Transcranial magnetic stimulation (TMS) is a non-invasive technique used to measure the in vivo neurophysiological parameters such as the cortical resting motor threshold (RMT) and central motor conduction time (CMCT). Previous studies have reported poor cortical excitability and prolongation of CMCT in Wilson’s disease (WD).Reference Bembenek, Kurczych and Czlonkowska1 However, the longitudinal changes of these measures and the effect of chelation therapy on them are not clear. The existing literature provides conflicting results. Few studies have reported improvement in CMCT with adequate chelation, whereas other studies, including a study from our group, reported no demonstrable improvement in the cortical excitability.Reference Bembenek, Kurczych and Czlonkowska1–Reference Hefter, Roick and Von Giesen4 However, these findings were based on recording from a few subjects with varying duration of disease and chelation status. Furthermore, evidence from clinical and neuroimaging studies support that adequate and long-term chelation improves the clinical and MRI abnormalities.Reference Roberts and Schilsky5, Reference Kim, Kim and Kim6 Despite the contradictory results from previous studies, we hypothesize that the neurophysiological measures may improve after adequate treatment. In this study of 18 subjects of neurological WD, we investigate the longitudinal changes of RMT and CMCT and also discuss the effect of chelation therapy.
This study was conducted at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore. All participants provided informed consent prior to recruitment. The neurophysiological and clinical variables measured included the RMT, CMCT, and Global Assessment Scale for Wilson Disease (GAS-WD) score. All patients were evaluated longitudinally at two occasions; prior to starting of chelation therapy (RMT 1 , CMCT1, and GAS-WD1) and in the subsequent follow-up (RMT2, CMCT2, and GAS-WD2). After the baseline measurement of RMT1, CMCT1, and GAS-WD1, patients were started on medications. The chelation regimen in subjects consisted of Penicillamine (12–15 mg/kg/day in 2–3 divided doses) and Zinc (150 mg/day of elemental zinc in 3 divided doses).Reference Roberts and Schilsky5 All medications were stopped for at least 24 hours to allow wash-out of the drug. The demographic details and drug profile of patients are provided in Table 1.
Table 1: Demography and treatment profile of patients with WD
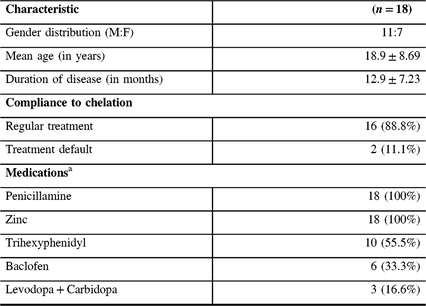
a Daily dose of medicines (minimum–maximum) – Penicillamine: 500–750 mg; Zinc: 100–150 mg; Trihexyphenidyl: 4–12 mg; Baclofen: 10–20 mg; Levodopa: 100–300 mg; Carbidopa: 25–75 mg. Recording during the follow-up was performed after withholding medication for at least 24 hours.
The recommendations of the IFCN committee were followed for all TMS measurements.Reference Rossini, Burke and Chen7 Measurements were obtained using a Magstim 200 stimulator and a hand-held, figure-of-eight coil while the subject relaxed in a comfortable chair in a quiet room. Two Ag–AgCl electrodes, arranged in a belly-tendon montage with the active electrode placed over the belly of the first dorsal interosseus (FDI) and the reference electrode placed on the metacarpophalangeal joint of the index finger, were used to record the motor evoked potentials (MEP).
The optimal “hotspot” was identified by placing the coil at a 45° angle over the left motor cortex. Stimulation of this “hotspot” elicited motor responses in the contralateral FDI muscle. Relative frequency method was followed for measuring the RMT. From a subthreshold stimulus of 30% of the maximal stimulator output (MSO), the intensity of the stimulus was increased in increments of 5% until the TMS consistently evoked MEPs with a peak-to-peak amplitude of more than 50 µV.Reference Rossini, Burke and Chen7 The stimulus intensity was sequentially decreased by 1% of MSO until <5 unsatisfactory MEPs were obtained in a trial of 10 recordings. This stimulus intensity plus one was regarded as the RMT. CMCT was determined by motor root stimulation at a suprathreshold stimulus (120% of RMT). The latency between cortical and motor root stimulation was reported as CMCT (in milliseconds).Reference Rossini, Burke and Chen7
Statistical analysis for quantifying significant differences in the RMT, CMCT, and GAS-WD score was performed using Wilcoxon signed rank test for non-parametric data. In subjects with unrecordable RMT, we used the 100% (MSO) as the imputed RMT for statistical analysis. Improvement in specific clinical symptoms was evaluated using McNemar’s test for paired proportions. Correlation (Spearman’s correlation) was performed to identify significant associations between the neurophysiological measures and clinical severity score. A threshold of p < 0.05 after Bonferroni correction for multiple comparisons was considered statistically significant.
The mean age of patients at recruitment was 18.94 ± 8.69 years and the mean duration between the first and second recordings was 12.94 ± 7.23 months. Among the subjects, 16 (88.8%) patients were on regular chelation therapy, whereas 2 subjects were treatment defaulters. At pre-chelation measurement, MEP was not recordable at 100% of MSO in three subjects. However, during the follow-up measurement, MEP was recordable in all subjects. On qualitative analysis, 14 subjects had decrease in the RMT and GAS-WD scores. The RMT and GAS-WD score worsened in two subjects who were also treatment defaulters. In yet another two subjects, the RMT and GAS-WD score worsened despite regular therapy. The CMCT value worsened in 11 subjects and improved in 7 subjects. The RMT, CMCT, GAS-WD score, and specific clinical signs in patients prior to chelation therapy and at the follow-up assessment are provided in Tables 2 and 3.
Table 2: Clinical and neurophysiological measures at baseline and at follow-up visit in WD
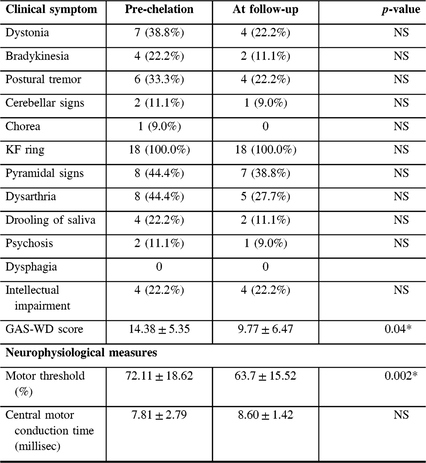
NS = Not significant. Data are based on the presence or absence of symptom. “*” denotes significant p-values after correction for multiple comparison.
Table 3: Longitudinal measurement of resting motor threshold and central motor conduction time in patients with WD
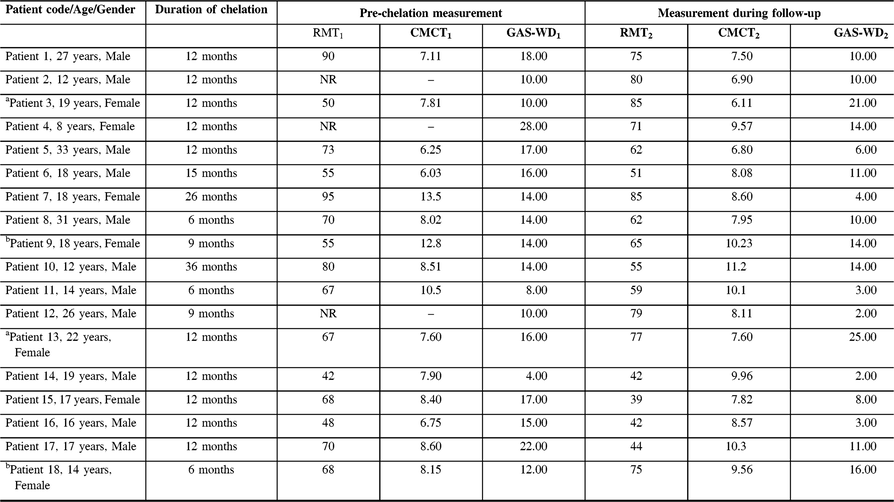
CMCT = Central motor conduction time; GAS-WD = Global Assessment Scale for Wilson Disease score; NR = Non recordable; RMT = Resting motor threshold.
aTreatment defaulters; bPatient on regular treatment with worsening of RMT and GAS-WD score.
Statistical analysis revealed a significant decrease in the mean RMT (72.11 ± 18.62 vs. 63.7 ± 15.52%; corrected p-value = 0.002) between the first and second assessments. Although statistically significant improvement was not obtained on comparisons of specific clinical features, the overall GAS-WD scores improved significantly (14.38 ± 5.35 vs. 9.77 ± 6.47; corrected p-value = 0.04). The CMCT, however, did not show any significant improvement (7.81 ± 2.79 vs. 8.60 ± 1.42; p-value = 0.2).
There were no significant correlations identified between the RMT, CMCT, and GAS-WD scores at any time point of measurement. However, a significant correlation was obtained between the change in the RMT (RMT1-2) and GAS-WD score (GAS-WD1-2) (r = 0.7, p-value = 0.002) after correction for the duration of chelation. There was no significant association of the change in CMCT values and GAS-WD scores.
This study demonstrates the longitudinal improvement in the RMT and GAS-WD scores in patients with WD while on chelation therapy. There was a significant association between the improvement in RMT and GAS-WD scores denoting the potential of RMT measurement as a neurophysiological biomarker in assessing chelation efficacy.
TMS enables the study of the functional properties of the motor cortex and corticospinal tract (CST). The factors affecting the RMT, CMCT, and their mechanism are not well understood. It is believed that RMT is a measure of the excitability of the cortico-cortical fibers projecting to the corticospinal neurons, whereas CMCT depicts the electrical conduction through the CST.Reference Bembenek, Kurczych and Czlonkowska1–Reference Hefter, Roick and Von Giesen4, Reference Kobayashi and Pascual-Leone8 The RMT changes observed in WD, hence, is a reflection of the cortico-motoneuronal dysfunction causing the decrease in the motoneuron excitability. The motoneuron excitability is influenced by the basal ganglia dysfunction and primary intracortical damage which are further dependent on copper toxicity in WD.Reference Bembenek, Kurczych and Czlonkowska1–Reference Hefter, Roick and Von Giesen4, Reference Kobayashi and Pascual-Leone8, Reference Trompetto, Avanzino and Marinelli9 Therefore, chelation therapy may decrease the concentration of circulating copper and improve the clinical abnormalities and basal ganglia dysfunction. This is further supported by a previous study that has demonstrated an inverse relationship between copper concentration and cortical excitability.Reference Tecchio, Assenza, Zappasodi, Mariani, Salustri and Squitti10 It is likely that adequate chelation may improve the RMT by decreasing the copper content and its detrimental sequelae, at least when adequately and timely treated. Furthermore, the changes in the RMT paralleled the changes in GAS-WD score both in treatment defaulters and in subjects who adhered to the chelation regimen. The worsening of RMT and GAS-WD score observed in two subjects while on regular treatment may be due to penicillamine-induced worsening.Reference Roberts and Schilsky5 Hence, our study demonstrates that longitudinal measurement of RMT may reflect the clinical status of the subject and may have clinical relevance in the evaluation of chelation efficacy in individual subjects. In contrast to our findings, few studies have not observed improvement in RMT following chelation therapy.Reference Bembenek, Kurczych and Czlonkowska1–Reference Perretti, Pellecchia, Lanzillo, Campanella and Santoro3 However, their conclusions were based on the follow-up measurements of one patient in Jhunjhunwala et al. and three subjects in Perretti et al.Reference Jhunjhunwala, Prashanth, Netravathi, Nagaraju and Pal2, Reference Perretti, Pellecchia, Lanzillo, Campanella and Santoro3 Nonetheless, it is possible that the timing and adherence to therapy may be key factors influencing improvement in RMT.
In contrast to RMT, lack of improvement in CMCT in our study may suggest irreversible damage to the CST. Previous neurophysiological studies using TMS in different diseases have demonstrated the marked prolongation of CMCT to indicate the loss of neurons or demyelination changes.Reference Bembenek, Kurczych and Czlonkowska1–Reference Hefter, Roick and Von Giesen4, Reference Kobayashi and Pascual-Leone8 However, mildly prolonged CMCT in our cohort probably may suggest a limited neuronal loss than demyelination.Reference Kobayashi and Pascual-Leone8, Reference Yook, Park, Ko and Seo11, Reference Jaiser, Barnes, Baker and Baker12 The involvement of CST in WD has been established by previous clinical, neurophysiological, and neuroimaging studies.Reference Bembenek, Kurczych and Czlonkowska1, Reference Hefter, Roick and Von Giesen4–Reference Kim, Kim and Kim6 However, reversibility of CST involvement is not well known. Longitudinal TMS studies by Perretti et al. (three subjects) and Hefter et al. (six subjects) have reported improvement in CMCT with adequate chelation.Reference Perretti, Pellecchia, Lanzillo, Campanella and Santoro3, Reference Hefter, Roick and Von Giesen4 The causes for this apparent disparity in our results are uncertain. However, it should be noted that, although not statistically significant, a majority of patients had an improvement of CMCT value from the baseline measurement in this study. It is possible that reversibility of CST involvement may be dependent on how early chelation therapy is instituted.
To conclude, this study evaluated longitudinal TMS changes in WD and demonstrated the reversible nature of copper-induced neurophysiological changes. Our results imply the reversible nature of cortico-motoneuronal dysfunction of the motor cortex and potentially irreversible nature of CST involvement in WD. However, comprehensive and prospectively designed studies on larger cohorts of patients are necessary to validate our findings.
Statement of Authorship
AS: Conception, execution, organization of the study, statistical analysis, and writing of first draft.
NK: Conception, execution of study, and writing of first draft.
KJ: Conception,organization, and execution of the study.
SP: Organization and critical review.
PKP: Conception, design, organization of the study, review, and Critique.
Disclosures
AS, KJ, NK, SP, and PKP have nothing to disclose.







