Background
Difficulties with motor skills can have serious consequences for a child's independence and daily functioning (Van der Linde et al., Reference Van der Linde, van Netten, Otten, Postema, Geuze and Schoemaker2015) and these negative effects can persist into adulthood (Kirby et al., Reference Kirby, Williams, Thomas and Hill2013; Kirby et al., Reference Kirby, Sugden and Purcell2014).
Difficulties with coordinated movement are often seen in combination with other neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). For example, it has been estimated that up to 50% of children with developmental coordination disorder (DCD), a neurodevelopmental disorder characterised by functional difficulties with coordinated movement, also possess a diagnosis of ADHD, usually of the inattentive subtype (Kadesjö and Gillberg, Reference Kadesjö and Gillberg1999; Kaiser et al., Reference Kaiser, Schoemaker, Albaret and Geuze2015). Individuals with DCD also often show problems in neurocognition, particularly in executive functioning (Wilson et al., Reference Wilson, Ruddock, Smits-Engelsman, Polatajko and Blank2013).
A range of genomic disorders such as those caused by sub-microscopic deletions or duplications of chromosomal regions including 1q21.1, 16p11.2 or 22q11.2 have been associated with the development of conditions such as ADHD, ASD, schizophrenia and intellectual disability (Torres et al., Reference Torres, Barbosa and Maciel2016; Chawner et al., Reference Chawner, Owen, Holmans, Raymond, Skuse, Hall and van den Bree2019). These chromosomal abnormalities are termed copy number variants (CNVs), as they change the number of copies of genes contained on the affected area of the chromosome. While there is strong evidence that many CNVs are associated with a high risk of developing neurodevelopmental disorder (ND), including psychopathology (referred to hereafter as ND-CNVs), penetrance is often incomplete, and expressivity is variable. This means that while some individuals will display many complex symptoms, others may show few or none (Crawford et al., Reference Crawford, Bracher-Smith, Owen, Kendall, Rees, Pardiñas, Einon, Escott-Price, Walters, O'Donovan, Owen and Kirov2018).
Previous research by our group has found that ~80% of children with 22q11.2 Deletion Syndrome (22q11.2DS) show poor coordination of movement (Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018). Our findings also indicated that the children who showed poorer motor coordination had higher risks of ADHD, ASD and anxiety symptoms and lower mean intelligence quotient (IQ). However, there is very little research into coordination difficulties in children with other ND-CNVs. It is therefore unclear if individuals with other ND-CNVs experience similar coordination difficulties, or if certain ND-CNVs confer greater risk for coordination difficulties than others. Similarly, it is unknown if the links between coordination difficulties and other neurodevelopmental symptoms that we found for 22q11.2DS are also present in other high-risk ND-CNVs. More generally, the links between coordination difficulties and other neurodevelopmental problems are not well understood, but it has been found that CNVs found in individuals with DCD are enriched for neurodevelopmental genes (Mosca et al., Reference Mosca, Langevin, Dewey, Innes, Lionel, Marshall, Scherer, Parboosingh and Bernier2016).
Finally, it is not known to what extent motor coordination disorder mediates the effects of carrying an ND-CNV on subsequent neurodevelopmental impairment. This idea is supported by theories that the early development of motor skills influences the development of other higher cognitive processes (Wilson, Reference Wilson2002). Motor skills develop very early in life and it follows that difficulties with interacting with and exploring one's environment will impact on the development of other skills. For example, it has been suggested that poor motor development will impair the development of skills such as the representation of abstract concepts (Piaget, Reference Piaget1954), mathematics (Giles et al., Reference Giles, Shire, Hill, Mushtaq, Waterman, Holt, Culmer, Williams, Wilkie and Mon-Williams2018) and language ability (Rowe et al., Reference Rowe, Özçalışkan and Goldin-Meadow2008).
With these ideas in mind, we assessed motor coordination, IQ and psychopathology in a large group of children with ND-CNVs in order to test the following hypotheses: (1) children with an ND-CNV will have poorer coordination ability (lower Developmental Coordination Disorder Questionnaire (DCDQ) scores) and will be more likely to have suspected DCD (measured by DCDQ cut-off criteria) than controls. We base this hypothesis on research where neurodevelopmental problems have been found to be associated with increased risk of motor coordination difficulties in non-genotyped samples (Kadesjö and Gillberg, Reference Kadesjö and Gillberg1999; Pratt and Hill, Reference Pratt and Hill2011; Skirbekk et al., Reference Skirbekk, Hansen, Oerbeck, Wentzel-Larsen and Kristensen2012; Kaiser et al., Reference Kaiser, Schoemaker, Albaret and Geuze2015; Sumner et al., Reference Sumner, Leonard and Hill2016), as well as evidence from 22q11.2DS (Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018); (2) motor coordination ability will differ across genotypes, as different ND-CNV's affect different genes in different areas of the genome; (3) poor coordination will be related to increased levels of psychopathology and lower IQ in children with ND-CNVs, similar to the pattern seen in non-CNV populations (Wilson et al., Reference Wilson, Ruddock, Smits-Engelsman, Polatajko and Blank2013; Harrowell et al., Reference Harrowell, Hollén, Lingam and Emond2017) as well as 22q11.2DS (Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018) and (4) the risk of psychopathology and low IQ posed by carrying a ND-CNV is partially indirect, via motor coordination ability, or in other words, motor coordination ability will mediate the relationship between the ND-CNV status and psychopathology and IQ. This hypothesis is supported by findings that appropriate development of motor skills is required for the development of higher-order cognitive skills (Wilson, Reference Wilson2002; Rowe et al., Reference Rowe, Özçalışkan and Goldin-Meadow2008; Giles et al., Reference Giles, Shire, Hill, Mushtaq, Waterman, Holt, Culmer, Williams, Wilkie and Mon-Williams2018).
Methods
Participants
One-hundred and sixty-nine participants with a range of ND-CNVs took part (67% male, median age: 8.88 years, range: 6.02–14.81, see online Supplementary Table S1 for CNVs included), as well as 72 closest-in-age unaffected siblings (controls; 54% male, median age: 10.41 years, range: 5.89–14.75). Families were recruited through UK Medical Genetics clinics as well as word of mouth and the charities Unique and MaxAppeal!. Informed and written consent was obtained prior to recruitment from the carers of the children and recruitment was carried out in agreement with protocols approved by the London Queens Square NRES Committee. Individual ND-CNV genotypes were established from medical records as well as in-house genotyping at the Cardiff University MRC Centre for Neuropsychiatric Genetics and Genomics using microarray analysis. Information about medical comorbidities including congenital heart defects, epilepsy and premature birth, along with medication use were collected by questionnaire.
Functional coordination impairment – the DCDQ
The DCDQ (Wilson et al., Reference Wilson, Crawford, Green, Roberts, Aylott and Kaplan2009) was completed by a single primary caregiver. It is designed to screen for functional motor coordination impairments in children 5–15 years old, and is widely used and validated (Wilson et al., Reference Wilson, Crawford, Green, Roberts, Aylott and Kaplan2009; Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018). In general, lower scores indicate greater coordination difficulties. Items probe fine and gross motor skills. It yields a total score as well as separate scores for three subscales: control during movement, fine motor/handwriting and general coordination. In addition, participants were categorised into those screening positive v. negative for ‘suspected DCD’ based on the DCDQ total score as compared with the appropriate age thresholds (Wilson et al., Reference Wilson, Crawford, Green, Roberts, Aylott and Kaplan2009). The DCDQ was used as a proxy for a more complete motor evaluation and not to primarily diagnose DCD. A diagnosis of DCD would require more in-depth motor assessments, including assessments of functional impairment.
IQ assessment
Full scale, verbal and performance IQ (FSIQ, VIQ and PIQ) was obtained by administering the Wechsler Abbreviated Scale of Intelligence (WASI), which comprises four subtests: matrix reasoning, block design, vocabulary and similarities (Wechsler, Reference Wechsler1999).
Psychiatric assessment
The social communication questionnaire (SCQ) (Rutter et al., Reference Rutter, Bailey and Lord2003) was used to screen for ASD trait symptoms. A score of 15 or greater is considered suggestive of ASD. The SCQ consists of three subscales: repetitive behaviour, social interactions and communication ability which are combined to give a total score.
ADHD, anxiety and ODD symptoms were assessed using the semi-structured Child and Adolescent Psychiatric Assessment (Angold et al., Reference Angold, Prendergast, Cox, Harrington, Simonoff and Rutter2009). The interview was conducted by trained psychologists with the primary caregiver. Interviews were audiotaped, and DSM-5 diagnosis obtained during consensus meetings led by a child and adolescent psychiatrist. We did not consider diagnoses to be mutually exclusive. Anxiety symptoms included any symptom of generalised anxiety disorder, social phobia, specific phobia, separation anxiety, panic disorder with and without agoraphobia, agoraphobia and obsessive-compulsive disorder.
Statistical analysis
To investigate coordination ability we conducted a linear mixed-effect model where the continuous DCDQ total score was predicted by the ND-CNV status with age as a covariate. This model included family membership as a random effect to take into account that the controls were siblings of the children with ND-CNVs.
As a sensitivity analysis, this mixed-effect model was also run with the following additional covariates: maternal education, family income, child gender, the presence of congenital heart defects, epilepsy, premature birth and the presence or absence of child medication use. This allowed us to establish whether these covariates played a role in the relationships between the DCDQ total score and ND-CNV status.
Additionally, we carried out a χ2 test in order to investigate the association between the presence or absence of an ND-CNV (children with an ND-CNV v. sibling controls) and screening positive or negative for suspected DCD.
An ANCOVA was used to investigate the extent to which the DCDQ total score was explained by the ND-CNV genotype. These analyses were based on the ND-CNV groups with 10 or more individuals available (15q11.2 deletion, 15q13.3 deletion, 16p11.2 deletion, 16p11.2 duplication, 1q21.1 deletion, 1q21.1 duplication, 22q11.2 deletion, and 22q11.2 duplication. Age was entered as a covariate. A similar ANCOVA was used to investigate the effect of deletion or duplication of the genetic material across the ND-CNV group, with age.
In order to investigate the relationship between the DCDQ score and psychopathology or IQ in children with ND-CNVs, hierarchical regression models were constructed where the DCDQ total score was predicted first by age, then the relevant psychopathology or IQ variable at the second step. For the analysis including IQ, we ran models with both standardised IQ (i.e. FSIQ, VIQ and PIQ scores) as well as raw IQ subtest scores which are not standardised by age. The latter were included because of the potential for coordination ability to impact on the IQ test performance, particularly in the block design task.
Additionally, mediation analyses (Baron and Kenny, Reference Baron and Kenny1986) were run to investigate whether the relationship between the ND-CNV status and psychopathology or IQ scores was mediated by motor coordination ability, with age as a covariate (Fig. 1). The R package ‘mediation’ v4.4.7 (Tingley et al., Reference Tingley, Yamamoto, Hirose, Keele and Imai2014) was used to conduct the mediation analyses. This provides point estimates of the average causal mediation effects (ACME), average direct effects (ADE) and total effects (ADE + ACME) and the proportion of the total effects that are accounted for by the indirect path (proportion mediated). Confidence intervals (CIs) and p-values were obtained using nonparametric bootstrapping with 5000 simulations (Imai et al., Reference Imai, Keele and Tingley2010a). The results of the analysis can be interpreted in the following ways. If a significant total effect, and direct effect (ADE) but insignificant indirect effect (ACME) are found, the mediator has no effect on the outcome. If a significant total effect, significant indirect effect, but insignificant direct effect is found, the mediator is fully mediating the effect of the independent variable on the outcome. Finally, if a significant total effect and significant direct and indirect effects are found, the mediating variable is a partial mediator of the effect of the independent variable on the outcome. Importantly, if the total effect is not significant, there is no evidence that the independent variable has an effect on the outcome.

Fig. 1. The mediation model we tested to investigate the associations between ND-CNV status (0 = sibling control; 1 = ND-CNV) and outcome, via a direct (path C) and indirect pathway (A, B). Pathways A and B estimate to what extent the link between the CNV status and outcome can be accounted for by an indirect link via a coordination ability (DCDQ Score) mediator.
To validate that the mediation analyses were robust to the mediation path being investigated, a second set of models investigating if FSIQ mediated the effect of the ND-CNV status on psychopathology were also constructed and tested using the same method.
Sensitivity analyses were carried out to ensure that indirect effects were robust to violations of the assumption of sequential ignorability (effect of unmeasured confounding variables). For these sensitivity analyses, the level of confounding due to unmeasured confounders was represented by the correlation between the residuals (error terms) from the models where the mediator and outcome variables are the dependant variable, denoted ρ (rho). If ρ = 0 there is no correlation between residuals which is interpreted as no unmeasured confounding. By varying levels of ρ between values of −1 and +1 we can explore the likelihood that any detected indirect effect is influenced by unmeasured confounders. The key outcome of these sensitivity analyses is the value of ρ where the indirect effect becomes non-significant, giving a measure of how strong the effect of any unmeasured confounding variables would need to be in order to invalidate the estimated indirect effect (ACME) (Imai et al., Reference Imai, Keele and Yamamoto2010b). We used as a rule of thumb that values of ρ over 0.3 indicate that the detected mediation effect is robust to unmeasured confounders.
All analyses were carried out on Mac OSX and R v3.5.3. The number of individuals with information for each assessment may differ, due to not successfully completing assessments.
For all models, individuals with missing data for any variables included in the model were excluded.
Results
Descriptive statistics for the individuals that took part in this study are presented in Table 1. Children with a ND-CNV were younger than the unaffected sibling controls but the proportion of males and females was similar. Due to this, and the fact that the DCDQ total score correlated with age (Spearman's r = 0.26, p < 0.001) in children with an ND-CNV, age was included as a covariate in all analyses. FSIQ scores could not be calculated for 12 individuals with an ND-CNV and three sibling controls, because they did not complete the WASI. A table of correlations between all quantitative variables after regressing out age from the DCDQ score (e.g. IQ and psychopathology symptom counts) is presented in online Supplementary Table S2.
Table 1. Demographic and summary statistics of participants

a Kruskal–Wallis rank sum test.
b Pearson's χ2 test.
c Linear model ANOVA.
Children with an ND-CNV were taking the following medications: sodium valproate (n = 8), methylphenidate (n = 6), carbamazepine (n = 3), risperidone (n = 3), atomoxetine (n = 2), levetiracetam (n = 2), ethosuximide (n = 2), clobazam (n = 1), clobazam (n = 1), lamotrigine (n = 1), fluvoxamine (n = 1), guanfacine (n = 1), fluoxetine (n = 1) and nitrazepam (n = 1). No other relevant medication use was noted. Notably, 54% of children with an ND-CNV had a congenital heart defect, 16% a history of seizures and 54% were born before 37 weeks.
Hypothesis 1 Do DCDQ scores differ between individuals with an ND-CNV and controls?
Children with an ND-CNV had lower DCDQ total and subscale scores than controls (Table 1). A linear mixed model where the DCDQ total score was predicted by the ND-CNV status and age with family membership as a random effect, found that the ND-CNV status was predictive of the DCDQ total score (b = 28.98, p < 0.001), along with age (b = 0.78, p = 0.022). Online Supplementary Table S3 includes mean DCDQ scores across various age and IQ ranges.
Addition of maternal education, family income, child gender, the presence of congenital heart defects, epilepsy, premature birth and child medication use in the mixed effect models as covariates indicated that they were not significant predictors of the DCDQ total score. Therefore, these were not included as covariates in any subsequent analyses.
Rates of suspected DCD were higher in individuals with an ND-CNV than controls. Ninety-one percent (154/169) of children with an ND-CNV and 19% (14/72) of controls screened positive for suspected DCD (OR = 42.53, χ2 = 122.86, p = <0.001). Across ND-CNV genotypes we studied, rates of suspected DCD were generally high. All (100%) children with 15q11.2 duplication, 16p11.2 distal duplication, 16p11.2 duplication, 1q21.1 deletion, 1q21.1 duplication, 22q11.2 distal deletion, deletion of 9q34.3 (Kleefstra Syndrome), TAR deletion and TAR duplication screened positive (online Supplementary Table S1).
Hypothesis 2 Does coordination score differ by genotype?
After including age, the ND-CNV genotype was not a significant predictor of the DCDQ score (F = 1.47, df = 7, p = 0.184, η 2 = 0.069) in those ND-CNV groups with 10 individuals or more. These findings are consistent with the null hypothesis that coordination ability is similar regardless of the ND-CNV genotype. In addition, type of CNV (i.e. deletion (n = 101) or duplication (n = 68) of the chromosomal material) had no effect on the DCDQ score (F = 0.67, df = 1, p = 0.413, η 2 = 0.003).
Hypothesis 3 Is coordination related to psychopathology and IQ in children with an ND-CNV?
Children with ND-CNV's displayed higher levels of psychopathology symptoms than siblings (Table 1). When investigating the links between coordination difficulties and psychopathology within the ND-CNV group, we found that worse coordination ability was associated with a greater number of ADHD and ASD trait symptoms, but not anxiety or ODD symptoms. In all models, age was a significant covariate, with older children having better coordination ability (Table 2).
Table 2. Regression results for the DCDQ score predicted by (A) ADHD symptom counts, (B) ASD trait symptom count, (C) anxiety symptoms and (D) oppositional defiant disorder (ODD) symptoms, with age as a covariate
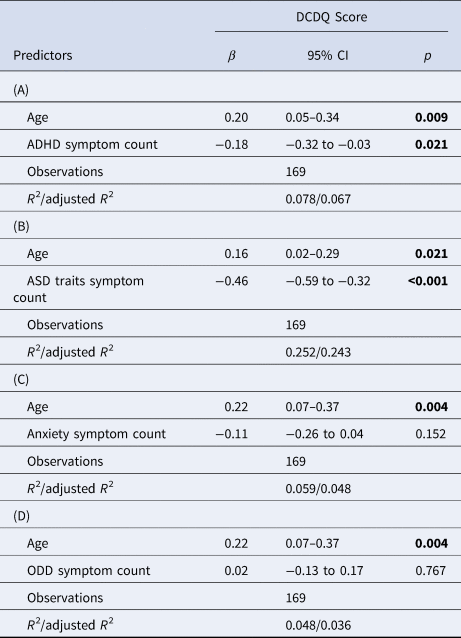
Bolded values indicate significant at p < 0.05.
Average FSIQ, PIQ and VIQ were lower in the ND-CNV group (Table 1). Within children with ND-CNV's, worse coordination was associated with lower FSIQ (β = 0.21, p = 0.011), PIQ (β = 0.20, p = 0.015) and VIQ (β = 0.036, p = 0.036), with age as a significant covariate (online Supplementary Table S4A–C). When investigating if the DCDQ score was associated with raw subtest scores, we found that poorer matrix reasoning (β = 0.18, p = 0.041), but not block design, similarities or vocabulary performance was associated with poorer coordination (online Supplementary Table S4D–G). In the ND-CNV group, there was no difference in DCDQ total scores between individuals with missing FSIQ scores and individuals with complete FSIQ data (W = 715, p = 0.165).
Hypothesis 4 Do coordination difficulties mediate the relationship between ND-CNV group status and psychopathology or IQ?
Mediation analysis indicated that coordination ability (DCDQ total score) was a full mediator of the effect of having an ND-CNV on anxiety symptoms (69% mediated) and a partial mediator of both ADHD symptoms (51% mediated) and ASD trait symptoms (66% mediated). No evidence for mediation was found for ODD symptoms (Table 3).
Table 3. Results of mediation analysis on the effect of having an ND-CNV on (A) ADHD symptom counts, (B) ASD trait symptom count, (C) anxiety symptoms and (D) ODD symptoms with coordination ability as a mediator
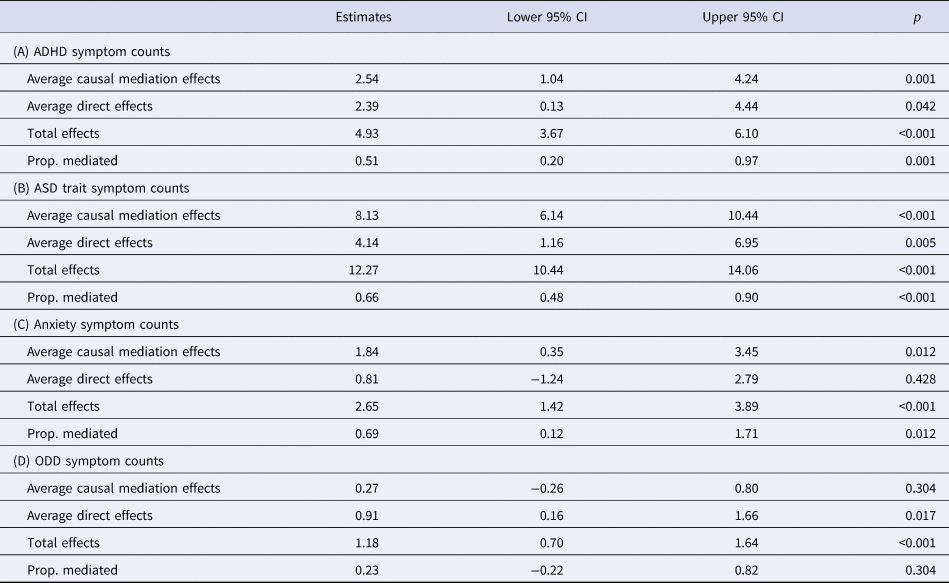
Average causal mediation effects corresponds to the indirect effect through coordination ability, while average direct effects correspond to the direct effect of having an ND-CNV on the psychopathology variable of interest.
Sensitivity analysis indicated that the detected mediation effect of coordination ability on ASD traits was robust to unmeasured confounding variables (ρ > 0.49). However, we found lower values for ρ in mediation models for ADHD (ρ > 0.24) and anxiety symptoms (ρ > 0.16), suggesting the mediation effects in these models may be more sensitive to confounding.
Mediation analysis also revealed that coordination ability was a partial mediator of FSIQ, PIQ and VIQ scores, mediating 40% of the effect of having an ND-CNV on FSIQ and PIQ, and 38% of the effect on VIQ (Table 4). However, we found low values of ρ for these models, ρ > 0.21 for FSIQ, ρ > 0.17 for VIQ and ρ > 0.19 for PIQ, suggesting that these models may be sensitive to confounding.
Table 4. Results of mediation analysis on the effect of having an ND-CNV on (A) full scale IQ, (B) performance IQ and (C) verbal IQ with coordination ability as a mediator
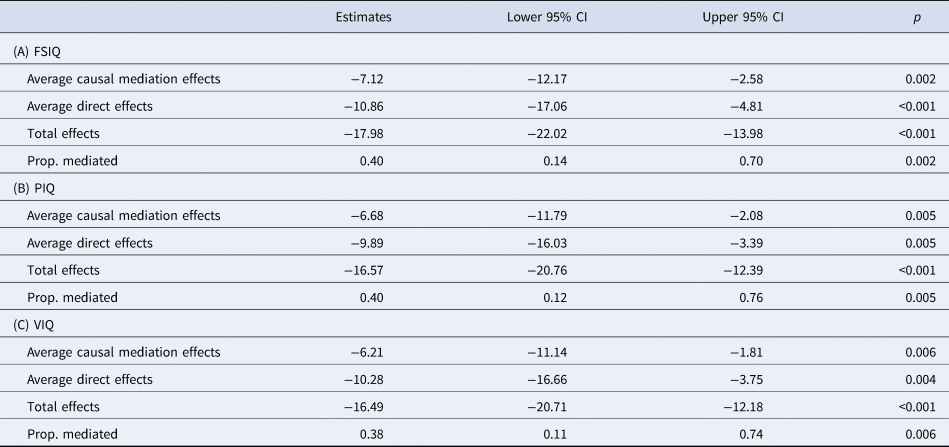
Average causal mediation effects correspond to the indirect effect through coordination ability, while average direct effects correspond to the direct effect of having an ND-CNV on the psychopathology variable of interest.
Previous studies have found no evidence that IQ is associated with levels of psychopathology in children with an ND-CNV (Niarchou et al., Reference Niarchou, Zammit, van Goozen, Thapar, Tierling, Owen and van den Bree2014). In order to validate that the mediation analyses were robust, we constructed a second set of models where FSIQ was included instead of coordination ability as a mediator of the effect of the ND-CNV status on psychopathology. In agreement with the previous findings, we found no evidence that FSIQ was a mediator of ADHD (ACME = 0.25, p = 0.549), ASD traits (ACME = 0.44, p = 0.466), anxiety (ACME = 0.22, p = 0.574) or ODD symptoms (ACME = 0.44, p = 0.478).
Discussion
This study shows that difficulties with coordination are common in children with ND-CNVs, with individuals with an ND-CNV having lower DCDQ total scores than controls, and a large proportion (91%) of children with an ND-CNV screening positive for suspected DCD. We also present evidence that coordination ability is associated with increased ADHD and ASD traits, and lower FSIQ, PIQ and VIQ scores. Coordination difficulties were elevated across all ND-CNV genotypes and genotype or CNV type (deletion or duplication) was not a significant predictor of coordination ability. Importantly, we find that coordination ability (DCDQ total score) is a partial mediator of the effect of ADHD symptoms and ASD traits, along with FSIQ, PIQ and VIQ, and a full mediator of anxiety symptoms, while we found no evidence for mediation of ODD symptoms.
The high rates of coordination difficulties across genotypes, and the lack of specificity of ND-CNV genotype on coordination ability, indicates that neuromotor deficits are a common outcome across ND-CNVs. We found no evidence for an effect of gender on coordination ability, which differs from studies in the general population where DCD is more commonly seen in boys (Tsiotra et al., Reference Tsiotra, Flouris, Koutedakis, Faught, Nevill, Lane and Skenteris2006; Missiuna et al., Reference Missiuna, Gaines, McLean, Delaat, Egan and Soucie2008). It is important to note that rates of premature birth were also high in the ND-CNV group at 54%. Prematurity has been linked to delays in motor development and coordination difficulties later in life (Goyen and Lui, Reference Goyen and Lui2009; Edwards et al., Reference Edwards, Berube, Erlandson, Haug, Johnstone, Meagher, Sarkodee-Adoo and Zwicker2011), but we found no effect of prematurity on the total DCD score. Additionally, while 19% of controls screened positive for suspected DCD, this is within the range of prevalence estimates for developmental coordination disorder in the general population, which range from 2–20%, depending on criteria used (Blank et al., Reference Blank, Barnett, Cairney, Green, Kirby, Polatajko, Rosenblum, Smits‐Engelsman, Sugden, Wilson and Vinçon2019).
Considering links with psychopathology, children with a ND-CNV showed elevated ADHD, ASD trait, anxiety and ODD symptoms compared to controls. Within the ND-CNV group, higher numbers of ADHD and ASD symptoms were associated with greater motor coordination difficulties (lower DCDQ total score), but this was not the case for anxiety or ODD. These results are similar to research in non-genotype selective samples, where high rates of coordination difficulties have been observed in children with ADHD (Watemberg et al., Reference Watemberg, Waiserberg, Zuk and Lerman-Sagie2007) and/or ASD (Sumner et al., Reference Sumner, Leonard and Hill2016), but differ from findings in children with 22q11.2 deletion, where an association with anxiety was found (Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018).
Additionally, the total DCDQ score was found to be a partial mediator of the effect of the ND-CNV status on ADHD symptoms and ASD traits and a full mediator of anxiety symptoms. No evidence for mediation was found for ODD symptoms. These results may suggest that coordination ability is intrinsically linked to the development of ADHD, ASD traits and anxiety symptoms, but not ODD symptoms. However, it is important to note that the identified mediating effects on ADHD and anxiety symptoms may need to be viewed with caution, as the models had reduced robustness against unmeasured confounding.
Importantly, there was no evidence that FSIQ mediated the effect of an ND-CNV on psychopathology. This agrees with previous work in 22q11.2 deletion, where it was found that FSIQ was not associated with levels of psychopathology (Niarchou et al., Reference Niarchou, Zammit, van Goozen, Thapar, Tierling, Owen and van den Bree2014). This helps us validate that our mediation analysis is not prone to detecting false positive mediating effects.
Poor coordination was also found to be associated with FSIQ, VIQ and PIQ in the ND-CNV group. This agrees with previous studies of children with coordination difficulties, not selected due to genotype, and with previous research in 22q11.2DS (Roizen et al., Reference Roizen, Higgins, Antshel, Fremont, Shprintzen and Kates2011; Cunningham et al., Reference Cunningham, Delport, Cumines, Busse, Linden, Hall, Owen and van den Bree2018), where associations between FSIQ and motor performance have been found. The presented results, and previous research in 22q11.2DS, support the idea that within ID populations, the level of intellectual impairment is associated with poorer motor coordination (Vuijk et al., Reference Vuijk, Hartman, Scherder and Visscher2010). It is of note that we found significant associations between coordination ability and raw scores on the matrix reasoning task, while no relationships were found between coordination ability and the block design, vocabulary or similarities tasks. The lack of association between DCDQ total scores and block design performance is somewhat unexpected as this WASI subtest requires movement (e.g. placing blocks in the correct place to complete a pattern). Our finding may suggest that in this population, performance on this task depends more on spatial reasoning than ability to manipulate the blocks.
While associations between vocabulary and gesture performance (a form of coordinated movement) have been found in infants, these relationships have not been thoroughly investigated in older children. We did not find any association between the vocabulary subtest performance and DCDQ total score, and it may be that the relationships between motor skills and verbal ability are weaker in older children. In addition, the vocabulary task does not have a movement component, so is unlikely to be confounded by poor coordination ability.
Mediation analysis also found that coordination ability was a partial mediator of the effect of the ND-CNV status on FSIQ, VIQ and PIQ. This agrees with theories that suggest that motor skills are required for the development of higher order cognitive skills, such as mathematical ability (Giles et al., Reference Giles, Shire, Hill, Mushtaq, Waterman, Holt, Culmer, Williams, Wilkie and Mon-Williams2018), which would be accounted for in PIQ or language development (Rowe et al., Reference Rowe, Özçalışkan and Goldin-Meadow2008) as accounted for by VIQ.
A number of different interpretations of our mediation findings are possible. First, aberrant development of motor coordination skills as a consequence of a ND-CNV may have cascading impacts on later development of other skills, like cognition, attention and social functioning. Second, motor coordination difficulties trigger environmental risk factors, such as social exclusion and bullying, which may increase the risk of psychopathology. Thirdly, both motor coordination problems and impairment in IQ and psychopathology may be the result of the same underlying genetic cause (pleiotropy), although the manifestation of the symptoms may occur at different stages during development. Such pleiotropic effects probably impact on brain development. For example, the cerebellum has been shown to be important in many motor as well as non-motor functions (Miall et al., Reference Miall, Reckess and Imamizu2001; Stoodley, Reference Stoodley2016) and, therefore, damage to this region has cross domain effects. It is also possible that multiple processes account for the findings, possibly at different developmental stages and in different ways for each trait. It is for example noteworthy that we did not find evidence of mediation of motor coordination problems for ODD.
We are not able to further distinguish between these hypotheses in the current study, and the cross-sectional design does not allow for insights into order of appearance of different impairments, but delays in the development of motor skills are often observed from very early in development. It would be fruitful to conduct a randomised control trial where an intervention for motor coordination difficulties is delivered at an early age to a high-risk group of children with ND-CNVs and changes in psychopathology and cognitive function are established.
This is the only study to investigate the relationships between coordination difficulties and psychopathology and IQ in children with CNV's that confer risk for psychiatric disorders. The presence of sibling controls for comparison, and detailed assessment of psychopathology are additional strengths. However, the DCDQ is a measure of overall coordination that is completed by a primary carer, and it therefore cannot allow for direct insights to be gained into underlying sensorimotor deficits that are present in the individual children. In addition, we are unable to distinguish sufficiently between motor difficulties due to a global motor delay, or due to a specific DCD like syndrome. To address these limitations, further research should investigate quality of movement using kinematic and clinical assessments of movement. An additional limitation is that the control group was significantly older than the ND-CNV group. However, this should have had limited effect on screening positive or negative for suspected DCD, as parents are always asked to rate children with respect to their peers, and screening is based on age dependent thresholds. Younger children require lower scores to screen positive than older children, allowing for a degree of internal control for improvements in ability due to age. In addition, age was included as a covariate in all analyses. It was not possible to conduct full physical and neurological assessments on the children. We did include congenital heart defects and epilepsy as covariates in our analyses, but we cannot rule out that other physical or neurological problems may affect coordination.
In summary, we found high rates of coordination difficulties in children with neurodevelopmental risk CNVs which constitute a significant portion of the caseload of clinical geneticists. These problems were elevated across all ND-CNV genotypes and associated with risk of IQ impairment and psychopathology. Furthermore, the association between ND-CNVs and anxiety symptoms was fully mediated by coordination ability, while the association between having an ND-CNV and lower FSIQ, PIQ, and VIQ scores, along with ADHD symptoms and ASD traits was partially mediated by coordination ability. The immediate clinical implication of these findings should be increased vigilance for motor impairments in children with ND-CNVs, so that appropriate support can be introduced as early as possible. It may be that motor interventions could help the development of cognitive skills and reduce risk for the development of psychopathology.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719003210.
Acknowledgements
We are very grateful for all the families that took part in the IMAGINE-ID study. We would like to thank Dr Samuel Chawner for valuable input and help. We would also like to thank the Core Services team at the MRC Centre for Neuropsychiatric Genetics and Genomics. This work was carried out in collaboration with the National Centre for Mental Health, a collaboration between Cardiff, Swansea and Bangor Universities and funded by Health & Care Research Wales (Welsh Government).
Financial support
This research was funded by the Medical Research Council (MR/N022572/1 and MR/L011166/1) and Doctoral Training Grant (MR/K51347/1), by the Baily Thomas Charitable Trust (2315/1), the Waterloo Foundation Changing Minds programme and Waterloo Foundation grant 918–1234, a Wellcome Trust ISSF grant, the National Institute for Mental Health (5UO1MH101724), Wellcome Trust Strategic Award (503147), Health & Care Research Wales (Welsh Government, 507556), Medical Research Council Centre grant (G0801418) and Medical Research Council Programme grant (G0800509).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








