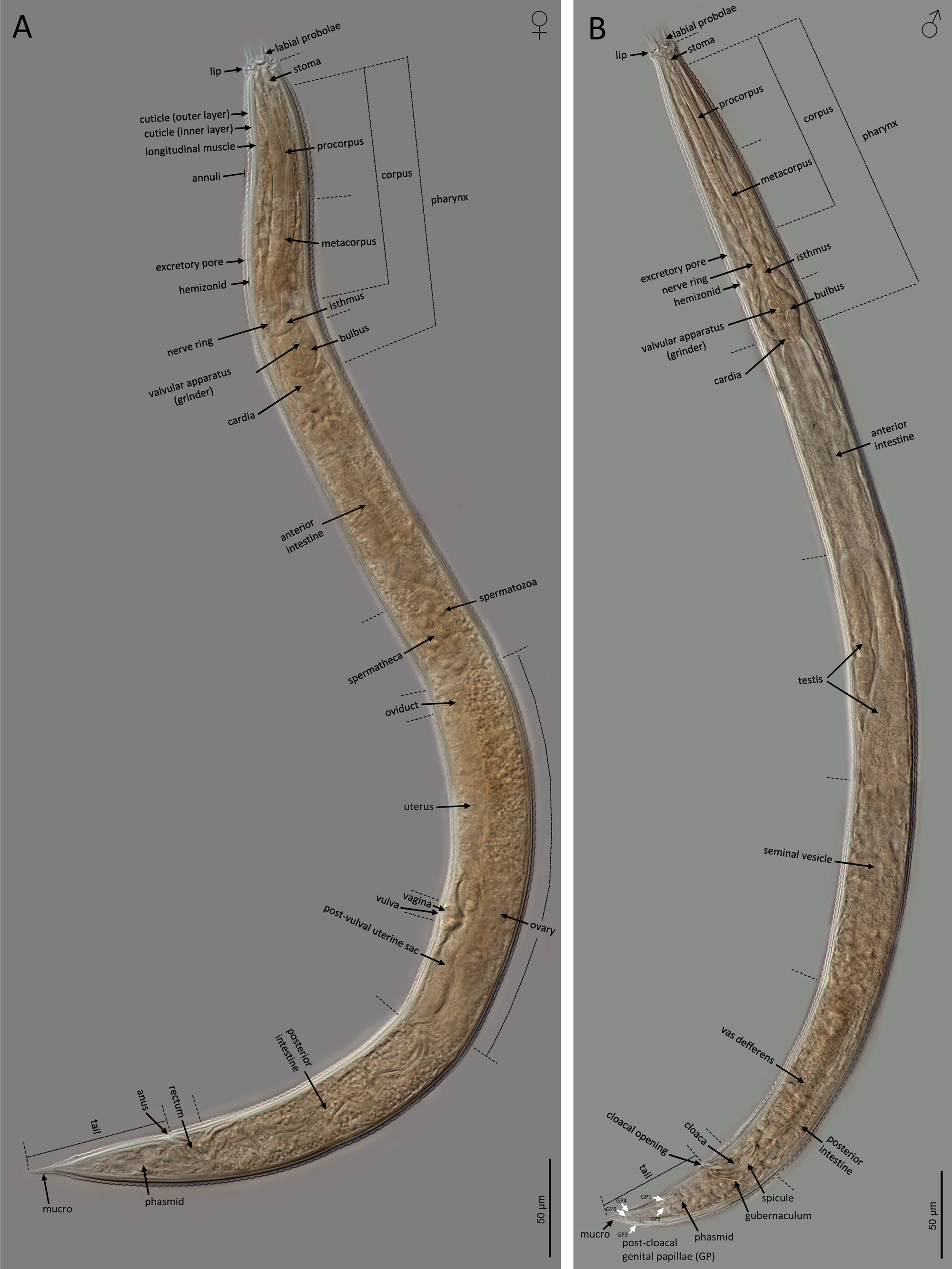
Fig. 2. LM of Nothacrobeles lanceolatus Abolafia & Peña-Santiago, 2003. (A) Female; (B) male.
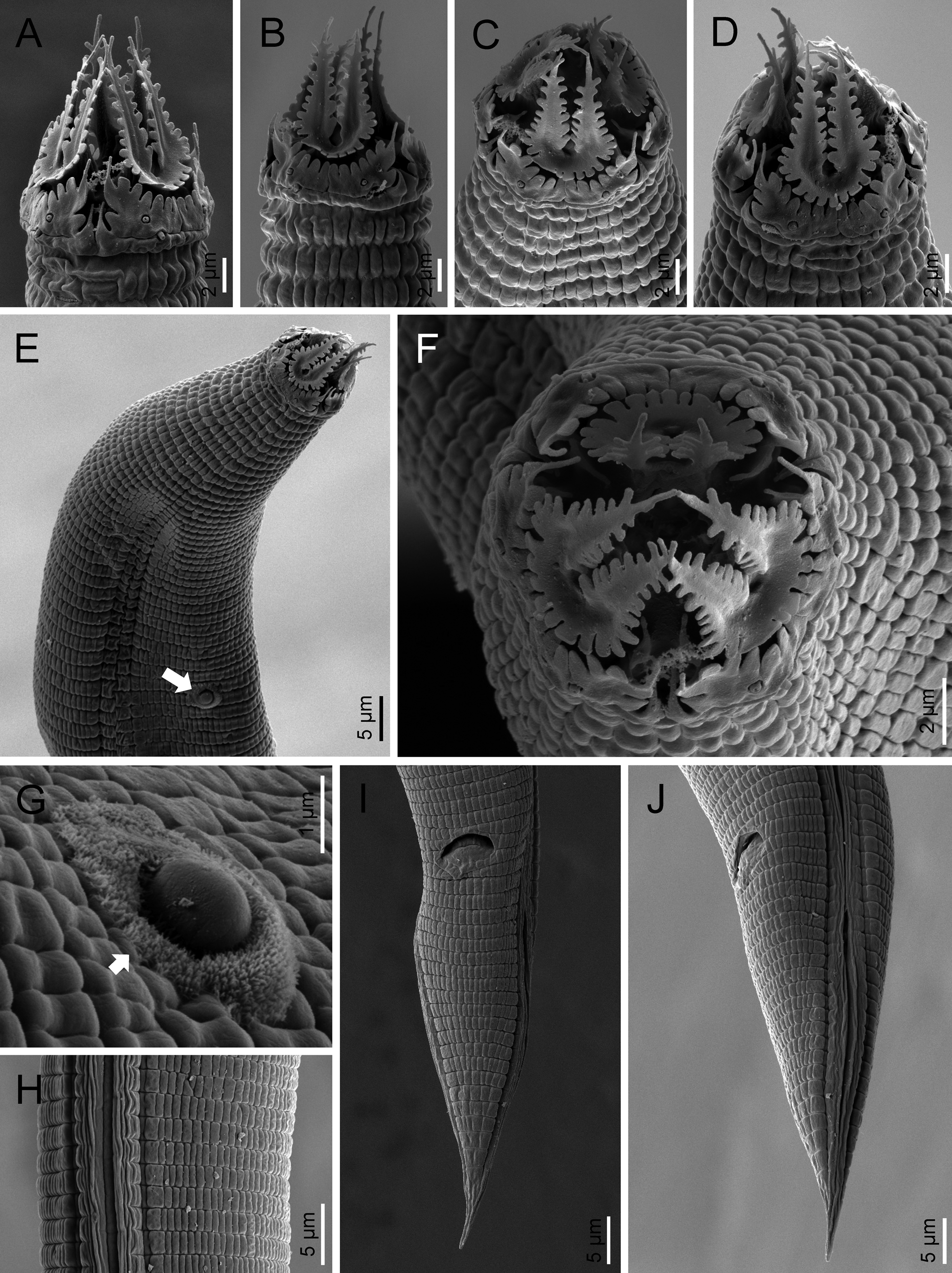
Fig. 3. SEM of Nothacrobeles lanceolatus Abolafia & Peña-Santiago, 2003. (A–D, F) Lip region in ventral, left lateral, subfrontal, right lateral and frontal view, respectively; (E) anterior region (arrow pointing the bacterium Pasteuria); (G) sporangium of Pasteuria (arrow) at cuticle; (H) lateral field; (I, J) tail in ventral and lateral view, respectively.
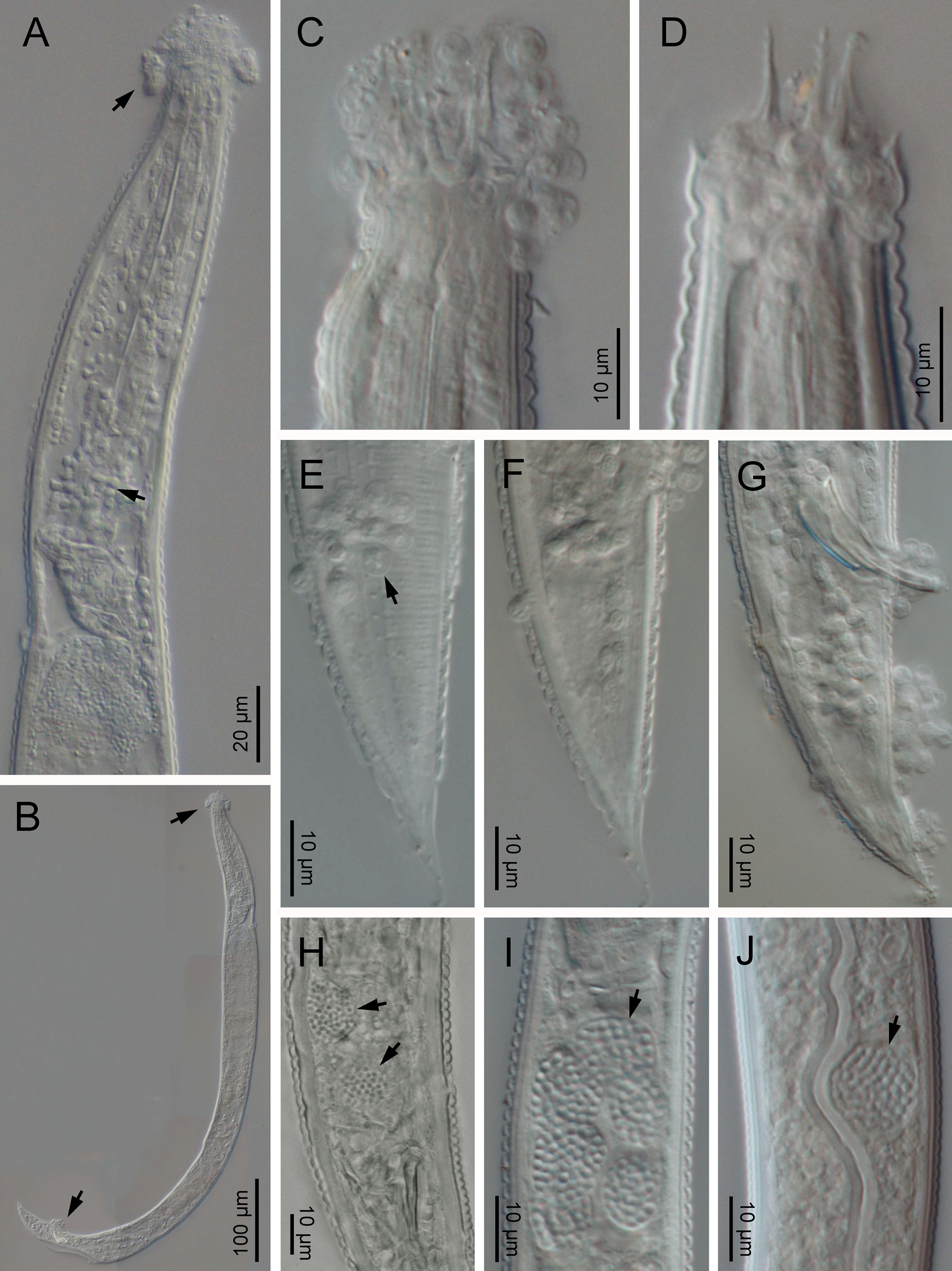
Fig. 4. LM of Nothacrobeles lanceolatus with fungi. (A–G) Zoospores of Catenaria (arrows) at anterior end and pharynx; (H–J) Nematocida (arrows) at intestine cells.
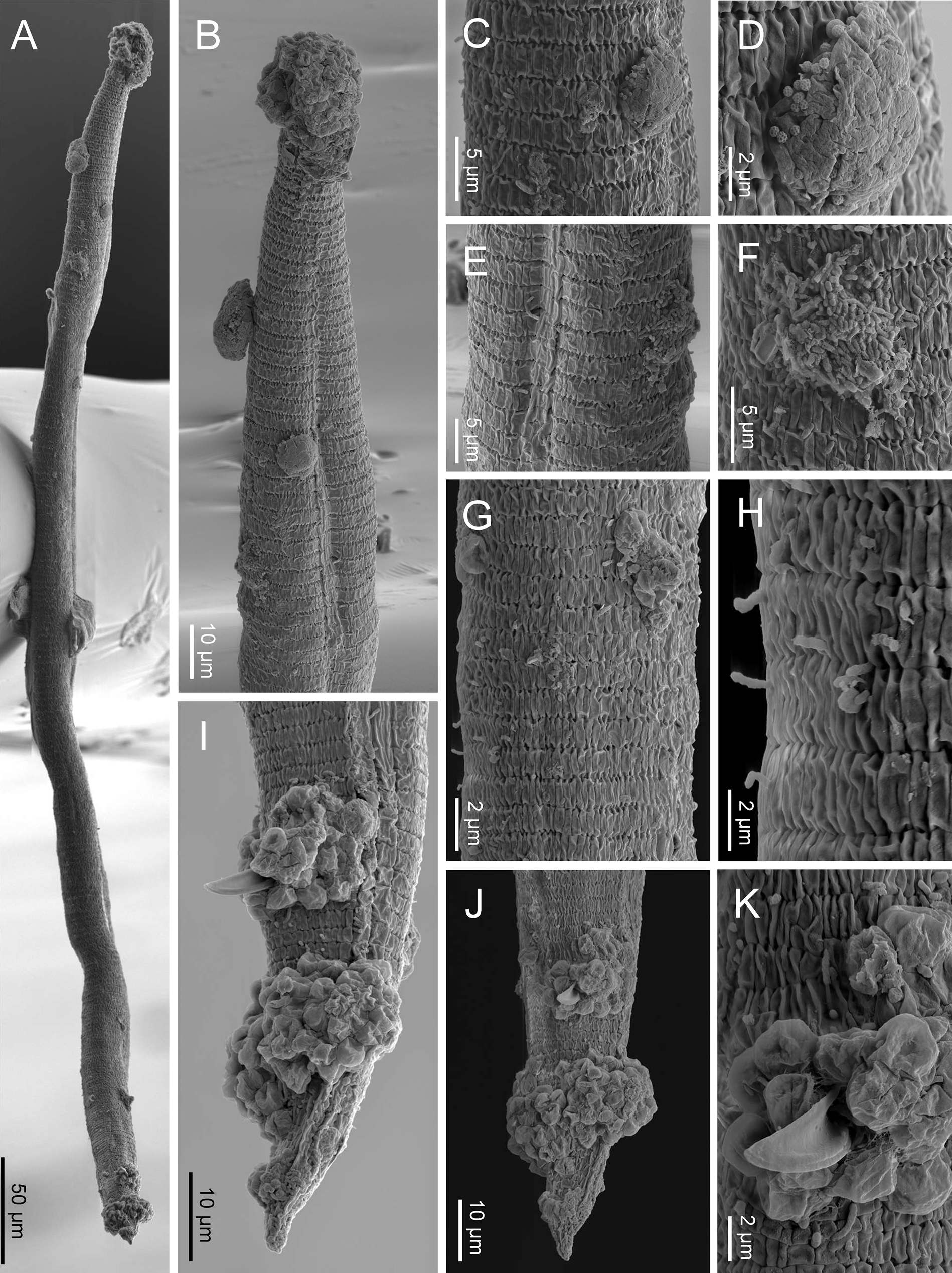
Fig. 5. SEM of Nothacrobeles lanceolatus with fungi and bacteria. (A) Entire body; (B) anterior end with zoospores of Catenaria; (C, D) cuticle with sticky mess with pollen grains; (E, F) excretory pore with bacteria; (G, H) sporangia of Catenaria emerging through the cuticle; (I, J) male tail in lateral and ventral views, respectively, with zoospores of Catenaria at cloaca and tail; (K) detail of cloaca showing the spicule and cloacal aperture surrounded by zoospores.
Female. Reproductive system monodephic–prodelphic, cephaloboid. Ovary long, without flexures with oocytes in only one row; oviduct very short; spermatheca well developed, swollen, 1.0–1.4 times the body diameter, with large spermatozoa; uterus with elongate tubular distal part having thicker walls and short proximal swollen part having thinner walls; vagina short, about one fourth of the body diameter; vulva a transversal slit; post-vulval uterine sac swollen, 1.4–2.2 times the corresponding body diameter long. Rectum shorter than the anal body diameter. Anus well developed, curved. Tail conical, spear-shaped, ending in an acute, conoid mucro, with cuticle slightly wider at posterior region. Phasmids located at 32–40% of tail length.
Male. Reproductive system monorchid, with testis ventrally reflexed anteriorly. Tail conical, ventrally curved with an acute mucro at the end. Phasmids located at 30–36% of tail length. Post-cloacal genital papillae five pairs, two at middle length of tail, one lateral and one ventrosublateral, and three near the tail tip, one subdorsal and two subventral. Spicules paired and symmetrical, ventrally curved having rounded manubrium, conoid calamus and ventrad curved lamina with reduced ventral velum and acute terminus. Gubernaculum well developed, almost straight with ventrad curved at its anterior part.
Molecular characterization
Four 18S rDNA sequences of N. lanceolatus were obtained having 849 bp (OK042922), 801 bp (OK042923), 742 bp (OK042924) and 795 bp (OK042925) from sand dunes in Artola-Cabopino, Málaga (Spain), being 100% similar between them, in a segment in common with 678 bp. On the other hand, two 28S rDNA sequences with 797 bp (OK042914, OK042915) are obtained from the same specimens being 100% similar.
Localities and habitats
The new examined populations of N. lanceolatus have been found in coastal sand dunes from several Spanish localities and beaches (fig. 1): El Altet Beach, Guardamar del Segura (province of Alicante); Salinas de Cabo de Gata, San José (province of Almería); Gavá (province of Barcelona); Barbate, Bolonia Beach, El Chato Beach, La Barrosa Beach, Rota, Valdelagrana (province of Cádiz); Prat de Cabanes-Torreblanca (province of Castellón); El Rompido, Isla Cristina, Islantilla, Punta Umbría (province of Huelva); Artola-Cabopino Beach, Torrox (province of Málaga); Calblanque Beach, San Pedro del Pinatar (province of Murcia); Deltebre (province of Tarragona); El Saler (province of Valencia). All of these populations are associated with a community of xerophilic plants, including Alyssum loiseleurii P. Fourn., Ammophila arenaria (L.) Link, Anthyllis cytisoides L., Carpobrotus edulis (L.) N.E. Br., Otanthus maritimus Hoffmanns. & Link and Pancratium maritimum L.
Remarks
Eight specimens (two males, three females and three juveniles) from two localities were found with bacteria or fungi feeding on them. Thus, one specimen collected from Islantilla (province of Huelva) was found with the bacterium of the genus Pasteuria Metchnikoff, 1888 (Firmicutes, Pasteuriaceae) adhered at the cuticle of the nematode (fig. 3e, g).
Also, two species of fungi have been found in the surface and inside the body of nematode specimens from Artola-Cabopino (province of Málaga). The fungus of the genus Catenaria Sorokin, 1876 (Blastocladiomycota, Blastocladiales) has been found in seven specimens of N. lanceolatus (figs 4a–g and 5a, b, g–k) appearing attached in groups of flagellate zoospores surrounding the oral, vulval and anal openings of the nematodes, generating long hyphae which penetrate inside the body along the digestive tract. Other fungus, found in two specimens, belonging to the genus Nematocida Troemel, Félix, Whiteman, Barrière & Ausubel, Reference Troemel, Félix, Whiteman, Barrière and Ausubel2008 (Microsporidia, Minisporida), an obligate intracellular parasite, appears inside some intestinal cells (fig. 4h–j). Unfortunately, it is unknown if these nematode specimens were parasitized in their natural habitat or during the extraction process, which maintains the soil samples in water for several days.
Discussion
Morphological and morphometrical analyses of N. lanceolatus
In general, the morphology of N. lanceolatus, in the present study, is similar to the type population described by Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2003) from Cabo de Gata-Níjar Natural Park. However, the morphometrical analysis shows some important differences, namely, the measurements of the type population are slightly smaller than in the specimens examined in the present study: body length (0.4–0.6 mm vs. 0.6–0.8 mm), labial probolae length (4–10 μm vs. 10–14 μm), female tail length (47–56 μm vs. 60–76 μm), male tail length (44–49 μm vs. 56–73 μm), post-vulval sac length (23–32 μm vs. 50–84 μm) and spicules length (27–29 μm vs. 31–41 μm). These differences are considered as geographical variability.
New geographical distribution of N. lanceolatus
After the original description provided by Abolafia & Peña-Santiago (Reference Abolafia and Peña-Santiago2003), N. lanceolatus appears with a more extended distribution along the sand dunes of Atlantic and Mediterranean Spanish coasts. All populations examined of this species appeared in xeric areas, which have soils with very low humidity and some salinity, the Cabo de Gata zone (Southern Iberian Peninsula) being the most arid area (Capel-Molina, Reference Capel-Molina and Capel-Molina1982) where the species has been found.
Other additional localities have been sampled in coastal dunes from the north Iberian Peninsula, as well as numerous samples across the Iberian geography, in several habitats, and this species was not found. For this reason, it could be considered as a bioindicator of these xeric habitats.
Molecular analysis of N. lanceolatus
In the phylogenetic tree based on 18S rDNA (fig. 6), the sequenced specimens of N. lanceolatus appear to be related with species of the genus Acrobeles von Linstow, 1877, having a similarity of 94.7% (36 bp differences among insertions, deletions and substitutions), specifically with members of the subgenus Seleborca, with 96.5% (24 bp differences) similarity, while those of the subgenus Acrobeles maintain 97.7% similarity (11 bp differences). Also, the genus Cervidellus Thorne, 1937 appears in the same clade, having 98.4% similarity (11 bp differences).

Fig. 6. Bayesian inference tree from known and the newly sequenced Nothacrobeles lanceolatus based on sequences of the 18S rDNA region. Bayesian posterior probabilities (%) are given for each clade. Scale bar shows the number of substitutions per site.
In the phylogenetic tree based on 28S rDNA (fig. 7), N. lanceolatus appears to be related with Nothacrobeles triniglarus Tandingan De Ley, De Ley, Baldwin, Mundo-Ocampo & Nadler, Reference Tandingan De Ley, De Ley, Baldwin, Mundo-Ocampo and Nadler1999 (DQ145646), which shares a similarity of 90.5% (64 bp differences). With Nothacrobeles sonorensis (Ragsdale, Mundo-Ocampo, Bumbarger & Baldwin, Reference Ragsdale, Mundo-Ocampo, Bumbarger and Baldwin2011) Ruiz-Cuenca & Abolafia, Reference Ruiz-Cuenca and Abolafia2020 (DQ145632) and Nothacrobeles borregi Poiras, Baldwin, Mundo-Ocampo & Bumbarger, Reference Poiras, Baldwin, Mundo-Ocampo and Bumbarger2002 (DQ145645) who appear further, N. lanceolatus shares a similarity of 84.3% (105 bp differences) and 81.1% (133 bp differences), respectively. Also, according to previous studies (Mehdizadeh & Shokoohi, Reference Mehdizadeh and Shokoohi2013; Abolafia, Divsalar, Panahi & Shokoohi, Reference Abolafia, Divsalar, Panahi and Shokoohi2014; Ruiz-Cuenca & Abolafia, Reference Ruiz-Cuenca and Abolafia2020), the distance with other species of the same genus is even longer: Nothacrobeles abolafiai Mehdizadeh & Shokoohi, Reference Mehdizadeh and Shokoohi2013 (KC182515), Nothacrobeles cancellatus (Thorne, Reference Thorne1925) Ruiz-Cuenca & Abolafia, Reference Ruiz-Cuenca and Abolafia2020 (HM439765), Nothacrobeles hebetocaudatus Abolafia, Divsalar, Panahi & Shokoohi, Reference Abolafia, Divsalar, Panahi and Shokoohi2014 (KJ08411) and Nothacrobeles spatulatus Tandingan De Ley, De Ley, Baldwin, Mundo-Ocampo & Nadler, Reference De Ley, Felix, Frisse, Nadler, Sternberg and Thomas1999 (DQ145644), with which N. lanceolatus shares a similarity of 83.1% (113 bp differences), 83.4% (111 bp differences), 75.5% (161 bp differences) and 79.5% (137 bp differences), respectively. Also, in this phylogenetic tree, the sequences of the Spanish Nothacrobeles appear near to genera Acrobeles and Cervidellus, with similarities of 76.9% (154 bp differences) and 73.9% (175 pb differences), respectively.
Phylogenetic relationships of the Nothacrobeles species
The phylogenetic tree based on 28S rDNA fragment (fig. 7) shows that the genus Nothacrobeles is clearly polyphyletic. Although only eight of the 21 species of the genus have been sequenced, the clades in the tree show an evolutionary development of the lip region. Thus, three species (N. abolafiai, N. cancellatus and N. hebetocaudatus), related with the genus Paracrobeles Heyns, 1968, have similar a lip region pattern appearing at the basal clades, all of which have with labial probolae smooth with long bifurcations and stipe swollen, probably a plesiomorphic condition. Nothacrobeles spatulatus, related with the genera Stegelleta Thorne, Reference Thorne1938 and Stegellina Andrássy, 1984, has low labial probolae lacking bifurcations and pinnae. Two species (N. borregi and N. sonorensis) have bifurcated labial probolae with smooth bifurcations and low stipe with an expansion on the outer side. Another two species (N. lanceolatus and N. triniglarus), related with the genera Cervidellus and Acrobeles, have labial probolae with long or short bifurcated prongs and basal concave expansion on the outer side, both species with pinnae. This last morphological pattern is a probable apomorphic condition, appearing also in species of the genus Acrobeles, while some species of the genus Cervidellus such as Cervidellus doorsselaeri (De Clerck & De Ley, 1990) Boström & De Ley, 1996, morphologically related with Acrobeles, present labial probolae with incipient lateral pinnae.

Fig. 7. Bayesian inference tree from known and the newly sequenced Nothacrobeles lanceolatus based on sequences of the 28S rDNA region. Bayesian posterior probabilities (%) are given for each clade. Scale bar shows the number of substitutions per site. Detail of the labial probolae of the Nothacrobeles species is included.
Acknowledgements
The authors thank the assistance of technical staff (Amparo Martínez-Morales) and ‘Centro de Instrumentación Científico-Técnica (CICT)’ from the University of Jaén for supplying equipment for obtaining SEM pictures.
Financial support
The authors thank the ‘University of Jaén/Caja Rural Jaén Foundation’, Spain, for the financial support received for the project entitled ‘Filogeografía de nematodos rabdítidos (Nematoda, Rhabditida) en ambientes xerofíticos del sur de la Península Ibérica’ (UJA2014/03/01) and the research activities ‘PAIUJA 2019/2020: EI_RNM02_2019’ and ‘PAIUJA 2021/2022: EI_RNM02_2021’ of the University of Jaén, Spain.
Conflicts of interest
None
Ethical standards
All procedures contributing to this study comply with the ethical standards of the relevant national and institutional guides on the care and use of animals.












