The relationship between anaemia, Fe status and HIV infection is complex and poorly understood. It is well established that anaemia is a common complication of HIV infection and a strong independent predictor of disease progression and mortality(Reference Sullivan1–Reference Kreuzer and Rockstroh4). Furthermore, correcting anaemia prolongs survival during HIV disease(Reference Sullivan1, Reference Moore, Keruly and Chaisson2). However, advanced HIV disease is often characterized by high Fe stores, which may be associated with more rapid progression and higher mortality among HIV-infected individuals(Reference Boelaert, Weinberg and Weinberg5, Reference Savarino, Pescarmona and Boelaert6). The hypothesized mechanism is that Fe deposits in the bone marrow, brain, liver and other organs(Reference de Monye, Karcher, Boelaert and Gordeuk7) increase HIV-1 transcription, stimulate microbial growth and impair host immune responses(Reference Boelaert, Weinberg and Weinberg5, Reference Weinberg, Boelaert and Weinberg8). It is uncertain if increased Fe stores are a cause of disease progression or a consequence, and few studies adequately control for the effect of inflammation on indicators of Fe status. We previously reported that severe anaemia was associated with an increased risk of sexual acquisition of HIV among women who were HIV-negative at delivery(Reference Humphrey, Hargrove and Malaba9), further strengthening our need to understand the full range of consequences of Fe deficiency, anaemia and Fe supplementation for both HIV-positive and -negative women living in HIV endemic areas(Reference Doherty, Weaver and Prentice10).
Much of the evidence that suggests a deleterious effect of Fe during HIV infection comes from studies among European and North American men, in whom Fe deficiency and anaemia are relatively rare (reviewed in references Reference Clark and Semba11 and Reference Gordeuk, Delanghe, Langlois and Boelaert12). However, the clinical and programmatic implications of potential harm from Fe supplementation during HIV infection are greatest for reproductive-aged women in sub-Saharan Africa (SSA), in whom HIV infection and Fe-deficiency anaemia most commonly coexist: 80 % of all HIV-positive women in the world live in SSA and at least half of all pregnant women in SSA are anaemic(Reference O’Brien, Kupka, Msamanga, Saathoff, Hunter and Fawzi3, Reference van den Broek, White and Neilson13, Reference Semba, Kumwenda, Hoover, Taha, Mtimavalye, Broadhead, Eisinger, Miotti and Chiphangwi14). Since the major cause of this anaemia is Fe deficiency, WHO has long recommended Fe/folic acid supplementation for all antenatal women.
The few published reports from SSA that examine the relationship between Fe status and HIV disease severity (as measured by either CD4 count or HIV-1 viral load (VL)) have had conflicting results(Reference Friis, Gomo, Nyazema, Ndhlovu, Krarup, Madsen and Michaelsen15–Reference Kupka, Msamanga, Mugusi, Petraro, Hunter and Fawzi18). Similarly, the association between elevated Fe stores (as measured by serum ferritin (SF)) and HIV-related mortality in developed and developing countries has also been conflicting(Reference Kupka, Msamanga, Mugusi, Petraro, Hunter and Fawzi18–Reference McDermid, Jaye, Schim van der Loeff, Todd, Bates, Austin, Jeffries, Awasana, Whittlex and Prentice20). The paucity of studies and the discordant findings available from SSA, together with the clear relevance to programmes recommending Fe supplementation to reproductive-aged women in HIV endemic areas, necessitate additional prospective studies examining the relationship between Fe stores and HIV disease severity and disease progression.
The objectives of the present study were to examine the relationship between Fe stores as measured by SF and (i) HIV plasma VL and (ii) HIV-related mortality by 12 months among HIV-positive postpartum women. Given the known influence of inflammation on Fe indicators, the acute-phase protein α1 acid glycoprotein (AGP) was concurrently measured to control for this effect.
Subjects and methods
Study population
The study was conducted using archived plasma and serum samples and data collected as part of ZVITAMBO, a clinical trial of postpartum maternal and neonatal vitamin A supplementation, previously reported(Reference Humphrey, Hargrove and Malaba9, Reference Humphrey, Iliff and Marinda21, Reference Zvandasara, Hargrove and Ntozini22). Briefly, 14 110 mother–infant pairs were recruited within 96 h of delivery at maternity clinics and hospitals in Harare between November 1997 and January 2000. Baseline characteristics were collected by questionnaire and transcription from hospital records. Maternal mid upper-arm circumference (MUAC) was measured and blood was collected by venepuncture into EDTA and plain (serum) tubes. Plasma and serum were separated and stored at −70°C until used. Mothers were tested for HIV at baseline using two ELISA kits run in parallel. Among women testing HIV-positive at baseline, CD4 cells were enumerated within 48 h of phlebotomy (Facscount; Becton Dickinson International, Erembodegem, Belgium). Hb was measured on the same day as blood collection among all women enrolled after 1 October 1999 (about 60 % of the total) using the HemoCue haemoglobinometer (HemoCue, Mission Viejo, CA, USA). Mothers were followed up at 6 weeks, 3 months and then 3 monthly up to 12 months. HIV testing and antiretroviral prophylaxis for HIV-positive antenatal women were not available in Harare public-sector facilities during ZVITAMBO recruitment.
For the present analysis, we drew on archived samples of HIV-positive mothers for whom Hb had been measured at baseline. To ensure that we could investigate the relationships between Fe status indicators across the spectrum of HIV infection, we selected all mothers with CD4 counts <200 cells/μl and a 20 % random sub-sample of mothers with CD4 counts of 200–400 and >400 cells/μl. Because there were fewer women with CD4 counts <200 cells/μl in the larger ZVITAMBO cohort, we over-sampled these women. If specimen volume was adequate, SF and serum transferrin receptor (TfR) concentrations were measured by enzyme immunoassay kits (Ramco Laboratories, Houston, TX, USA), and AGP was measured by radial immunodiffusion kits (Kent Laboratories, Bellingham, WA, USA). All assays had a CV less than 10 %. AGP was chosen as an indicator of inflammation because several studies suggest that it best reflects the gradual increase and extended elevation of SF that accompanies the inflammatory response compared with other acute-phase proteins(Reference Thurnham, Mburu, Mwaniki and De Wagt23). Our final sample size of HIV-positive women for whom we had Hb, SF, TfR, AGP and CD4 measurements was 643. HIV-1 VL was available on a random sub-set of 375 women.
Because women were recruited into the study immediately after delivery and this was the first point of contact between study staff and study participants, no accurate information on Fe supplementation during pregnancy is available. According to the 1999 Zimbabwe Demographic Health Survey, pregnant women were recommended to take Fe tablets during pregnancy. However, nationally, less than 6 % of pregnant women took ninety or more Fe tablets during their pregnancy, and in Harare this figure was only 1 %(24).
Ethical review
The Medical Research Council of Zimbabwe, the Committee on Human Research of The Johns Hopkins Bloomberg School of Public Health, and the University Committee on Human Subject at Cornell University approved the study.
Statistical methods
All statistical analyses were carried out using the STATA version 8 statistical software package (Stata Corporation, College Station, TX, USA). SF and TfR distributions were normalized by log10 transformation; geometric means and 95 % confidence intervals are reported. An AGP cut-off of >1 g/l was used to define inflammation(Reference Filteau, Morris, Abbott, Tomkins, Kirkwood, Arthur, Ross, Gyapong and Raynes25); an Hb cut-off of <110 g/l was used to define anaemia(26).
To examine how concentrations of different Fe status indicators varied across the spectrum of HIV infection, ANOVA was used to compare continuous Fe status variables across CD4 categories and HIV RNA quartiles; the χ 2 test was used to compare proportions with inflammation across these CD4 categories. Pairwise comparisons between each pair of means were examined using a post hoc Bonferroni test. We used multivariate linear regression analysis to estimate the association between maternal Fe status and VL. Dummy variables were used to test the statistical effect of categorical variables. Both main effects and interaction between variables were assessed. Where significant interactions were found with a categorical variable, we stratified our analyses.
To examine the association between maternal Fe stores during the immediate postpartum period and mortality over the subsequent 12 months, multivariate Cox proportional hazards models were used in which indictors of Fe status were modelled as the independent variable and death as the dependent variable. Other factors that were found to significantly predict death in a larger group of HIV-positive women in the ZVITAMBO trial were included in the model(Reference Zvandasara, Hargrove and Ntozini22). These included maternal age, CD4 count, MUAC, marital status and education. Vitamin A supplementation had no effect on maternal mortality in this trial(Reference Zvandasara, Hargrove and Ntozini22); therefore the treatment arms of the ZVITAMBO trial were not included in our models. Women were censored at the time of death where it occurred ≤365 d postpartum, at the time of last contact for women lost to follow-up prior to 12 months, or at 12 months for women known to be alive at 12 months. Kaplan–Meier survival analysis was used to compare the survival curves across SF categories.
Cause of death was determined from medical records if available or by review of verbal autopsy by the study gynaecologist. Multiple causes were recorded and not ranked. Women whose cause of death could not be ascertained or for whom death was deemed unrelated to HIV were not counted as events in the analyses. Death was categorized as HIV-related if the cause was an AIDS-defining opportunistic infection or malignancy, or if the stated cause was non-specific infection or organ failure. All deaths among patients with CD4 count <200 cells/μl were defined as HIV-related. This methodology has been described elsewhere(Reference Cohen, French, Benning, Kovacs, Anastos, Young, Minkoff and Hessol27).
Results
Baseline characteristics
The prevalence of anaemia was 47 %, with a mean Hb concentration of 110 (sd 19) g/l. Thirty-seven per cent of women had AGP > 1 g/l and were classified as experiencing an acute-phase response (geometric mean AGP concentration: 0·91 g/l). Fe deficiency, as defined by SF concentration <15 μg/l in individuals without an acute-phase response (geometric mean SF concentration: 18 μg/l) and <30 μg/l in individuals experiencing an acute-phase response (geometric mean SF concentration: 39 μg/l)(26), was 42 %; 24 % of women were classified as being Fe-deficient and anaemic (IDA) using SF and Hb. Using TfR to define Fe deficiency, the prevalence of Fe deficiency (TfR > 8·3 μg/ml) was 34 % and the prevalence of IDA was 22 %.
Cross-sectional association of maternal Fe status indicators with viral load and CD4 count
Baseline values of Hb, SF, TfR and AGP were compared across four pre-defined CD4+ count categories (Table 1). TfR concentrations did not differ across the groups, but Hb concentrations were significantly higher among women with higher CD4 cell counts, whereas SF and AGP concentrations were significantly lower among women with higher CD4 cell counts. Similar results were obtained when comparing these indicators across quartiles of VL (data not shown).
Table 1 Iron status indicators and acute-phase protein concentrations by CD4 count category: sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000

SF, serum ferritin; TfR, transferrin receptor; AGP, α1 acid glycoprotein.
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0·05).
*P values for Hb, TfR and AGP determined by ANOVA; P value for SF determined by Kruskal–Wallis test.
In our initial multivariate model, after controlling for AGP and CD4, SF was directly and Hb was indirectly associated with VL (data not shown). Other covariates associated with VL in univariate analyses were not retained in the model if they had a significance level of P > 0·10 and their inclusion did not significantly alter the SF coefficient. The inclusion of AGP in the final model significantly attenuated the association between SF and VL. The inclusion of AGP in the final model significantly attenuated the association between SF and VL. A log10 increase in SF was associated with a 2-fold higher VL (β = 0·300, P = 0·002) and each g/dl increase in Hb was associated with an 18 % lower VL (β = −0·88, P < 0·001).
Further analysis revealed that the positive association between SF and VL was significantly modified by the presence of anaemia (interaction P = 0·029).
No association was found between TfR and VL. Among non-anaemic women, one log10 increase in SF was associated with a 2·27-fold higher VL (β = 0·356, P = 0·019; Table 2). The inclusion of AGP into the model significantly attenuated the association between SF and VL. There was no association between increasing SF and VL among anaemic women (P = 0·152).
Table 2 Predictors of HIV-1 viral load stratified by the presence or absence of anaemia (multivariate analysis): sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000
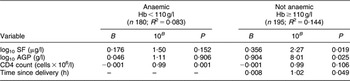
SF, serum ferritin; TfR, transferrin receptor; AGP, α1 acid glycoprotein.
Additional variables assessed in the model were age, birth weight, parity and time elapsed between delivery and sample blood draw; time elapsed was retained in the model.
To further provide insight into the association between SF and VL, we divided SF into three categories: <15, 15–45 and >45 μg/l (Fig. 1). After controlling for AGP and CD4 count, anaemic women had significantly higher VL than non-anaemic women within the SF < 15 μg/l (2·8-fold higher, P = 0·003) and SF = 15–45 μg/l (1·9-fold higher, P = 0·033) categories, but not in the SF > 45 μg/l category (P = 0·404). Additionally, among non-anaemic women, those with SF > 45 μg/l had a 2·9-fold (95 % CI 1·44, 5·83) higher VL compared with women with SF < 15 μg/l. There was no difference in VL among women with SF = 15–45 μg/l v. those with SF < 15 μg/l. In contrast, among anaemic women, VL was not significantly different in any of the three SF categories. When excluding all women with elevated AGP concentrations, non-anaemic women with SF > 45 μg/l had a >2·5-fold higher VL (P = 0·023) than those with SF < 15 μg/l.
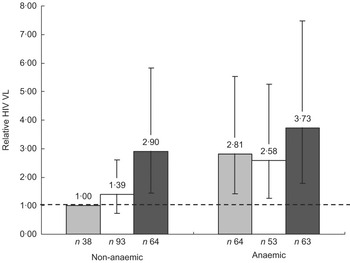
Fig. 1 Relative plasma HIV-1 viral load (VL) by anaemia status (anaemic, Hb < 110 g/l; non-anaemic, Hb ≥ 110 g/l) and category of serum ferritin (SF) concentration (▒, <15 μg/l; ␣, 15–45 μg/l; █, >45 μg/l): sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000. Values are relative VL concentrations compared with the reference category of non-anaemic women with SF < 15 μg/l. Vertical bars are 95% CI around the relative VL
A second method by which we attempted to control for inflammation was to stratify the analysis according to AGP < 1 g/l v. AGP > 1 g/l (Table 3). Among women without evidence of inflammation (AGP < 1 g/l), one log10 increase in SF was significantly associated with a 2·33-fold higher VL (β = 0·369, P = 0·013) after controlling for AGP, Hb, CD4 count and birth weight, which was retained in the model. In a similar model but among women with AGP > 1 g/l, there was no association between SF and VL (β = 0·191, P = 0·148).
Table 3 Predictors of HIV-1 viral load stratified by the presence or absence of an acute-phase response (multivariate analysis): sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000
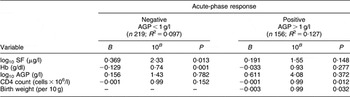
AGP, α1 acid glycoprotein; SF, serum ferritin; TfR, transferrin receptor.
Additional variables assessed in the model were age, birth weight, parity and time elapsed since delivery; birth weight was retained in the model.
In these models, in addition to stratifying by elevated AGP concentrations, we further included AGP as a continuous variable as there remained a range of AGP concentration among women in these strata. When AGP was not further included as a covariate in models restricted to women with AGP < 1 g/l, the association between SF and VL was not altered (β = 0·376, P = 0·009; data not shown). However, when AGP was not included in the models restricted to women with AGP > 1 g/dl, the relationship between SF and VL did change and was significant (β = 0·253, P = 0·026).
Association between maternal Fe status indicators and maternal mortality
Of the 643 women who had complete Fe status measurements, twenty-two were lost to follow-up immediately after recruitment and thirty died during the postpartum year. Cause of death was unknown for four deaths, which were excluded from the analysis. The final sample size included 617 women and twenty-six HIV-related deaths. The cause of death was listed as tuberculosis and/or pneumonia in 81 % (twenty-one deaths) of all cases.
SF was positively and significantly associated with mortality (Table 4). The association between SF and mortality was attenuated but remained significant when AGP was included in the model. After adjusting for AGP, CD4 count, Hb, MUAC, age, educational status and marital status, each log10 increase in SF was associated with a >4-fold greater risk of death during the subsequent year (P = 0·002); this association was greater for non-anaemic women (hazard ratio (HR) = 12·95; 95 % CI 1·75, 95·66; P = 0·012) than for anaemic women (HR = 3·21; 95 % CI 1·04, 9·91; P = 0·042), but the interaction term was not significant (P = 0·181). We were unable to stratify by AGP < 1 g/l v. AGP > 1 g/l in our analysis as twenty-one of the twenty-six deaths (81 %) occurred among women with elevated AGP concentrations. As previously seen in the larger ZVITAMBO cohort(Reference Zvandasara, Hargrove and Ntozini22), Hb was inversely associated with mortality. We found no association between mortality and TfR. In the larger ZVITAMBO cohort of approximately 4500 HIV-positive women, increasing maternal age, having at least a high school level of education and being in a stable union/marriage were all protective against mortality.
Table 4 Predictors of death by 12 months (n 617; twenty-six HIV-related deaths): sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000
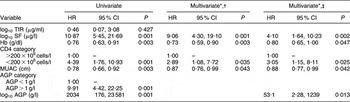
HR, hazard ratio; TfR, transferrin receptor; MAUC, mid upper-arm circumference; SF, serum ferritin; AGP, α1 acid glycoprotein.
*All multivariate models included maternal age (years), marital status (coded 0 for stable union, 1 for other) and maternal education (coded 0 for some secondary education, 1 for no secondary education).
†Model does not control for AGP.
‡Model controls for AGP as a continuous (log10) variable.
Separate Kaplan–Meier survival curves were constructed for each of our three SF categories (Fig. 2). In pairwise log-rank tests, the SF > 45 μg/l category was significantly different from each of the other categories (P < 0·001 for the difference with SF < 15 μg/l and SF = 15–45 μg/l), which were not different from each other (P = 0·569). When SF categories were modelled as dummy variables in a multivariate Cox model that controlled for AGP, CD4 count, Hb, MUAC, age, educational status and marital status, the risk of death was >5-fold higher (HR = 5·45; 95 % CI 1·16, 25·74; P = 0·032) at SF > 45 μg/l compared with SF < 15 μg/l (data not shown). There was no statistically significant difference in mortality at SF levels of 15–45 μg/l compared with the reference category of SF<15 μg/l (HR = 1·50; 95 % CI 0·27, 8·41).
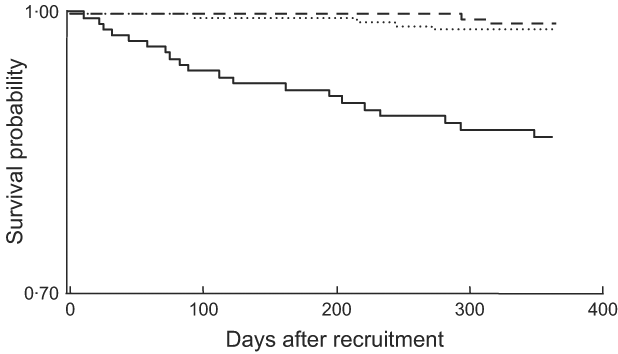
Fig. 2 Kaplan–Meier estimates of the proportion alive by 12 months by category of serum ferritin concentration (– – –, <15 μg/l; ……, 15–45 μg/l; ——, >45 μg/l): sub-sample of postpartum HIV-positive women in the ZVITAMBO clinical trial, Harare, Zimbabwe, November 1997–January 2000. Log-rank test: χ 2 = 41·89, P < 0·001
Discussion
In the present study of postpartum HIV-positive women, SF was independently associated with HIV-1 VL and HIV-related maternal mortality during the subsequent year. Controlling for the effects of inflammation on SF using AGP attenuated, but did not eliminate, these associations. These associations were limited to women with SF > 45 μg/l, a level which we consider more than adequate from an Fe nutrition perspective given that the median SF among adult women aged 20–55 years was 28 μg/l in the second National Health and Nutrition Examination Survey(Reference Gibson28). Our SF categories were chosen to be informative of Fe nutrition and represent low or depleted Fe stores (<15 μg/l), a normal range (15–45 μg/l) and a high Fe storage category (>45 μg/l). Our high Fe storage category would otherwise not be considered high, and is well below what would be considered Fe overload (SF > 150 μg/l(26)); however, given that these women are in the immediate postpartum period (a physiological period when Fe stores would normally be very low), we consider this level to be high and more than adequate.
We believe, in line with other investigators, that the absence of any association between TfR and VL or mortality may be a result of the dual nature of TfR(Reference Gordeuk, Onojobi and Schneider19, Reference McDermid, Jaye, Schim van der Loeff, Todd, Bates, Austin, Jeffries, Awasana, Whittlex and Prentice20). TfR is both an indicator of the severity of Fe insufficiency at the tissue level, as well as a marker of the intensity of erythropoeisis; the latter may be the stronger determinant. Furthermore, TfR concentrations are a marker of Fe insufficiency (and not of excess or elevated Fe) only when Fe stores have been exhausted and no other causes of abnormal erythropoeisis are present, which is likely not the case with HIV-infected populations.
Association between Fe status indicators and HIV-1 viral load
Our data suggest that high Fe stores (measured by SF) may be detrimental, but only among non-anaemic women in whom there is presumably sufficient Fe for the formation of red blood cells. Among these women, excess storage Fe may be enhancing viral replication; the plausible biological role of this potential effect is well documented(Reference Boelaert, Weinberg and Weinberg5, Reference Savarino, Pescarmona and Boelaert6, Reference Georgiou, van der Bruggen, Oudshoorn, Nottet, Marx and van Asbeck29, Reference van Asbeck, Georgiou, van der Bruggen, Oudshoorn, Nottet and Marx30). However, among anaemic women increasing SF was not associated with viral load or mortality. It may be that in HIV-positive anaemic women, Fe is being prioritized for red blood cell production and is thereby not available for viral replication.
We recognize that our use of SF must be interpreted with caution as SF is likely elevated in the face of inflammation. There is no established method of controlling for the positive acute-phase nature of SF, but we addressed this by attempting to control for inflammation in two ways. First, we included AGP as a covariate in our regression models. In these models the inclusion of AGP attenuated the association between SF and VL, but this association remained statistically significant. Clearly, this apparent association is in part due to inflammation. Second, we stratified by AGP concentrations of <1 g/l v. >1 g/l. We assumed among women with AGP < 1 g/l that SF primarily reflects Fe stores, while among women with AGP > 1 g/l, SF may primarily reflect the acute-phase response and may or may not also reflect raised Fe stores. For women with low AGP, SF predicted VL significantly and the magnitude of this effect did not change after further adjusting for AGP, supporting our view that SF reflects Fe stores and not inflammation among these women. In contrast, among women with elevated AGP concentrations, SF significantly predicted VL only when AGP was not included as a covariate in regression models; adjusting for AGP greatly attenuated this association which was no longer significant.
There are three plausible explanations for our results. First, it is possible that AGP does not adequately control for the acute-phase response and that some reverse causality remains (i.e. HIV infection drives up SF via inflammation). When examining the correlation between SF and AGP, we see that this relationship is much stronger at elevated AGP (>1 g/l) concentrations (r = 0·423, P < 0·0 0 1) compared with normal AGP (<1 g/l) concentrations (r = 0·142, P = 0·034). Furthermore, our regression analyses described earlier support the inclusion of AGP as a continuous variable to control for the effect of inflammation on SF as this clearly attenuates the association between SF and VL. Therefore, we believe that AGP does capture some of the contribution of elevated SF to VL owing to inflammation (although it may not fully do so) and that its inclusion in our models helps better relate SF to true Fe stores. A second inference that can be proposed is that AGP does in fact control for the acute-phase response, but that advancing HIV disease in turn causes sequestration of Fe leading to increased SF concentrations. Finally, it is plausible that increasing SF as a function of truly increasing Fe stores causes an increase in VL in non-anaemic women. This inference suggests that there are real adverse consequences of increasing Fe stores with regard to increasing VL. The cross-sectional design of the study prevents us from distinguishing our second v. our third inference. What remains undeterminable is the direction of the observed association between Fe stores and disease progression; cross-sectional studies cannot rule out the possibility that increased storage Fe (i.e. high SF or bone-marrow Fe) is a consequence of HIV progression, rather than its cause.
Three other African studies have directly investigated the relationship between SF and VL. In a cross-sectional analysis of HIV-infected pregnant women in Zimbabwe, investigators showed that women with severely depleted Fe stores (SF < 6 μg/l) had approximately one-third the VL of women with non-depleted Fe stores (SF ≥ 24 μg/l) after controlling for inflammation(Reference Friis, Gomo, Nyazema, Ndhlovu, Krarup, Madsen and Michaelsen15). Similarly, among HIV-infected pregnant women in Tanzania, investigators found a strong positive association between SF and VL in both the presence and the absence of inflammation(Reference Kupka, Msamanga, Mugusi, Petraro, Hunter and Fawzi18). In contrast, a study in Malawi found no relationship between indicators of Fe status (Hb, SF, TfR) and VL in a cross-sectional study of HIV-positive pregnant women(Reference Semba, Taha, Kumwenda, Mtimavalye, Broadhead, Miotti and Chiphangwi17).
Association between Fe status indicators and mortality
In the present study population, increasing SF and decreasing Hb were associated with significant increases in the risk of death by 12 months among HIV-positive postpartum Zimbabwean women. We note that AGP is itself a very strong predictor of death, and that the addition of AGP into the model greatly attenuated the associated risk between increasing SF and death. When AGP was included, further adjusting for CD4 count only marginally attenuated the risk associated with SF. We could not stratify our analyses by elevated AGP concentration as 81 % of deaths occurred in women who had elevated AGP concentration at baseline.
These results suggest that while decreasing Hb is associated with increased risk of death, an expected and previously described result(Reference O’Brien, Kupka, Msamanga, Saathoff, Hunter and Fawzi3, Reference Zvandasara, Hargrove and Ntozini22), increasing Fe stores may also be associated with increased risk of death. We observed a log10 increase in SF to be associated with a >4-fold increase in the risk of death.
There are several studies in developed countries that show an association between SF and HIV-related mortality(Reference de Monye, Karcher, Boelaert and Gordeuk7, Reference Costagliola, de Montalembert and Lefrere31–Reference Delanghe, Langlois and Boelaert33). More recently, an association between SF and mortality has been shown in a retrospective case–control study of an HIV-infected, highly active antiretroviral therapy-naïve population in the USA(Reference Gordeuk, Onojobi and Schneider19). The investigators found that a log10 increase in SF was associated with a marginally significant 1·67-fold increase in the odds of death. Two longitudinal studies from SSA examining the association between SF and disease progression and/or mortality have been published recently, with conflicting findings. Among HIV-infected Gambian adults(Reference McDermid, Jaye, Schim van der Loeff, Todd, Bates, Austin, Jeffries, Awasana, Whittlex and Prentice20), elevated SF predicted a 40 % increase in mortality over 11·5 years. Among pregnant Tanzanian(Reference Kupka, Msamanga, Mugusi, Petraro, Hunter and Fawzi18) women elevated SF was not associated with a statistically significant increase in the risk of disease progression, although the authors argue that the elevated point estimates coupled with low statistical power do not rule out the possible adverse effects of Fe stores.
Our results support the hypothesis that increasing SF may be associated with a more rapid progression of HIV-1 infection, leading to death. The mechanisms by which this might occur include direct cytotoxicity and immune dysfunction, enhancement of viral replication, and increased susceptibility to certain opportunistic infections and neoplasia(Reference McDermid and Prentice34). A well-recognized consequence of Fe loading is the stimulation of the growth of micro-organisms, including opportunistic pathogens that are associated with HIV infection(Reference Boelaert, Weinberg and Weinberg5, Reference Boelaert35, Reference Weinberg36). There is concern that acquisition of Fe by these pathogens and subsequent increase in microbial growth may itself upregulate transcription of HIV and therefore aid in disease progression(Reference Weinberg, Boelaert and Weinberg8). Additionally, because Fe loading has been shown to directly inhibit various immune responses(Reference Brock37, Reference Weiss38), it is plausible that increasing Fe stores has the ability to compromise host responses to HIV infection leading to more rapid disease progression.
The strength of the present analysis is its prospective design which limits the reverse causality argument of advanced HIV disease causing Fe sequestration and in turn increased SF that is inherent in a cross-sectional design. However, like with the adverse association between SF and VL, we cannot rule out the possibility that the use of AGP does not fully control for the effect of inflammation on SF. Clearly the use of AGP attenuates the observed association between SF and mortality, but what remains uncertain is if it eliminates the effect of inflammation on SF.
Conclusions
These results are consistent with the hypothesis that high Fe stores have adverse consequences within the context of HIV infection. The association with decreasing Hb was as expected. Controlling for AGP greatly attenuated the association between SF and both VL and progression, but these risks remained and were found at SF levels >45 μg/l. We are unable to determine if the adverse associations that we see at elevated SF concentrations are a consequence of Fe sequestration with advanced infection (in cross-sectional analyses) or if residual effects of the acute-phase response remain after controlling for AGP (in both cross-sectional and prospective analyses).
If adverse consequences are real, our data suggest they occur at a level of Fe stores that is more than adequate from an Fe nutrition perspective. These observations are consistent with the idea that, across the spectrum of Fe status, there exists a range associated with optimal host protection. However, we are unable to generalize our findings to other HIV-infected populations such as adult men and children. Therefore, additional studies are needed in HIV-infected populations to elucidate the potential benefit of treating and preventing anaemia with different dosages of Fe supplementation v. increasing Fe stores to a level that could be potentially hazardous. This is especially important in populations where health systems lack the capacity for targeted supplementation based on screening for Fe deficiency and anaemia.
Acknowledgements
Conflict of interest statement: We declare that there are no conflicts of interest.
Contributors: R.R., J.H.H. and R.J.S. designed the study, interpreted the data and wrote the manuscript. R.R. and R.N. were responsible for data management and analysis. R.R. and K.M. carried out laboratory analysis. All authors contributed to the final manuscript.
Funding support: The ZVITAMBO project was supported by the Canadian International Development Agency (CIDA; R/C Project 690/M3688), the United States Agency for International Development (USAID; cooperative agreement number HRN-A-00-97-00015-00 between Johns Hopkins University and the Office of Health and Nutrition–USAID) and a grant from the Bill and Melinda Gates Foundation (Seattle, WA, USA). Additional funding was received from the Nestlé Foundation (Lausanne, Switzerland), the Rockefeller Foundation (New York, NY, USA) and Cornell University (Ithaca, NY, USA). The Regional Network on AIDS, Livelihoods, and Food Security (RENEWAL) provided support for the time of the first author in preparing this manuscript for submission.










