Introduction
There has been a trend toward delaying motherhood in developed countries, resulting in an average age of 26.3 years for mothers delivering their first-born child in the USA in 2014. Reference Bichell6 Unfortunately, this delay in motherhood has led to an overlap with the period of increasing natural incidences of certain cancers. At present, the cumulative incidence of cancers in pregnant women has been estimated to be at around 1 in 1000–2000 pregnancies, Reference Aronowitz3 further creating a unique and challenging clinical scenario of pregnancy-associated secondary brain tumors (PASBT). Among these, there has been an increased incidence of breast, melanoma, and lung cancer, all of which have propensities to metastasize to the brain. Reference Ostrom, Wright and Barnholtz-Sloan35
There are obvious risks to the fetus when treating expectant mothers diagnosed with cancer using chemoradiation. Beyond that, there are elevated anesthesia risks and other comorbidities associated with many non-obstetrics-related surgical procedures when performed during pregnancy. Reference Cordeiro and Gemignani12,Reference Greskovich and Macklis14,Reference Morris28 It is noteworthy that there are currently no guidelines regarding the neurosurgical treatment of PASBT. To address this void, we performed a systematic review of the literature with a focus on PASBT to coalesce common and unique challenges in this setting and to discuss key aspects in managing these patients from a neurosurgeon’s perspective.
Materials and Methods
Data Sources
A PRISMA-guided literature search in MEDLINE (PubMed) was conducted using the search phrases “brain”, “intracranial”, “intracerebral”, “cancer”, “metastasis”, “pregnancy”.
An additional search was conducted using Google Scholar to avoid missing articles that may have lacked one or more of the above search terms.
Search Criteria
Studies had to meet the following criteria to be included in our analysis: (1) Published in the MRI era between January 1990 and August 2018; (2) English language; (3) Human subjects; and (4) Peer-reviewed publications. A PRISMA workflow diagram is provided in Figure 1.
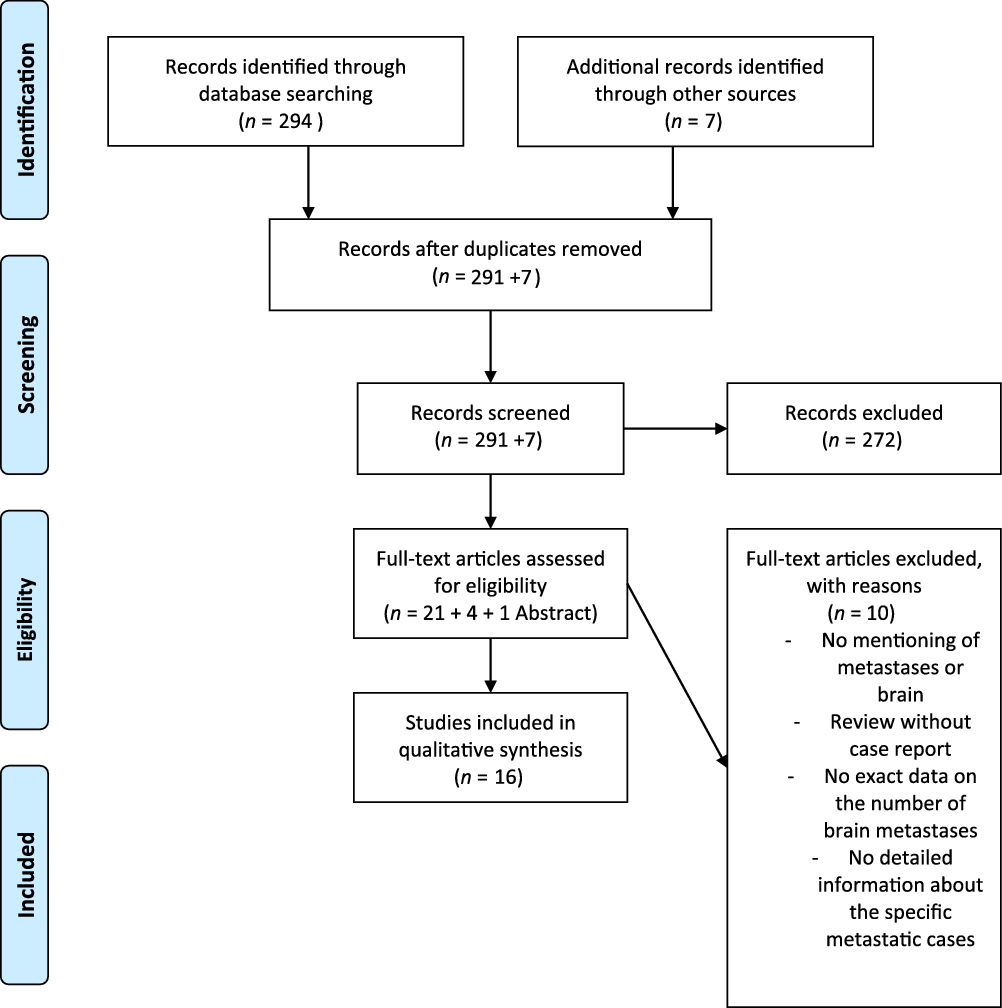
Figure 1: PRISMA 2009 flow diagram.
Data Extraction
The following information had to be available in selected articles and was extracted from all suitable publications identified in our PubMed search: (a) Classification of primary malignancy; (b) Description of brain metastases; (c) Diagnostic method and criteria for detection of brain metastases; (d) Information regarding pregnancy status and management, including outcome; (e) Description of details regarding treatment of brain metastases during pregnancy; (f) Age of the mother; (g) Gestational age (GA) of the fetus at diagnosis and delivery; (h) Method of delivery.
Results
A total of 301 studies were retrieved after the application of our PRISMA algorithm (Figure 1). Of these, 16 fulfilled all inclusion criteria, and 2 studies presented more than 1 case. This resulted in a pooled cohort of 22 cases of PASBT, which we included in our final analysis. Individual cases and reports are summarized in detail in Table 1.
Table 1: Summary table of relevant cases of PASBT identified through our PRISMA search
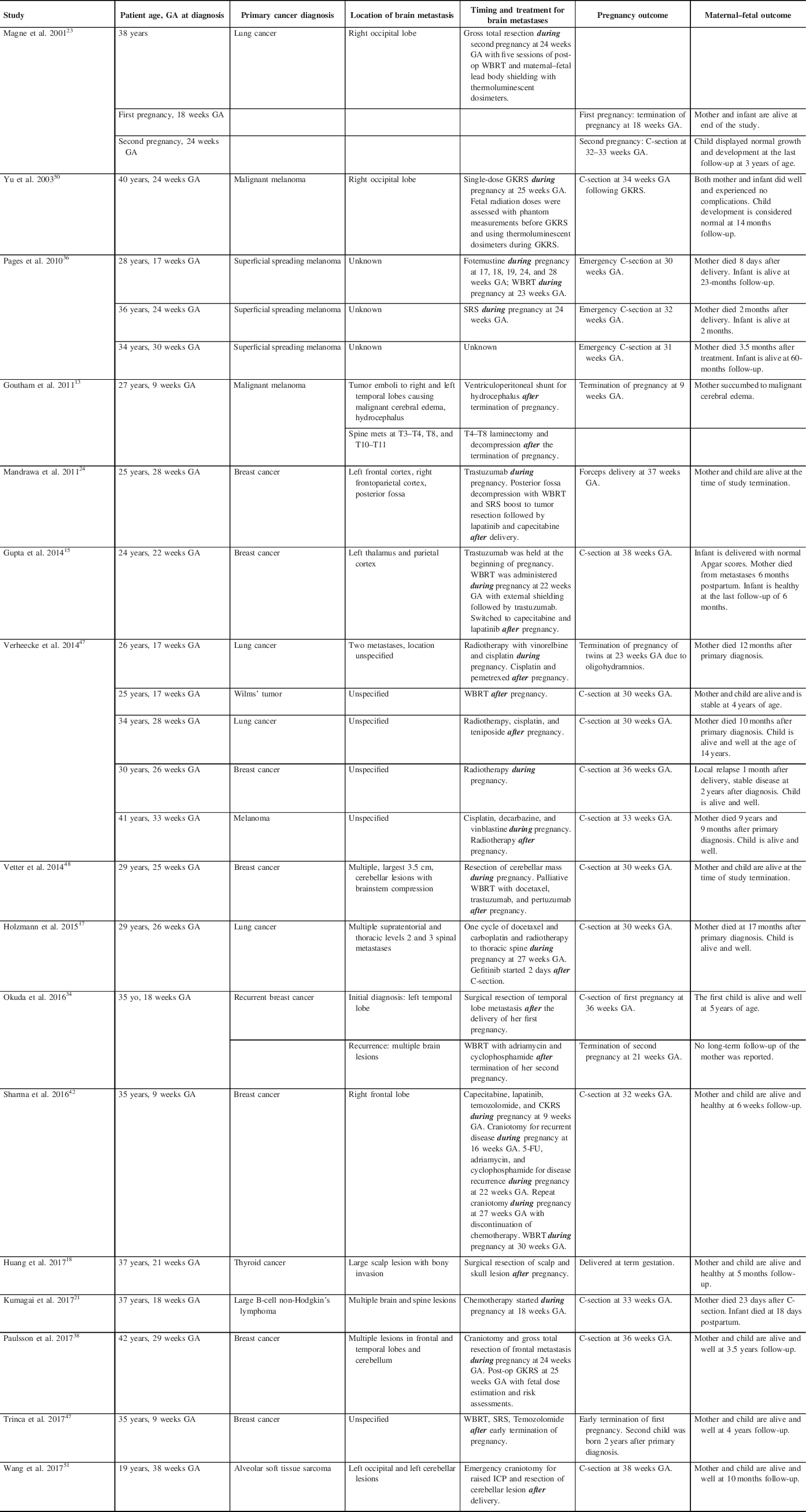
C-section = cesarean section; CKRS = CyberKnife Radiosurgery; GA = gestational age; GKRS = Gamma Knife Radiosurgery; ICP = intracranial pressure; SRS = stereotactic radiosurgery; WBRT = whole-brain radiotherapy; 5-FU = 5-Fluorouracil.
Tumor Diagnoses
Eight of the 22 patients (36.36%) had breast cancer: Six with intraductal/infiltrative breast cancer and two with triple-negative breast cancer. Six patients (27.27%) presented with a primary skin tumor: three of those were classified as superficial spreading melanoma, and three as malignant melanomas. Five patients (22.73%) had lung cancer and the remaining three patients (13.64%) had various pathologies, including one Wilms’ tumor; one follicular thyroid carcinoma; and one primary mediastinal large B-cell non-Hodgkin’s lymphoma.
Chemotherapy Only
One patient presenting with spine and brain metastases due to large B-cell non-Hodgkin’s lymphoma at 18 weeks GA was treated with chemotherapy alone during the pregnancy. Reference Kumagai, Koishi, Takahashi and Suzuki21
Radiation Therapy Only
Four patients (18.18%) received single modality radiotherapy for the treatment of their brain metastases: three patients received stereotactic radiosurgery (SRS) during their pregnancy at 24 weeks GA, Reference Pages, Robert and Thomas36 25 weeks GA Reference Yu, Jozsef, Apuzzo, MacPherson and Petrovich50 and 26 weeks GA. Reference Verheecke, Halaska and Lok47 One patient received whole-brain radiotherapy (WBRT) after her elective C-section at 30 weeks GA. Reference Verheecke, Halaska and Lok47
Neurosurgical Procedure Only
Three patients (13.64%) had neurosurgical interventions after their pregnancies: One patient had an insertion of a ventriculoperitoneal (VP) shunt after early termination of her pregnancy at 9 weeks GAReference Goutham, Kumar, Anil, Peter, Ravi and Chakravorthy13; Two patients had surgical resection of their tumors after successful term deliveries.Reference Huang, Cheng, Chen, Hsu, Liu and Chen18,Reference Okuda, Yamamoto, Matsuo, Kataoka and Kitawaki34
Chemoradiation Only
Seven patients (31.82%) received combination chemotherapy and radiation. Five patients received chemotherapy and radiation both during and after their pregnancies, Reference Gupta, Jain and McDunn15,Reference Holzmann, Kropfmuller and Schinko17,Reference Magne, Marcie, Pignol, Casagrande and Lagrange23,Reference Pages, Robert and Thomas36,Reference Verheecke, Halaska and Lok47 while two patients received treatments after their pregnancies. Reference Okuda, Yamamoto, Matsuo, Kataoka and Kitawaki34,Reference Trinca, Inacio, Timoteo and Dinis46
Combined Neurosurgical Intervention and Chemoradiation Therapy
Four patients had combined interventions: One patient had chemotherapy during her pregnancy followed by neurosurgical resection and chemoradiation after successful delivery of her child Reference Mandrawa, Stewart, Fabinyi and Walker24 ; the second patient received surgical resection of her metastasis during pregnancy followed by WBRT and chemotherapy after successful delivery of her child Reference Wang, Cui, Yan, Jin and Hong49 ; the third patient received chemoradiation prior to neurosurgical resection followed by further resection at the time of recurrence and WBRT during her pregnancy with the successful delivery of a healthy fetus. Reference Stovall, Blackwell and Cundiff42 Timing of the above interventions with maternal/fetal outcomes is summarized in Table 1.
Discussion
The detection of brain metastasis during pregnancy is a rare occurrence. However, the risks surrounding the treatment of the mother’s primary tumor and metastatic disease have to be balanced against maintaining the health and viability of the fetus and those competing treatment goals pose significant challenges to the neurosurgeon.
It is estimated that 20% of cancer patients will develop brain metastases. Reference Achrol, Rennert and Anders1,Reference Nayak, Lee and Wen30 Of these, lung (20–56%), breast (5–20%), and melanoma (7–16%) most commonly metastasize to the brain with the prevalence of renal cell carcinoma and colorectal cancer on the rise. Reference Achrol, Rennert and Anders1,Reference Barnholtz-Sloan, Sloan, Davis, Vigneau, Lai and Sawaya5 Lung cancer is the most frequent to metastasize to the brain irrespective of gender, but breast cancer metastases are the most common occurrence in women. Reference Achrol, Rennert and Anders1 Risk of brain metastases also varies with age and the type of primary, with breast cancer brain metastases being reported as highest in younger patients (20–39 years of age), lung cancer brain metastases the highest in middle-aged patients (40–49 years of age), and melanoma, renal cell carcinoma, and colorectal cancer brain metastases highest in older patients (50–59 years of age). Reference Barnholtz-Sloan, Sloan, Davis, Vigneau, Lai and Sawaya5,Reference Holzmann, Kropfmuller and Schinko17,Reference Hung, Liu and Shiau19 Interestingly, the increased risk of brain metastases in patients younger than 35 years of age was independent of cancer subtype, however, triple-negative or HER2-positive breast cancer subtypes were associated with a higher risk of brain metastases in patients older than 35 years of age. Reference Achrol, Rennert and Anders1 These epidemiologic findings highlight the need for neurosurgical guidelines for the management of patients with PASBT, as the neurological disease causes up to 25% mortality in patients with brain metastases. Reference Neal, Chan and Lucas31,Reference Tabouret, Chinot, Metellus, Tallet, Viens and Goncalves44
Guiding Algorithms
Safe Dosing of Radiation Therapy for Brain Metastases During Pregnancy
In a general cancer patient population of male and female nonpregnant individuals, there is Level 1 evidence that surgery followed by WBRT is recommended as first-line treatment in all patients with single brain metastases with a favorable performance status and limited extracranial disease, as outlined in the 2019 guidelines put out by the Congress of Neurological Surgeons. Reference Nahed, Alvarez-Breckenridge and Brastianos29 However, SRS to the tumor bed has recently been shown to have equivalent survival outcomes and just slightly inferior local control rates, and can thus be used as an effective alternative to WBRT (median survival of SRS = 12.2 months vs. WBRT = 11.6 months). Reference McTyre, Johnson and Ruiz27 This may be an appealing treatment alternative in this setting to attenuate neurocognitive deficits associated with WBRT, especially poignant in young expectant mothers with well-controlled systemic disease. Reference Brown, Ballman and Cerhan8 Modern shielding techniques can scatter radiation and shield the fetus from the adverse effects of WBRT, including mental retardation, organ malformations, and radiation-induced childhood malignancies. Reference Greskovich and Macklis14,Reference Magne, Marcie, Pignol, Casagrande and Lagrange23,Reference Parsai, Nalk, Djemil and Chao37,Reference Yu, Jozsef, Apuzzo, MacPherson and Petrovich50
Timing of Neurosurgical Intervention During Pregnancy
Of the 11 cases requiring neurosurgical intervention, only 1 case required emergent craniotomy to alleviate increased intracranial pressure due to tumor mass effect. Both the mother and her fetus survived the surgery and the fetus was subsequently delivered via cesarean section at 38 weeks GA. Reference Wang, Cui, Yan, Jin and Hong49 Surgery should be delayed if possible until after the first trimester to minimize miscarriage risk, which can be as high as 10.5%. Reference Cohen-Gadol, Friedman, Friedman, Tubbs, Munis and Meyer10,Reference Cohen-Kerem, Railton, Oren, Lishner and Koren11 However, it is generally considered to be safe during the second and third trimesters with recommended dosimetry guidelines for safety. Reference Greskovich and Macklis14,Reference Paulsson, Braunstein and Phillips38,Reference Stovall, Blackwell and Cundiff42,Reference Yu, Jozsef, Apuzzo, MacPherson and Petrovich50
To compare our study results to a larger patient population, we turned to a retrospective cohort study of pregnancy-related hospitalizations between 1998 and 2009 gathered from the Nationwide Inpatient Sample (NISQIP), a database of nonfederal US hospitalizations. Here, a recent study identified 19.75 million pregnancy-associated admissions, 397 associated with malignant brain tumors, including 165 associated deliveries (44%). Reference Terry, Barker and Leffert45 Patients with malignant tumors had more pregnancy complications including preterm labor, intrauterine growth restriction, and stillbirths. The rate of craniotomies for malignant tumors was 31.4% although the study did not differentiate between primary or metastatic brain malignancies. This was much lower than the 50% rate of craniotomies from our systematic review, however, this may be due to a lower rate of metastatic brain tumors in the former study. There was also a higher rate of C-sections amongst pregnant women with malignant tumors compared to the normal population, similar to the 65% of cases that required C-sections in our systematic review.
Our systematic review also identified one patient who required VP shunting for hydrocephalus. Reference Goutham, Kumar, Anil, Peter, Ravi and Chakravorthy13 A retrospective series of 77 pregnancies in 37 women with VP shunts showed that rates of shunt revisions were 10 times higher during pregnancy or the following 6 months ensuing likely due to raised intra-abdominal pressures causing distal shunt failure. Reference Bradley, Liakos and McAllister7 This had led some neurosurgeons to consider ventriculoatrial (VA) shunts for pregnant women, but VA shunts carry significant risks of arrhythmias, infection, valvular dysfunction, and high morbidity associated with revisions. The neurosurgeon should, therefore, closely monitor the pregnant patient for signs and symptoms of shunt failure in the peripartum and postpartum periods. Reference Tabouret, Chinot, Metellus, Tallet, Viens and Goncalves44
Timing of Use of Antileptic Drugs During Pregnancy
Important to the neurosurgical management of patients with supratentorial tumors is the use of antiepileptic drugs (AEDs) to manage symptomatic lesions presenting with seizures. Older generation AEDs such as valproic acid and phenobarbital have been associated with increased rates of teratogenicity and congenital malformations (up to 9.3% and 5.5%, respectively) such as cardiac defects, cleft lip/palate, neural tube defects, and dysmorphic syndromes. Reference Hernandez-Diaz, Smith and Shen16 However, newer agents such as lamotrigine and levetiracetam (currently, the most commonly used AED in neurosurgery) have improved safety profiles for use in pregnancy with much lower reported rates of congenital malformations between 0.7% and 2.4%. Reference Hernandez-Diaz, Smith and Shen16,Reference Mawhinney, Craig and Morrow25 Doses of AEDs should be tapered to the minimally effective dose to achieve seizure control and used for the shortest period of time possible during pregnancy. Reference Hernandez-Diaz, Smith and Shen16
Surgical Considerations of the Pregnant Patient During Neurosurgical Procedures
Unique challenges exist during the positioning of the patient for non-obstetric surgeries during pregnancy. Prevention of positioning injuries to mother and fetus needs to be observed and the operating table should be centered and parallel to the longest wall in the room to minimize the need for relocation prior to the patient entering the operating room. Reference Barnett, Bartalot, Nezhat, Nezhat, Kavic, Lanzafame, Lindsay and Polk4 When possible, the patient should be maintained in the left lateral recumbent position using a positioning wedge to avoid a flat supine position once transferred onto the operating table with attention to the application of non-constricting safety straps to not harm, yet prevent the patient from falling off the table. 2 A uterine displacing wedge or chests rolls should be used to reduce pressure from the pregnant uterus on the vena cava and straps across the chest are discouraged to avoid restricting respiratory effectiveness. Reference Carzo, Viveiro, Rothrock and McEwen9 Pressure points on the mother’s body should be well padded to prevent ulcers and peripheral neuropathies during surgery. Hypothermia should be carefully avoided (e.g. with the use of a warming blanket). Intraoperative electronic fetal monitoring past 26 weeks GA may be applied if the fetus is viable, if the monitor does not physically interfere with the procedure, if an obstetrician is available with prior consent obtained to perform an emergency C-section for fetal distress, and if the type of surgery is safe for the interruption to perform an emergency delivery if warranted. Reference Barnett, Bartalot, Nezhat, Nezhat, Kavic, Lanzafame, Lindsay and Polk4,Reference Reitman and Flood40
Anesthetic Considerations for the Pregnant Patient
Special anesthetic considerations are also required for non-obstetric procedures during pregnancy in order to avoid the complications of fetal loss, premature labor, and delivery. Cardiopulmonary changes in maternal physiology during pregnancy can lead to unintended general hypotension, difficult laryngeal intubation with potential airway edema, hypoxemia, and increased uterine irritability leading to preterm labor. Reference Nejdlova and Johnson32 The mother should be informed regarding the low risk of teratogenicity associated with anesthetic agents and a neonatal team should be available and present should premature labor be anticipated. Regional anesthesia is preferred over general anesthesia where it is feasible to minimize drug exposure to the fetal and allow the mother to maintain her own airway, however, there is a lack of evidence supporting these claims in the form of randomized clinical trials.
In the case of neurosurgical procedures, general anesthesia can rarely be avoided. Reference Ni Mhuireachtaigh and O’Gorman33 Maintenance of PCO2 during the surgery can also be challenging as ventilation should be aimed to keep levels within the normal range of pregnancy, however, hyperventilation may be required to decrease central vasodilation as a means of lowering intracranial pressures during tumor resection. Extubation should be performed with a full anesthetic reversal and preferably in the lateral position to avoid aspiration. Reference Sharma, Nguyen, Lozen and Mueller41
Suggested Algorithm for the Treatment of Brain Metastases in the Pregnant Patient
We recently published a case series reviewing the surgical management of 104 meningioma patients during pregnancy and proposed an algorithm dividing the date of diagnosis with GA of the fetus for the surgical management of these patients into two groups. Reference Laviv, Bayoumi, Mahadevan, Young, Boone and Kasper22 Using a similar algorithm based on studies in our systematic review and current literature, we propose dividing patients with PASBT into three groups in order to guide the timing of neurosurgical interventions:
-
1) Group A – Patients at ≤ 13 weeks GA
-
Surgery and/or systemic chemotherapy or radiotherapy should be delayed past the first and second trimesters if possible to prevent teratogenic effects of anesthetic drugs, chemotherapy, and radiation, as well as premature labor caused by anesthesia-induced hypotension to the placenta and uterus.
-
If patients require radiation therapy, SRS is preferred over WBRT to reduce long-term neurocognitive dysfunction to the mother and congenital malformations to the fetus. Body shielding should be used to minimize radiation exposure to the fetus.
-
If surgery and/or chemoradiation cannot be avoided, then a frank discussion should be had with the patient explaining adverse effects to the fetus and options including early termination of pregnancy.
-
Any practical aspects of survival intervention (positioning/pinning) are usually not compromised yet in this patient subgroup, as the pregnant uterus remains small and patients can even be positioned prone without significant concerns.
-
-
2) Group B – Patients between 13 and 26 weeks GA
-
The same algorithm as in Group A can be applied to Group B patients but if the patient is diagnosed or becomes symptomatic at around 26 weeks GA, the option of C-section to deliver the fetus with support in a neonatal intensive care unit should be presented to the patient prior to treatment for her symptomatic brain metastases. Here, and only if circumstances allow, the goal for initial treatment would remain to bring the baby as close to 30 weeks of GA as possible, since the relative risk reduction of infant mortality per week is greatest before that date. Reference Szuflita, Boulter, Gihooly, Neal, Nezhat, Kavic, Lanzafame, Lindsay and Polk43
-
If surgical intervention is required, the left lateral decubitus or supine position is the preferred way to perform surgery at this stage.
-
-
3) Group C – Patients at ≥ 26 weeks GA
-
Symptomatic patients requiring surgery and/or chemoradiation should be presented with the delivery of their fetus via C-section or forceps-assisted delivery (to avoid raised intracranial pressures that may be associated with straining during labor) prior to their neurosurgical intervention.
-
Late into the third trimester, the teratogenic effects of chemotherapy and radiation exposure are much lessened and may allow for treatment of the mother without needing to deliver the fetus but risks need to be discussed with the mother.
-
As above, if surgical intervention is required, the left lateral decubitus or supine position is the preferred way to perform surgery at this stage.
-
Finally, current advances in targeted therapies for the treatment of breast, lung, and melanoma cancers may dramatically change the way we approach the treatment of PASBT in the future. While there remains a paucity of information regarding the long-term teratogenicity of targeted therapies, the HER2-neu receptor antagonist trastuzumab has been used for the treatment of breast cancer during pregnancy. Reference Mandrawa, Stewart, Fabinyi and Walker24 The BRAF inhibitor dabrafenib is classified as a category D agent and therefore contraindicated for the treatment of metastatic melanoma during pregnancy. Reference McGettigan26 Similarly, there is a lack of data pertaining to fetal safety for the EGFR inhibitor erlotinib, currently, the standard of care treatment for EGFR-mutated lung cancer in the USA, Reference Ji, Schwartz, Hartford, Ramsey, Phillips and Verschraegen20 hence conventional standard chemotherapy remains recommended in the treatment of pregnant patients with lung cancer. We eagerly await longitudinal data using immunotherapies and other novel modalities for the treatment of brain metastases, which may be added to increase the neurosurgeon’s armamentarium in managing PABST. Reference Achrol, Rennert and Anders1
Conclusion
In conclusion, neurosurgical management of PASBT is a challenging problem and should involve a multidisciplinary team of neuro-oncologists, anesthesiologists, obstetricians, neonatologists, and ethicists in order to fully inform the mother of the risks to herself and her unborn fetus. The increasing ability of systemic targeted therapies to effectively control a patient’s primary disease combined with research into understanding the process of brain metastasis formation will hopefully lead to novel therapies, which are both effective and safe for the health and viability of the mother and fetus.
Acknowledgements
We would like to thank the administrative staff in the Division of Neurosurgery at McMaster University Faculty of Health Sciences, in particular Ms. Kim Morris and Ms. Christina Vandervlist for their assistance in the final submission process of this manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author Contributions
P.J.P, F.C.L. designed, wrote, and analyzed the data. S.S., B.C.Y., and Y.L. edited the manuscript. E.M.K. supervised, designed, and edited the manuscript.






