Probiotic lactobacilli have been widely defined as living micro-organisms with low or no pathogenicity that exert beneficial effects on human health by improving the balance of gut indigenous microbiota when they are administered in adequate amountsReference Salminen, Bouley, Boutron-Ruault, Cummings, Franck, Gibson, Isolauri, Moreau, Roberfroid and Rowland1, Reference Erickson and Hubbard2. These micro-organisms have been associated with the modulation of immune functions because they participate in the enhancement of both humoral and cell-mediated immune responsesReference Gill, Rutherfurd, Prasad and Gopal3, Reference Vaarala4, conferring a protective effect against pathogens. For this reason, lactic acid bacteria (LAB) are considered as micro-organisms capable of preventing infection by pathogenic bacteria, offering an alternative to traditional therapies for the prevention or treatment of intestinal infections. The antimicrobial activities of probiotics have been evaluated against Escherichia coli, Salmonella, Listeria species, Helicobacter pylori Reference Alvarez-Olmos and Oberhelman5, Reference Cross6 and Candida albicans Reference Elahi, Pang, Ashman and Clancy7. Therefore, there is a large body of evidence showing the involvement of LAB in the modulation of the intestinal mechanisms of defence against pathogenic bacteria in in vivo modelsReference Perdigon, de Macías, Alvarez, Oliver and de Ruiz Holgado8, Reference Hudault, Lievin, Bernet-Camard and Servin9. In human subjects, pathogen challenge investigation is obviously not possible, but clinical studies have demonstrated an increase of Ig secretion following exposure to attenuated or non-virulent strains of enteric pathogensReference Isolauri, Joensuu, Suomalainen, Luomala and Vesikari10. The mechanisms by which probiotics exert a protective effect are not clearly elucidated; however, antimicrobial characteristics may be attributed to different factors such as organic acid production, NEFA, hydrogen peroxide, bacteriocin-like compoundsReference Reid11 or modulation of immune responseReference Matsuzaki and Chin12. This last aspect acquires a clinical importance because LAB may prevent the attachment of pathogen micro-organisms by competitive inhibition for microbial adhesion sites favouring the elimination of these detrimental agentsReference Reid and Burton13.
Lactobacilli isolated from the human and mouse gastrointestinal tracts are considered a part of commensal microbiota with beneficial effects on health, including enhanced lymphocyte proliferationReference Kirjavainen, El-Nezami, Salminen, Ahokas and Wright14, innate and acquired immunityReference Gill, Rutherfurd, Prasad and Gopal3 and anti-inflammatory cytokine productionReference Pessi, Sutas, Hurme and Isolauri15. Therefore, there are numerous application areas for use of LAB both in industry and human health, including the preservation of foods and use as probiotics.
Lactobacillus plantarum constitutes the dominant species in fermented food products such as sour dough, green olives or natural wines, because this micro-organism tolerates a lower pH than other bacteria. This bacterium is more common in the commensal microbiota of vegetarian people than in the microbiota of omnivoresReference Bengmark16 and it is known to produce important antimicrobial substancesReference van Reenen, Dicks and Chikindas17. The present study was designed to determine whether the oral administration of L. plantarum influences immune resistance in a murine model infected with Listeria monocytogenes by an intravenous route. L. monocytogenes, a food-borne pathogen of major importance for man, has been extensively used for some years as an experimental model to better define molecular pathogenesis and cellular immunity involved in the host defence against an intracellular pathogenReference Shen, Tato and Fan18, since protective immunity to L. monocytogenes requires a coordinated action between many cell types and the production of numerous cytokinesReference Edelson and Unanue19.
In the present study, a strain of L. plantarum was orally administered to Balb/c mice, prior to L. monocytogenes infection. Our purpose was to evaluate the protective effects of L. plantarum after oral administration of this probiotic. Therefore, in the current study we investigated the effects of the administration of L. plantarum on resistance to infection in an experimental model based on the exposure to L. monocytogenes, an intracellular pathogen. In addition, we examined the action of L. plantarum administration on the pro-inflammatory cytokine production during the course of infection with L. monocytogenes.
Materials and methods
Animals and treatment with Lactobacillus plantarum
BALB/c mice, 8 to 10 weeks old, were purchased from the University of Jaen (breeding colony of Servicios Técnicos de Investigación, Universidad de Jaén). Mice were housed in cages in an environmentally controlled room at a temperature of 24°C with a 12 h light–dark cycle and divided into two groups: L. plantarum-treated mice or sham-treated mice (received PBS). Mice were randomly allocated to receive daily L. plantarum or sterile PBS by oral route for 4 weeks, respectively. Each group was allowed access ad libitum to water as well as to standard mouse chow (PanLab A04 mouse maintenance diets, Barcelona, Spain). The animal procedures complied with the national and European Union legislation on the care and use of animals and related codes of practice (86/609/EEC).
Bacteria preparation
L. plantarum isolated from kefirReference Bujalance, Jimenez-Valera, Moreno and Ruiz-Bravo20 (kindly provided by Dr. Ruiz-Bravo, Facultad de Farmacia, Universidad de Granada, Spain) was grown overnight at 37°C in Mann Rogosa Sharpe (MRS) broth medium (Scharlau Chemie, Barcelona, Spain). The number and viability of LAB were determined by aerobic culturing on MRS plates. A clinical isolate of L. monocytogenes was grown in blood tryptic soya agar medium (Scharlau Chemie) for 24 h at 37°C. At the end of the experimental feeding period, each mouse was intravenously infected with a virulent strain of L. monocytogenes, injected through the tail vein.
Analysis of acid and bile salt tolerance
To test acid tolerance, we used the assay described by Chou & Weimer with minor modificationsReference Chou and Weimer21. L. plantarum was grown for 24 h at 37°C and, subsequently, cells were collected by centrifugation at 4300 g for 10 min at 4°C, washed three times in sterile saline solution and inoculated (1 %) onto MRS broth acidified with concentrated hydrochloric acid to pH 3·5 or non-acidified MRS broth to pH 6·8. Counts of viable cells were performed at 0 and 90 min, by pour plating onto MRS agar (pH 6·8). To confirm the resistance to acids of this strain, because variances in acid tolerance may occur, individual colonies were grown in acidified MRS broth (pH 3·5) and incubated at 37°C for 0, 24, 48 and 72 h. The growth of bacteria was quantified spectrophotometrically (BioRad, Hercules, CA, USA) at 650 nm. These cells were then removed to tubes for the determination of bile salt tolerance. Acidified MRS broth (pH 4·0) was inoculated with the cells and each tube contained a concentration of bovine bile (0, 0·5, 1·0, 2·0 and 4·0 %, w/v) (Sigma Chemical, St. Louis, MO, USA). Tubes were incubated at 37°C for 30 min and dilutions were plated onto MRS agar and incubated at 37°C for 24 h for colony forming unit (c.f.u.) counts. Results are expressed as log10 c.f.u./ml.
Oral feeding, Listeria monocytogenes infection and blood collection
Mice were fed a dose of 5 × 107 c.f.u. viable L. plantarum in 100 μl PBS by gastric intubation using a feeding needle (822-gauge stainless steel) with a spherical tip (Harvard Apparatus Ltd, Edenbridge Kent, UK), every day for 4 weeks (five in each time point). Similarly, sham-treated mice received the same amount (100 μl) of sterile PBS each day. Three independent control and treated groups (sham- or L. plantarum-treated mice, respectively) were challenged with a similar amount of L. monocytogenes 1 d after the last administration of lactobacilli, in order to examine host resistance to this infectious micro-organism. Two levels of infection have been used. To measure survival, mice were intravenously injected with a lethal dose of 106 c.f.u./ml. To recover viable bacteria from both liver and spleen and to determine pro-inflammatory cytokine production from the sera, mice were intravenously injected with a sub-lethal dose of 105 c.f.u./ml. At each time-point (from 1 to 8 d), mice were killed to determine the level of colonization by L. monocytogenes. Peripheral blood was isolated at 0 (before infection), 2, 4, 6 and 8 d after experimental infection with this pathogen for the determination of cytokine secretion (five in each time point). Mice were anaesthetized with diethyl ether and blood was drawn from the retro-orbital plexus into tubes containing heparin (20 U/ml blood). Serum was obtained after centrifugation of the tubes at 1500 g for 30 min. Finally, serum samples were stored at − 80°C for subsequent analysis.
Survival analysis
Mice fed orally with L. plantarum or sterile PBS were infected with a virulent strain of L. monocytogenes (fifteen in each group). For the determination of mice survival, L. monocytogenes was suspended in PBS at a concentration of 106 c.f.u./ml. Then, this bacterial suspension (100 μl) was injected into each mouse through the tail vein. The survival was monitored every 8 h for 8 d after the L. monocytogenes challenge. Mice found to be moribund were killed by cervical dislocation. The mice were closely monitored during the next 12 h as this is the predicted life expectancy for this lethal dose of bacteria. Results were expressed as percentage of survival.
Determination of numbers of viable bacteria in spleens and livers
To this assay, a L. monocytogenes cell suspension at 105 c.f.u./ml (100 μl) was inoculated through the tail vein (five in each time point). The spleens and livers of infected animals were aseptically isolated in sterile PBS and weighed under sterile conditions. Then, spleen and liver cells were prepared by homogenizing these organs between frosted-glass slides in distilled water under sterile conditions. To evaluate the extent of systemic listerial infection, the number of viable bacteria within spleens and livers were determined at various time points from 1 to 8 d after L. monocytogenes challenge. Thus, cells were disrupted by treatment with distilled water in order to lyse host cells and release intracellular bacteria. Then, serial 10-fold dilutions of each sample were made and an aliquot (10 μl) of each dilution was transferred onto blood tryptic soya agar medium to determine the number of live L. monocytogenes in the spleens and livers. Plates were incubated at 37°C for 24 h. Finally, the number of c.f.u. was counted and the values were expressed as log10 viable bacteria. The limit of detection was fixed approximately at 102 c.f.u. bacteria/organ.
Cytokine production
ELISA kits were used for determination of pro-inflammatory cytokine concentrations such as IL-1β (R&D Systems, Minneapolis, MN, USA), IL-6 (R&D Systems) and TNF-α (BioSource, Camarillo, CA, USA) in the sera samples. Results were calculated against standard curves generated using known amounts of recombinant cytokines in accordance with the manufacturer's instructions in a microplate reader (BioRad) at a wavelength of 450 nm. Limits of detection for these assays were < 3 pg/ml (IL-1β), 1·6 pg/ml (IL-6) and < 3 pg/ml (TNF-α). Samples were assayed in duplicate.
Statistical analysis
Results are expressed as means with their standard errors of the mean. The main effects of treatment and time of infection were compared using two-way ANOVA. Significant differences between treatments were identified by the least significant difference test at each time point. The effects of acids and bile salts in in vitro assays were evaluated with the Student's t test. Survival curves of L. monocytogenes-infected mice were compared using the Kaplan–Meier log rank test. The survival data from three independent experiments were pooled for statistical analysis. Viable bacteria counts from spleens or livers were log10 transformed prior to analysis. Data were analysed using SPSS Version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences were considered as statistically significant at a value of P < 0·05.
Results
Acid and bile salt tolerance
The L. plantarum strain tested in the present study was acid-tolerant to pH 3·5 for 90 min at 37°C (Table 1). In addition, the transference of this strain from MRS agar plates to acidified MRS broth for 0 to 72 h showed that the cell viability was not modified in acid medium. Fig. 1 illustrates the resistance of L. plantarum to the action of different concentrations of bovine bile, when this strain was grown on MRS broth at pH 4·0 for 30 min. Counts of viable L. plantarum after the treatment with different concentrations of bile salts has demonstrated a significant reduction of cell viability (P < 0·05, treated v. untreated cultures), but there was not a dose dependency. In fact, the cell viability was maintained to approximately 105 c.f.u. after the treatment with bile salt from 0·5 to 4·0 % (w/v). As a result, this strain may be considered as acid- and bile-tolerant.
Table 1 Survival of Lactobacillus plantarum on acidified Mann Rogosa Sharpe (MRS) agar (pH 3·5) and non-acidified MRS agar (pH 6·8)*
(Values are means with their standard errors of the mean of two independent determinations in triplicate)

c.f.u., colony forming unit.
* For details of animals and procedures, see Materials and methods.
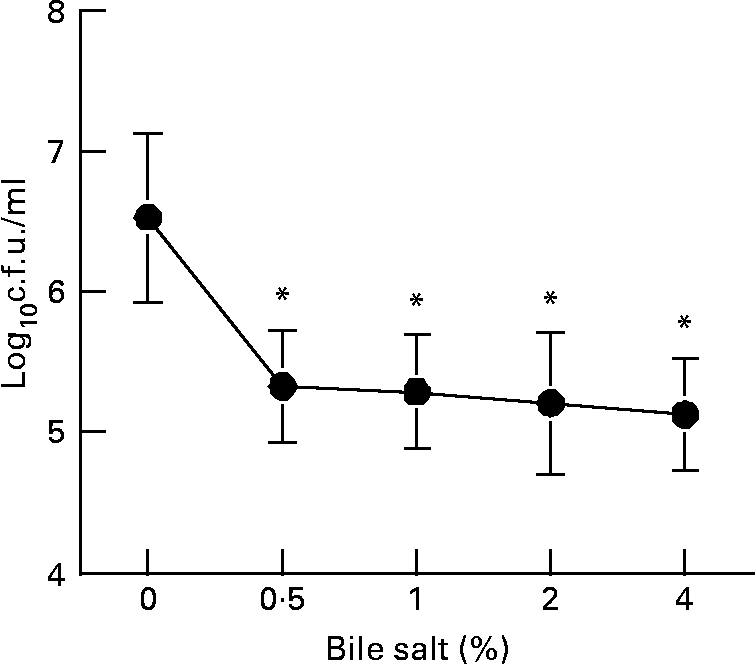
Fig. 1 Determination of bile salt tolerance. Acidified Mann Rogosa Sharpe (MRS) broth (pH 4·0) was inoculated with the cells and determination of bile salt tolerance was carried out at different concentrations from 0 to 4 %. Cells were incubated at 37°C for 30 min and dilutions were plated onto MRS agar plates and incubated at 37°C for 24 h for the counts of colony forming units (c.f.u.). Results are means with their standard errors of the mean of two independent determinations in triplicate. Values were significantly different from untreated bacteria (0 %) as calculated with the Student's t test: *P < 0·05. For details of animals and procedures, see Materials and methods.
Body, spleen and liver weights
The oral administration of L. plantarum did not affect the body weight of Balb/c mice that were intravenously infected with 105 c.f.u./ml L. monocytogenes. Initially, the spleen weights of mice treated with L. plantarum were significantly reduced from 0 to 4 d after experimental infection with L. monocytogenes (P < 0·05). On the other hand, no differences in the liver weights were observed (Table 2).
Table 2 Effect of oral administration of Lactobacillus plantarum for 4 weeks on the body, spleen and liver weights of mice experimentally infected with Listeria monocytogenes for 8 d*
(Values are means with their standard errors of the mean of three independent experiments (five in each time point) and were analysed by two-way ANOVA)
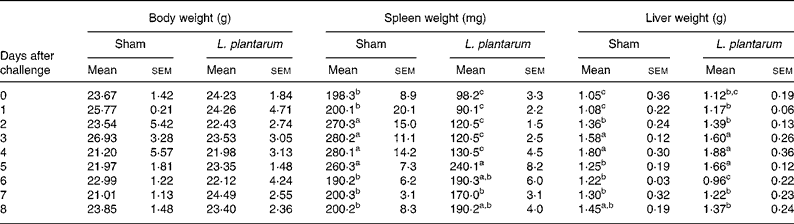
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* For details of animals and procedures, see Materials and methods.
Survival of mice
The protective effects of L. plantarum were evaluated by measuring the survival time of Balb/c mice that received a daily dose of L. plantarum or PBS (sham-treated mice as controls) (Fig. 2). At 24 h of feeding treatment with either PBS or L. plantarum, animals were infected with a lethal dose of L. monocytogenes. After challenge with L. monocytogenes, most deaths occurred between 4 and 7 d post-infection. At day 8 after the challenge, 60 % of mice treated with L. plantarum survived to infection, whereas 40 % of control mice survived. Nevertheless, we did not find any significant difference between survival of mice orally administered L. plantarum and sham-treated mice (P = 0·13).
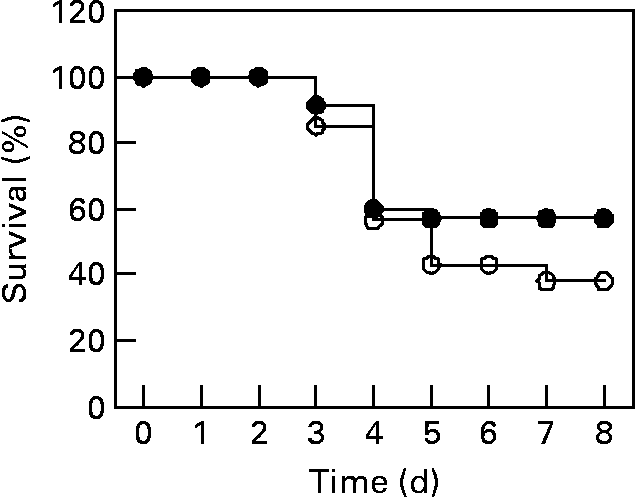
Fig. 2 Measurement of survival percentage of mice orally administered Lactobacillus plantarum and challenged with Listeria monocytogenes. Balb/c mice were treated with L. plantarum or received PBS (sham-treated mice) for 4 weeks (fifteen in each group) and subsequently infected with L. monocytogenes (106 colony forming units (c.f.u.)/ ml). ●, Represents the survival percentages of mice treated with L. plantarum (experimental mice); ○, represents the survival percentages of sham-treated mice. The data represent the pooled result of three experiments. For details of animals and procedures, see Materials and methods.
Bacterial clearance from spleens and livers
To verify the capacity of the immune system to eliminate L. monocytogenes from spleens or livers, these organs were homogenized and viable bacteria were isolated. The recovery of viable L. monocytogenes from spleens was significantly lower (P < 0·05) in L. plantarum-treated mice compared with sham-treated mice (i.e. 10-, 5-fold at 1 and 2 d after the challenge, respectively), whereas it was significantly higher (P < 0·05) in L. plantarum-treated mice compared with sham-treated mice (i.e. 8-, 75-fold at 3 and 4 d after the challenge, respectively) (Fig. 3(A)). Finally, L. monocytogenes was not detected in spleens of both experimental and control groups at day 5 after the challenge. Bacterial recovery from livers was similar at day 1 of experimental infection in both the experimental and control groups, whereas the counts of L. monocytogenes were significantly higher (P < 0·05) in L. plantarum-treated mice compared with sham-treated mice (i.e. 5-, 6- and 10-fold at 2, 3 and 4 d after the challenge, respectively) (Fig. 3(B)). At day 5 post-infection, the bacteria were completely eliminated from livers of L. plantarum-treated mice, whereas 102 c.f.u. were counted in the livers of the control group, although this micro-organism was absolutely eliminated from liver at day 7 after the challenge (Fig. 3(B)).
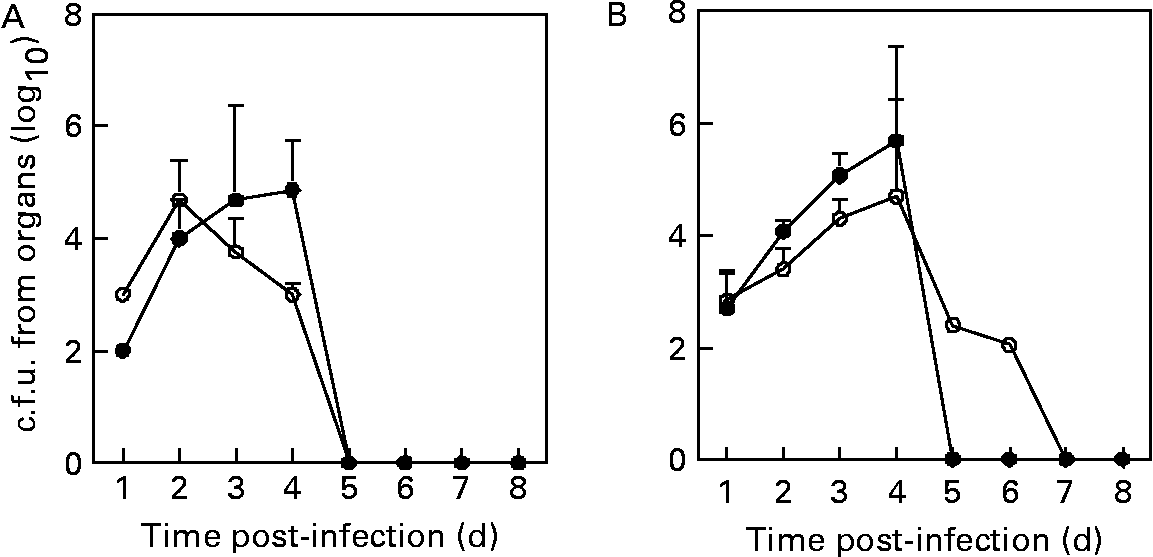
Fig. 3 Recovery of viable Listeria monocytogenes from spleens (A) and livers (B) of mice orally fed with Lactobacillus plantarum or treated with PBS (sham-treated mice). Balb/c mice were treated with L. plantarum (●) for 4 weeks (five in each time point) and subsequently infected with L. monocytogenes (105 colony forming units (c.f.u.)/ml). Sham-treated mice received orally sterile PBS (○) for 4 weeks (five in each time point) and subsequently infected with L. monocytogenes (105 c.f.u./ml). The number of bacterial colonies was counted and the results were expressed as log10 viable bacteria. Results are means with their standard errors of the means of two identical experiments. Data were analysed by two-way ANOVA. Mean values with different superscript letters were considered to be significantly different (P < 0·05). For details of animals and procedures, see Materials and methods.
Measurement of pro-inflammatory cytokine production
In general, the oral administration of L. plantarum to mice experimentally infected with L. monocytogenes produced a reduction of IL-1β and IL-6 concentration in the serum of mice treated with L. plantarum (Fig. 4). The alteration of both IL-1β and IL-6 production depends on the treatment (P < 0·05) and on the time of challenge with L. monocytogenes (P < 0·05). IL-1β production in the Lactobacillus-treated group was significantly reduced before infection with L. monocytogenes (day 0), compared with sham-treated mice (P < 0·05), but levels of this cytokine increased at day 2 post-infection. Again, the secretion of this cytokine was significantly reduced with regard to control mice at 4 and 6 d after the challenge with L. monocytogenes (P < 0·01) (Fig. 4(A)). On the other hand, the production of another pro-inflammatory cytokine such as IL-6 was quantified in the serum from these mice. The levels of this cytokine decreased progressively in comparison with the values from the control group at day 4 after the challenge with L. monocytogenes (P < 0·01) (Fig. 4(B)). Finally, the production of another important pro-inflammatory cytokine such as TNF-α was also assessed. Results from values of mice orally treated with L. plantarum have not indicated any significant differences with regard to values from non-treated mice in the different time intervals of infection, although an important increase of TNF production was observed at day 2 of challenge in both sham- and L. plantarum-treated mice (P < 0·05) (Fig. 4(C)).
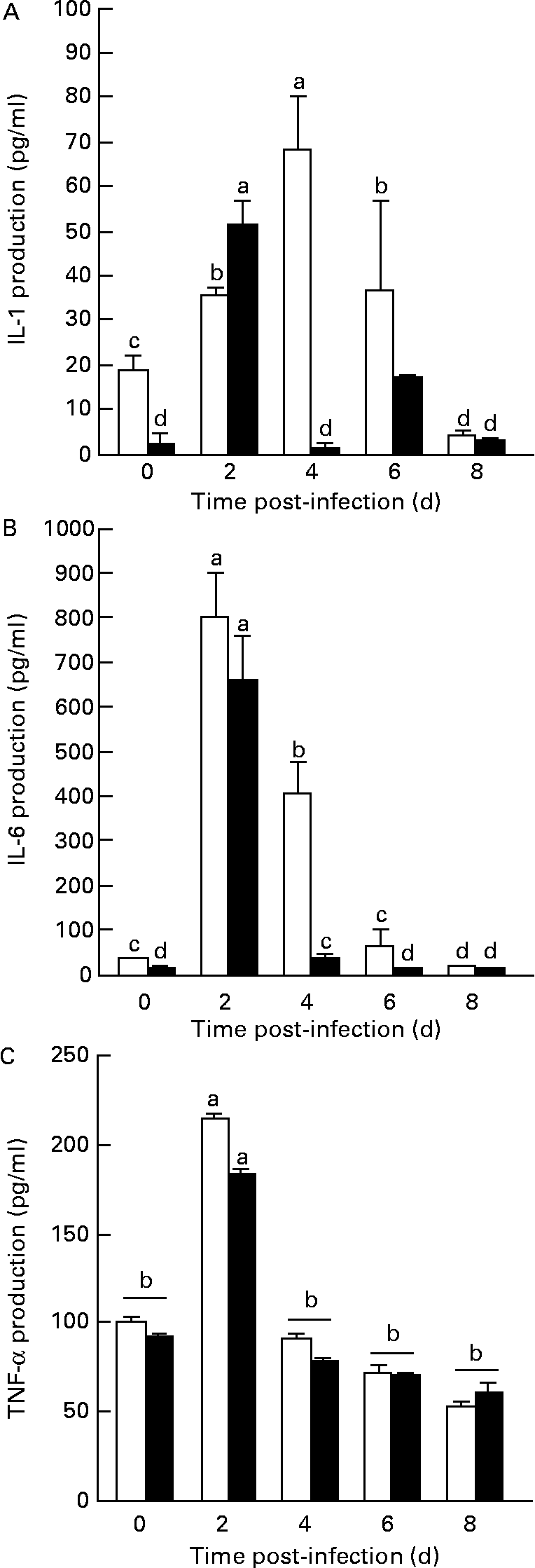
Fig. 4 Measurement of IL-1β, IL-6 and TNF-α production from the sera of peripheral blood of mice orally administered Lactobacillus plantarum or PBS (sham-treated mice) and challenged with Listeria monocytogenes. BALB/c mice (five in each time point) received sterile PBS (□) or were orally administered L. plantarum (■) for 4 weeks and subsequently were challenged with L. monocytogenes (105 colony forming units (c.f.u.)/ml). The production of IL-1β (A), IL-6 (B) and TNF-α (C) from the animal sera was measured from 0 to 8 d after experimental infection. Quantification of IL-1β, IL-6 and TNF-α levels in experimental samples were made by extrapolation from ELISA results, using various concentrations of rIL-1β, rIL-6 and r-TNF-α as a standard, respectively. Results are means with their standard errors of the means of two identical experiments. Data were analysed by two-way ANOVA. Mean values with different superscript letters were considered to be significantly different (P < 0·05). For details of animals and procedures, see Materials and methods.
Discussion
Health benefits attributable to the consumption of LAB have been extensively reported in recent yearsReference Reid, Jass, Sebulsky and McCormick22. Based on these properties, probiotics have been applied as micro-organisms that exert a favourable influence on the host by improving the indigenous microbiotaReference Erickson and Hubbard2, Reference Holzapfel, Haberer, Snel, Schillinger and Huis in't Veld23. It is well recognized that the organisms most commonly used as probiotics are LAB (lactobacilli and bifidobacteria), which appear to be promising candidates in clinical practice for the treatment of disorders caused by intestinal abnormal microbiotaReference Salminen, Isolauri and Salminen24. Hence, probiotics constitute a valuable option that may contribute to improve gut mucosal barrier functionsReference Alvarez-Olmos and Oberhelman5, because antigenic components from lactobacilli can enter epithelia cells, establishing contact with immune cells and stimulating the intestinal mucosal immune system without safety problemsReference Galdeano and Perdigon25.
In addition, LAB act as immunomodulatory agents capable of exerting a crucial role in the host immune defence against infectious micro-organisms. Therefore, an initial objective in the present study was focused on the examination of in vivo action of L. plantarum and its ability to modify host resistance against an infectious micro-organism. For this reason, we have used a murine model infected with L. monocytogenes, a Gram positive pathogen that serves as an important model for understanding host immune resistance against intracellular bacteria. In addition, from a clinical point of view, L. monocytogenes exerts an adverse effect in elderly, newborns and immunocompromised individuals, who are more susceptible to infection by this micro-organism.
Before arriving in the intestinal tract, probiotic micro-organisms must resist transit through the stomachReference Dunne, O'Mahony and Murphy26; therefore, resistance to low pH and bile are considered as essential properties of probiotics. Despite the reported beneficial effects of oral probiotics on the immune system functions, in the present study, the oral administration of an acid- and bile-resistant strain of L. plantarum has not produced an efficient elimination of L. monocytogenes from both the liver and spleen of mice, suggesting an absence of a protective activity against this pathogen after oral administration of L. plantarum. In fact, an early investigation suggested that L. plantarum together with other species of lactobacilli do not enhance the immune resistance against L. monocytogenes infectionReference Sato27, but it prevents colonization of pathogenic micro-organisms by competitive inhibition for microbial adhesion sitesReference Mack, Michail, Wei, McDougall and Hollingsworth28. However, this strain has been recently reported to promote adhesion to epithelial cells, although it was unable to establish a continuous colonization in the gastrointestinal tractReference Bujalance, Moreno, Jimenez-Valera and Ruiz-Bravo29. In the current study, the impact of L. plantarum on the survival against a lethal dose of L. monocytogenes has produced a non-statistical significant increase of survival in comparison with values from sham-treated mice. Similarly, other early studies also reported an enhancement of resistance to L. monocytogenes infection in mice that received an intravenous or a subcutaneous injection of L. casei Reference Nomoto, Miake, Hashimoto, Yokokura, Mutai, Yoshikai and Nomoto30, Reference Yokokura, Nomoto, Shimizu and Nomoto31.
In spite of the fact that the oral administrations of another LAB, such as L. casei, significantly improves host resistance against oral L. monocytogenes infectionReference de Waard, Garssen, Bokken and Vos32, the present findings show that the oral administration of L. plantarum is unable to ameliorate the elimination of L. monocytogenes from spleens and livers of mice experimentally infected. Indeed, the present data have shown that spleen weight was initially reduced before the inoculation of L. monocytogenes and after the challenge with this pathogen. In addition, a recent study has indicated that in vitro cultures of L. plantarum inhibit the growth of L. monocytogenes, but the presence of L. plantarum in the gut of gnotobiotic rats facilitates L. monocytogenes colonizationReference Bernbom, Licht, Saadbye, Vogensen and Norrung33. This fact corroborates the reduced capacity of L. plantarum to stimulate host immune response.
Numerous investigations have reported that LAB are involved in an increase of cytokine secretionReference Erickson and Hubbard2, Reference Heumann, Barras, Severin, Glauser and Tomasz34, Reference Nicaise, Gleizes, Forestier, Quero and Labarre35. Nonetheless, L. plantarum administered to Balb/c by an oral route for 4 weeks significantly decreases the production of pro-inflammatory cytokines such as IL-1β and IL-6, but does not alter the secretion of TNF-α. We hypothesize that L. plantarum could modify cytokine profile reducing T-helper 1 cell response.
According to the results, L. plantarum does not enhance immune resistance against an intravenous infection with the intracellular pathogen L. monocytogenes. In addition, the production of pro-inflammatory cytokines such as IL-1β or IL-6 was strongly inhibited in mice orally fed with L. plantarum, whereas TNF-α production was not altered after the oral administration of L. plantarum. Based on the present results, we conclude that the L. plantarum strain isolated from kefir is characterized by showing a resistance to bile salts and acids, without conferring a determinant protective role, because it does not appear to participate in the elimination of L. monocytogenes. However, the reduction of IL-1 and IL-6 levels leads to the hypothesis that L. plantarum may represent an important model to reduce inflammatory disorders. On the basis of this last argument, we suggest that further studies should determine the action of L. plantarum and its application in clinical practice as an immunomodulatory micro-organism that diminishes the inflammatory response without severely reducing the host resistance to the infection. Hence, L. plantarum is not related to an impairment of host resistance against L. monocytogenes because it does not exert adverse effects on survival and on the clearance of L. monocytogenes from liver and spleen. However, oral administration of L. plantarum is associated with an important reduction of pro-inflammatory IL secretion. Indeed, L. plantarum and other lactobacilli species have been reported as probiotics able to strengthen anti-inflammatory cytokine synthesis, decreasing expression of pro-inflammatory interferon-γ and stimulating secretory IgA productionReference Pessi, Sutas, Hurme and Isolauri15, Reference Pathmakanthan, Li, Cowie and Hawkey36, Reference Di Giacinto, Marinaro, Sanchez, Strober and Boirivant37.
Taking into account the high incidence of allergies, inflammatory bowel disease, urogenital infections and other disorders in the gut and urogenital tract, the action of probiotic micro-organisms may play an essential role in contributing to their resolution. Therefore, further studies are needed to determine the influence of an oral administration of L. plantarum in the production of cytokines with an anti-inflammatory function and to understand the impact of probiotic treatment upon the modulation of host immune resistance against an infectious micro-organism.
Acknowledgements
This research was supported by Consejería de Innovación y Universidades, Junta de Andalucia, Spain (group PAI, CTS105). E.P. receives a pre-doctoral fellowship from Ministerio de Educación y Ciencia, Spain. No conflict of interest was reported.










