Vitamin A deficiency is a leading cause of morbidity and mortality, especially in young children and pregnant and lactating women(Reference Black, Allen and Bhutta1). Food-based interventions focused on alleviating vitamin A deficiency in susceptible populations have advantages over supplementation and fortification programmes, especially in rural areas, because they can provide a sustainable source of a variety of nutrients and other phytochemicals without the recurring transport and administration costs of these other methods(Reference Sayre, Beeching and Cahoon2).
Cassava is a root vegetable, specifically a starchy tuber, with many positive attributes. It can survive droughts, is inexpensive, resistant to pests and easy to grow. Although it is a valuable source of energy, typically it is a poor source of provitamin A carotenoids(Reference Montagnac, Davis and Tanumihardjo3, Reference Gegios, Amthor and Maziya-Dixon4). Not coincidently, it is a staple crop in three regions where vitamin A deficiency is prevalent: Africa, South America and Southeast Asia(Reference Sayre, Beeching and Cahoon2, Reference Nhassico, Mubquingue and Cliff5).
Recently, multi-national non-governmental organisations (especially HarvestPlus) have enhanced the provitamin A carotenoid content of cassava, either through traditional plant-breeding (HarvestPlus) or bioengineering (BioCassavaPlus)(Reference Sayre, Beeching and Cahoon2, Reference Bouis, Hotz and McClafferty6, Reference Pfeiffer and McClafferty7). Their efforts have resulted in yellow-orange-fleshed cassava cultivars with moderately high concentrations of β-carotene and other provitamin A carotenoids. Only HarvestPlus varieties have been disseminated in countries to date.
Cassava contains cyanogenic compounds that require processing in order to make it safe for consumption. Unfortunately, the type of processing, as well as cooking temperature and time(Reference Bradbury and Denton8), can also decrease the retention of carotenoids(Reference Chavez, Sánchez and Ceballos9, Reference Vimala, Thushara and Nambisan10).
In general, carotenoid bioavailability in food is considered to be low, with the bioconversion rate of β-carotene estimated to be as low as 12 μg to 1 μg retinol(Reference Yeum and Russell11). However, biofortified cassava enriched with provitamin A carotenoids has successfully maintained vitamin A status in Mongolian gerbils(Reference Howe, Maziya-Dixon and Tanumihardjo12). Thus, more information is required on the bioavailability and bioconversion of carotenoids from cassava in human subjects. Also, since provitamin A bioavailability generally increases with the addition of fat(Reference Yeum and Russell11), the effect of consuming varying amounts of fat with biofortified cassava is of interest.
Both common white and provitamin A-enriched cassava were fed to ten healthy well-nourished American women. The objectives of the present study were to estimate the effectiveness of biofortified cassava for increasing provitamin A carotenoid and vitamin A concentrations in healthy adult women, and to determine whether increased fat in the diet improved these concentrations.
Experimental methods
Subjects
A total of twelve healthy, non-smoking, non-pregnant women aged 21–44 years were enrolled in the study. A woman was judged as ‘healthy’ if she had a BMI of 18–30 kg/m2, blood pressure less than 135/90 mmHg and TAG, cholesterol, total protein, electrolytes, kidney and liver function tests (such as blood urea N), Hb, haematocrit, erythrocytes and leucocytes within the clinically normal ranges (Table 1). Exclusion criteria included the use of medications that affect retinoid, carotenoid or cholesterol absorption from food such as fat-, TAG- or cholesterol-lowering medications, medicines containing high dosages of retinoids, vitamin A or carotenoid supplements, illegal drugs or tobacco. In addition, subjects could not be allergic to cassava, peanuts or peanut oil. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of California, Davis Institutional Review Board. Written informed consent was obtained from all subjects.
Table 1 Subject demographics and blood chemistries (Mean values, ranges and standard deviations)

NA, not available.
* Normal range for females.
† Normal range from 10 to 49 years old.
Cassava preparation
Biofortified cassava, cross-bred to contain high amounts of β-carotene (Genotype GM 905-69), was provided by the International Center for Tropical Agriculture (CIAT). GM905-69 was derived from crosses among cassava genotypes from South and Central America that naturally contain little β-carotene, because the African germplasm lacked enough genetic variability to allow for the development of β-carotene-biofortified cassava. Typical unfortified white cassava was purchased from Las Montañas Supermarket. Initial weights of the cassava varieties were 3·44 and 3·38 kg for the biofortified and unfortified white cassava, respectively. Upon arrival at the Western Human Nutrition Research Center, roots were washed, peeled and flash frozen and then stored in a food-safe freezer at − 20°C in the Metabolic Kitchen and Human Feeding Laboratory until use. For preparation, roots were thawed overnight at 4°C and then rinsed twice with deionised water. Tips from the distal and proximal ends were removed (1–2 cm) and discarded and the roots were diced (about 1 cm3). Deionised water (four volumes) was added to the chopped roots and stirred. The roots were then refrigerated overnight for 12 h, after which they were drained to remove cyanogenic glycosides. Another round of four volumes of deionised water was added and subsequently drained after 2 h. This process was repeated every 2 h for 8 h. Following the last draining, the cassava cubes were rinsed with deionised water and lightly simmered (95°C) in ten volumes of deionised water for 30 min. Constant stirring of the cassava helped to ensure its homogeneity and minimised variations in carotenoid concentration. Cooked cassava was drained, cooled and aliquots were stored in 50 ml polypropylene screw-capped tubes wrapped in aluminium foil under N2 at − 20°C in the food-safe freezer. All procedures were conducted under dim lights to minimise light exposure. Fig. 1 shows the biofortified cassava before and after preparation.

Fig. 1 Appearance of biofortified cassava before and after processing, including the drained water after simmering.
Cassava preparations were analysed for cyanogenic glycosides by two methods. Each step of the cassava preparation was monitored by our laboratory using a La Motte colorimetric assay (LaMotte). Cassava preparations (1 g) were placed in a 15 ml test-tube, sliced into small pieces, mashed with a mortar and then mixed with 7 ml deionised water by vortexing for 1 min. Preparations were left at room temperature for 20 min, then vortexed for 20 s and filtered through a 0·2 μm Pall Gelman Acrodisc (Sigma Aldrich) into a LaMotte test-tube. The water extract was tested using the LaMotte Cyanide in Water Test Kit, according to the manufacturer's specifications. For confirmation, the final prepared samples were sent to an outside laboratory (Applied Specialization and Consulting LLC) and analysed using alkaline hydrolysis, distillation and ion chromatography with pulsed amperometric detection.
Dietary protocols
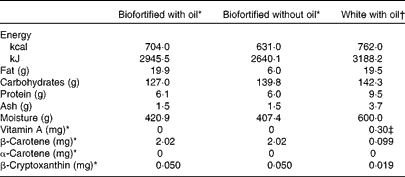
Each subject consumed three randomised dietary interventions of cassava porridge separated by 2-week washout periods. Before each intervention, subjects were required to eat a diet low in vitamin A and carotenoids (Table 2). On the first 4 d, subjects were instructed on how to reduce their dietary intake of provitamin A carotenoids and vitamin A (the dietary guidance for the subjects is provided as Supplementary material, available online) and were required to complete a 4 d food record to assess their adherence with the dietary restriction. On the 3 d leading up to each intervention, subjects were provided with nutrient-controlled research meals and required to eat only from what was provided to them, but were not required to eat everything. The nutrient-controlled meals were designed by a registered dietitian and were prepared in the Metabolic Kitchen of the Western Human Nutrition Research Center, then given to the subjects for reheating. The meals were low in fat, protein, carotenoids and vitamin A. Nutrient data were calculated using the Nutrition Data System for Research (The Nutrition Coordinating Center, University of Minnesota) software. The United States Department of Agriculture Nutrient Data Laboratory is the primary source of the nutrient values and nutrient composition in this database, which contains information on over 18 000 foods. A total of five aliquots were collected from each of the test meals and analysed for vitamin A and provitamin A content. The extraction procedure used was similar to that described by Howe et al. (Reference Howe, Maziya-Dixon and Tanumihardjo12). Briefly, approximately 1 g homogenised cassava was mixed with 6 ml ethanol (containing butylated hydroxytoluene 0·05 % (w/v)) and 120 μl potassium hydroxide (80 % (w/v)) and saponified at 60°C for 10 min, before 3 ml deionised water were added and it was extracted twice with 3 ml hexane. The diet consumed on the 3 d prior to the test day contained 107·8 μg β-carotene, 181·7 μg β-cryptoxanthin and 0 μg α-carotene and retinol by analysis (Table 3). Total calculated energy was 9113 kJ (2178 kcal), with 67·7 g protein (12 % of energy), 24 g fat (9·7 % of energy) and 428·9 g carbohydrate (78·1 % of energy) (Table 3).
On each test day, subjects consumed one of the following randomised porridge meals for breakfast: (1) β-carotene-biofortified cassava porridge with oil, containing 100 g drained cassava, 15 ml added rapeseed or peanut oil (20 g total fat) and approximately 2 mg β-carotene; (2) biofortified cassava porridge without added oil (6 g total fat); (3) unfortified white cassava porridge with added rapeseed or peanut oil (20 g total fat) containing a reference dose of 0·3 mg pure food-grade retinyl palmitate. The test meals were consumed under supervision by the Metabolic Kitchen and Human Feeding Laboratory staff.
We attempted to make this a double-blind study, wherein the rest of the foods in the porridge masked the differences in colour between yellow-orange biofortified and white unfortified cassava. However, small differences in the colour of the porridges could be detected by a trained eye. Furthermore, adding oil changed the viscosity of the porridge slightly. Therefore, the present study can best be called a single-blind study, with the researchers blinded, conducted under controlled conditions.
The cassava porridge consisted of 100 g cassava, 40 g quick-cooking unfortified oatmeal (Quaker Oats), 150 g canned pears in light syrup (Dole), 0·5 g salt (Sysco), 21·0 g honey (Sue Bee), 18·9 g raisins (Sysco) and 245·5 g unfortified rice milk (Hain Celestial Group). For the first two subjects, 14·0 g peanut oil was added to the test meals that included additional oil. However, subjects complained about the taste and viscosity of the porridges prepared with peanut oil (Hain Celestial Group) and had difficulty eating them. Subsequently, the peanut oil was replaced by 14·0 g rapeseed oil (Sysco) and added to the test meals. Food-grade retinyl palmitate, kindly donated by Kazi Jamil of the International Centre for Diarrhoeal Disease Research, was diluted in peanut oil. Each of the porridges was prepared immediately prior to consumption.
A proximate analysis was performed on each of the biofortified cassava porridges (Covance), but was calculated for the unfortified white cassava porridge using the Nutrition Data System for Research (The Nutrition Coordinating Center, University of Minnesota) software (Table 2). Macronutrient and micronutrient concentrations for Fe, phytate, vitamin C and other micronutrients and food constituents of interest were also estimated with the Nutrition Data System for Research (The Nutrition Coordinating Center, University of Minnesota) software (Table 3). The three test meals were served in random order. An evening meal that was low in fat, vitamin A and provitamin A carotenoids was fed 9.5 h after the test meal. The evening meal provided the same nutrient specification as the foods provided during the 3 d before the test.
Table 2 Composition of cassava porridge meals (per 100 g)
* As analysed.
† As calculated using Nutrition Data System for Research (The Nutrition Coordinating Center, University of Minnesota) software.
‡ Vitamin A was added in the form of a reference dose of retinyl palmitate.
Table 3 Estimated macro- and micronutrient composition for run-in menu, test day meal excluding porridge and for white cassava porridge alone*

* Values by calculation using the Nutrition Data System for Research version 2009 (The Nutrition Coordinating Center, University of Minnesota). Data are based on typical (non-biofortified) white cassava.
† Vitamin A was added in the form of a reference dose of retinyl palmitate, but was not included in this table.
Isolation of postprandial lipoprotein fraction
Throughout all blood processing procedures and analyses, laboratory workers were blinded to the dietary treatment received by the subject. Blood was typically collected by a catheter inserted into an anticubital vein. Approximately 30 min before the porridge test meal, the catheter was inserted and a baseline sample of 13 ml blood was collected. Baseline blood was used for complete blood count analysis performed on a CellDyn 3200 (Abbott Laboratories), as well as for carotenoid and vitamin A tests. Postprandial blood samples (10 ml) were collected at 2, 3·5, 5, 7·25 and 9·5 h after the test meal. After the 9·5 h blood draw, the catheter was withdrawn.
Blood was transferred into EDTA vacutainers, wrapped in foil to protect from light and placed on ice. Plasma was separated by centrifugation at 1300 g for 10 min at 4°C on a Sorvall DuPont RC-3C (Thermo-Fisher Scientific). All plasma handling was carried out under gold fluorescent lights in order to protect these compounds.
The TAG-rich lipoprotein (TRL) fraction, containing newly absorbed carotenoids and retinyl palmitate, was separated from plasma by ultracentrifugation(Reference van Vliet, Schreurs and van den Berg13). A 1 ml aliquot of plasma was overlaid with NaCl salt solution (density = 1·006 kg/l) in 2·2 ml polyallomer ultracentrifuge tubes (Beckman Coulter, Inc.). Samples were ultracentrifuged at 100 000 g for 20 min at 4°C in a Beckman Coulter Optima TLX (Beckman Coulter, Inc.), with the use of a swing-out rotor-type Beckman TLA 100 (Beckman Coulter, Inc.). Tubes were removed from the ultracentrifuge, placed in a Beckman Centritube Slicer (Beckman Coulter, Inc.) and sliced at a fixed position. These procedures resulted in a reproducible TRL fraction consisting of chylomicrons and large VLDL. Approximately 100 μl of the plasma TRL fraction was removed and dispensed into a 15 ml test-tube, along with 100 μl of echinenone as an internal standard, and immediately deproteinised with 1 ml methanol before it was extracted twice with 1 ml hexane. The hexane layers were dried under N2 and reconstituted in 100 μl 90:10 (v/v) methanol–isopropanol. TAG, cholesterol and HDL concentrations were measured at each blood draw time point using a clinical chemistry analyser (Integra 400 Plus, Roche Diagnostics), while LDL concentrations were measured by difference.
Analytical procedures
Food samples and the plasma TRL fraction were analysed by HPLC using reversed-phase chromatography with coulometric array electrochemical detection. Carotenoids and retinoids were separated by an ESA MD-150 column (150 mm × 3·2 mm; Dionex (ESA)(14)). The HPLC consisted of an ESA model 582 solvent delivery system, 542 autosampler and 5600 Coularray electrochemical detector, with a CH30 Eppendorf column heater (Eppendorf). The gradient mobile phases used were: solvent A (methanol–0·2 m-aqueous ammonium acetate, 90:10 (v/v), pH 4) and solvent B (methanol–isopropanol–1 m aqueous ammonium acetate, 78:20:2 (by vol.), pH 4). The column was maintained at 37°C throughout. The following gradient was used: the mobile phase was maintained at 0 % solvent B from 0 to 10 min, before increasing linearly from 10 to 20 min to 80 % B and from 20 to 27 min to 100 % B. It was then abruptly changed to 0 % B at 27 min and maintained until the runtime ended at 32 min. Cell potential settings were 200, 400, 500 and 700 mV. Flow rate was 0·8 ml/min and the injection volume was 20 μl. All samples were analysed in duplicate. ESA Coularray software version 3.1 (Thermo Fisher Scientific) was used to collect and integrate all chromatographic data. β-Carotene and echinenone responded predominantly at 400 mV and retinyl palmitate at 700 mV.
Methanol, isopropanol and ammonium acetate were purchased from Thermo Fisher Scientific. The calibration standards β-carotene, retinol and retinyl palmitate were purchased from Sigma-Aldrich and β-cryptoxanthin and α-carotene standards were purchased from Santa Cruz Biochemicals. Echinenone, an internal calibration standard, was purchased from Carotenature. A pooled plasma sample purchased from UTAK was used to evaluate inter-assay precision of the plasma TRL fraction. Inter-assay precision ranged between 5 and 11 % for carotenoids and retinoids.
Data analysis
Areas under the concentration–time curve (AUC) were calculated using trapezoidal approximation after subtracting initial fasting concentrations for retinyl palmitate and TAG, while the AUC for β-carotene was calculated by subtracting the unfortified white cassava control group concentrations at each time point.
Many retinyl esters in the plasma TRL fraction were too low in concentration to quantify accurately. As the postprandial retinyl ester profile is relatively constant, retinyl palmitate, the most common retinyl ester, can be used to estimate total retinyl ester formation(Reference Berr and Kern15, Reference Li, Nugroho and Rocheford16). With retinyl palmitate absorption typically ranging between 75 and 99 %(Reference Sivakumar and Reddy17, Reference Biesalski18), we assumed a mid-range recovery of 90 % retinyl palmitate.
To quantify the bioavailability of β-carotene from the cassava porridges, fractional absorption was calculated as described by O'Neill & Thurnham(Reference O'Neill and Thurnham19). Absorption calculations were estimated using the assumption that the t 1/2 of β-carotene, retinyl palmitate and chylomicrons were equivalent (0·192 h)(Reference Cortner, Coates and Le20, Reference Goodman, Blomstrand and Werner21). The plasma volume (ml) = 927+(31·47 × body weight in kg)(Reference Grundy and Mok22) and the molecular mass was 536·9 for β-carotene.
The percentage of β-carotene absorbed was calculated in two ways using the sum of β-carotene plus retinyl palmitate AUC(Reference van Vliet, Schreurs and van den Berg13, Reference O'Neill and Thurnham19). The first calculation assumed that 1 mol of β-carotene formed 1 mol of retinyl palmitate, which would happen by eccentric cleavage(Reference Harrison23). The second calculation assumed central cleavage of β-carotene, the major path of retinyl palmitate formation. In central cleavage, 2 mol of retinyl palmitate are formed from 1 mol of β-carotene(Reference Harrison23).
To quantify bioconversion, vitamin A equivalence was calculated as described by Li et al. (Reference Li, Nugroho and Rocheford16). The retinyl palmitate AUC values were converted to mass (nmol) retinyl palmitate in the entire plasma pool by multiplying the TRL retinyl palmitate concentration (nmol/l plasma) by the calculated plasma volume (0·0427 litres × kg body weight) of each subject(Reference Boer24, Reference Tang, Qin and Dolnikowski25).
Vitamin A (nmol) formed from the biofortified cassava = (retinyl palmitate AUC after ingestion of the biofortified cassava/retinyl palmitate AUC after ingestion of the white cassava with retinyl palmitate reference dose) × the vitamin A reference dose of 1047·1 nmol.
Bioconversion factor for β-carotene in biofortified cassava = β-carotene equivalents in biofortified cassava (nmol) × molecular weight (MW) β-carotene (536·8)/vitamin A formed from β-carotene equivalents (nmol) × MW retinol (286·5).
Differences in AUC and plasma TRL fraction values were analysed using repeated-measures ANOVA, while vitamin A equivalence differences were analysed using paired Student t tests. One-tailed t tests were used for a priori hypotheses, including the greater values of β-carotene and retinyl palmitate expected in the biofortified meal with oil v. without oil and the higher levels of β-carotene expected in the biofortified meals v. the non-fortified white cassava. Two tailed t tests were used when there was no hypothesis regarding the direction of the effect. P values < 0·05 were considered to be statistically significant. Statistical analyses were performed using Statistical Analysis Systems statistical software (Windows version 9.3; SAS Institute, Inc.).
Results
Subject characteristics
Healthy premenopausal women with normal BMI, cholesterol and TAG were recruited for the present study. Their blood chemistries and demographic characteristics are shown in Table 1. Although the cassava meals were large (approximately 34 % of a 8368 kJ (2000 kcal) diet), ten of twelve subjects completed the study and only one left because of portion size. BMI, cholesterol and TAG concentrations did not change during the study.
Two women left the study prior to completion, one for scheduling conflicts, while the other disliked the porridge portion size served. Table 1 shows the demographic characteristics of the subjects who completed the study.
Composition of test meal
Biofortified cassava had pale cream-orange coloured flesh before processing and thus appeared to be an unpromising food source for enhancing carotenoids. However, gentle simmering appeared to release carotenoids from the food matrix, resulting in a yellow-orange appearance (Fig. 1).
The carotenoid concentrations and proximate analysis measurements for the biofortified cassava porridges are shown in Table 2. The macro- and micronutrient composition of these porridges and of the entire run-in and test day meals was also calculated with Nutrition Data System for Research (The Nutrition Coordinating Center, University of Minnesota) software (Table 3). The composition of these meals did not differ substantially, except for the increase of fat in the biofortified cassava with oil (BFO) test meal. The meals provided were low in vitamin A, provitamin A carotenoids and fat, as planned.
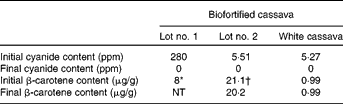
Two lots of cassava were shipped from CIAT, 4 months apart. The first lot was used for method development and the second for the intervention study. Despite being from the same cultivar, they had substantially different carotenoid and cyanogenic glycoside concentrations. The β-carotene concentration of the first lot was 8·0 μg/g fresh weight, while that of the second lot was 21·1 μg/g after transporting, freezing and thawing. Cassava roots were not waxed during shipment or storage, and the second lot appeared to have lost moisture, with a higher percentage dry weight (40.9 %) and carotenoid concentration than typical. The β-carotene concentrations for the second lot of cassava during processing are shown in Table 4. Despite the developing yellow-orange colour brought on by simmering, the β-carotene concentration in cassava actually decreased to 20·2 μg/g, a loss of 4 % during processing and cooking.
Table 4 Comparison of change in β-carotene and cyanide concentrations before and after processing
ppm, Parts per million; NT, not tested.
* Fresh weight.
† Weight after transport, storage, freezing and thawing.
The small amount of β-carotene in the white cassava appeared to decrease less than 1 % during these procedures. These relatively small losses were probably due to the precautions taken to preserve β-carotene content during processing, such as heating the cassava to a temperature that resulted in only a slight simmer and later reheating it for the least amount of time necessary to reach minimal reheating temperature of 74°C just prior to feeding.
The initial cyanide content of the biofortified cassava for the first lot was 282 parts per million (ppm) and 5·5 ppm for the second lot, a 50-fold difference. Low cyanogenic glycoside concentrations were also found in the locally purchased white cassava (5·3 ppm). The washing procedures developed for the present study removed almost all of the cyanogenic glycosides from the cassava. The effects of all processing procedures, including transportation, freezing and thawing, storage, washing and simmering, on cyanogen concentrations are also shown in Table 4. Both testing methods showed non-detectable cyanide concentrations in our final prepared products (by both the in-house test and the confirmatory testing by Applied Specialization and Consulting).
Postprandial TAG response
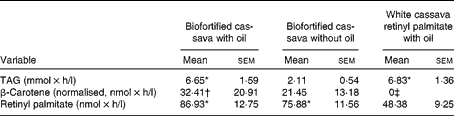
The difference in TAG AUC after consuming the high-fat BFO (P< 0·0002) and unfortified white cassava porridge with retinyl palmitate (WC+RP; P< 0·0001) meals was significant compared with the low-fat (BF, biofortified cassava without oil) meal (Table 5). After consuming the BFO and WC+RP meals, plasma TRL fraction concentrations (nmol/l) rose within 2 h and maintained high concentrations for several hours before decreasing (Fig. 2).
Table 5 AUC values for TAG, β-carotene and retinyl palmitate in TAG-rich lipoprotein layer after consumption of cassava porridges (Mean values with their standard errors)
* Mean value was significantly different from the biofortified cassava without oil meal (P< 0·0002).
† Mean value was significantly different from the white cassava meal (P= 0·05).
‡ Not detectable.

Fig. 2 Change in TAG content in plasma TAG-rich lipoproteins after subjects ingested biofortified cassava with oil (![]() ) containing 20 g total fat, biofortified cassava without oil (
) containing 20 g total fat, biofortified cassava without oil (![]() ) containing 6 g total fat or an unfortified white cassava meal with oil (retinyl palmitate,
) containing 6 g total fat or an unfortified white cassava meal with oil (retinyl palmitate, ![]() ) containing 20 g total fat. Values are means, with their standard errors represented by vertical bars.
) containing 20 g total fat. Values are means, with their standard errors represented by vertical bars.
Postprandial β-carotene and retinyl palmitate response
The chromatography method used in the present study was sensitive enough to measure β-carotene and retinyl palmitate in the TRL fraction of plasma, but could not quantify all the less common retinyl esters and carotenoids found in plasma (data not shown). The TRL fraction had properties characteristic of chylomicrons and large VLDL(Reference Berr, Eckel and Kern26–Reference Brown, Ferruzzi and Nguyen30). This is appropriate, as recently consumed β-carotene and retinyl palmitate are found in both of these lipoprotein fractions(Reference Li, Nugroho and Rocheford16, Reference Hu, Jandacek and White28, Reference Paetau, Chen and Goh31).
The plasma TRL fraction β-carotene concentrations were greater in the BFO meal than in the WC+RP meal (P= 0·05; Table 5). However, the BF meal was non-significantly different from the WC+RP meal (P= 0·16). β-Carotene concentrations were highest in the BFO meal, as expected, but the effect of added oil compared with the BF meal was not significant (P< 0·4). The high β-carotene concentration after consuming the BFO meal at 2 h suggests that added oil may have resulted in faster absorption and metabolism of the β-carotene in the biofortified cassava (Fig. 3).
Retinyl palmitate concentrations increased with all treatments, because a retinyl palmitate reference dose was included in the WC+RP meal (Fig. 4). The retinyl palmitate AUC for the BFO and BF meals were significantly greater than that of WC+RP (P< 0·02 and P< 0·05, respectively; Table 5). The AUC for the BFO and BF meals were compared with baseline (as retinyl palmitate would not be expected to be generated by WC alone, without the retinyl palmitate reference dose). Both increased significantly (P< 0·0001 for both). The retinyl palmitate AUC for the BFO meal did not differ from the BF meal (P< 0·35).
If we calculate the fractional absorption rate of β-carotene and retinyl palmitate assuming eccentric cleavage, it was 33·6 % for the BFO meal and 27·4 % for the BF meal. Assuming central cleavage, fractional absorption was 21·4 % for the BFO meal and 16·7 % for the BF meal.
The mean vitamin A equivalence values for each of the biofortified cassava meals are shown in Table 6. The mean amount of β-carotene in the biofortified cassava, with the vitamin A activity equivalent of 1 μg retinol, ranged from 0·3 to 10·6 and 1·4 to 12·1 μg for the BFO and BF meals, respectively. The vitamin A equivalence values for the BFO and BF meals was non-significantly different (P< 0·44).

* Calculated as μg of β-carotene equivalents in biofortified cassava without oil cassava/μg of retinol formed.
† The present US Institute of Medicine retinol activity equivalents state that 12 μg of food-derived β-carotene equivalents form 1 μg retinol(Reference Yeum and Russell11).
Discussion
The present study measured the effect of meals containing β-carotene-biofortified cassava on the plasma TRL fraction response of β-carotene and retinyl palmitate in healthy, well-nourished women. It used ultracentrifugation to isolate the plasma TRL fraction, which allows one to separate and measure newly absorbed β-carotene and newly formed retinyl palmitate to evaluate the bioavailability and bioconversion of β-carotene from biofortified cassava in human subjects. One important result from the present study is that feeding biofortified cassava increased β-carotene and retinyl palmitate in the plasma TRL fraction of these women. A second important result is that β-carotene from biofortified cassava, with or without added oil, was efficiently converted into vitamin A, with an average vitamin A equivalence of 4·4:1.
The inclusion of fat in a meal can increase carotenoid bioavailability(Reference Yeum and Russell11). However, there is considerable debate about the effect of different concentrations of fat on carotenoid absorption(Reference Yeum and Russell11). In the present study, we compared the effects of a low-fat meal (6 g) with a higher-fat meal (20 g). Similar to other high-fat v. low-fat meal studies, the higher amount of fat included in the BFO and WC+RP meals resulted in a significantly higher TAG AUC in the TRL plasma fraction compared with the low-fat meal(Reference Brown, Ferruzzi and Nguyen30). Despite this result, there was no significant difference in the AUC for β-carotene or retinyl palmitate after consuming the higher-fat BFO meal (v. the lower-fat BF meal). However, the AUC for β-carotene was 66 % higher in the BFO meal (v. the BF meal). Thus, the present results are somewhat equivocal, as they suggest a real difference that did not attain statistical significance because of our small number of subjects and the large variation in β-carotene concentrations commonly observed in human studies. The results from other investigations on the effect of different amounts of fat on carotenoid absorption are also mixed. Some studies indicate that only a small amount of fat (3–5 g) is required to substantially increase carotenoid bioavailability(Reference Jalal, Nesheim and Agus32, Reference Jayarajan, Reddy and Mohanram33) and that β-carotene bioavailability does not significantly improve when consumed with a high-fat v. a low fat meal(Reference Roodenburg, Leenen and van het Hof34). Other studies show improved absorption of β-carotene with high fat v. low fat intake(Reference Brown, Ferruzzi and Nguyen30, Reference Dmitrov, Meyer and Ullrey35). Further studies are necessary to determine the effects of adding fat to the diet.
Interestingly, although the two biofortified cassava meals had similar bioavailability overall, the rate at which β-carotene appeared in the plasma TRL fraction appeared to be different (Fig. 3). However, the difference at this time point was not statistically significant (P< 0·21), presumably because of the large variation in β-carotene concentrations in the group and the low number of subjects. Although other studies have reported rapid β-carotene appearances in the plasma TRL fraction(Reference van Vliet, Schreurs and van den Berg13, Reference Cardinault, Tyssandier and Grolier36–Reference van den Berg and van Vliet38), their peak concentrations appeared later. It is possible that the increased amount of fat in the BFO resulted in β-carotene being packaged into chylomicrons and secreted out of the enterocyte at a faster rate, as TAG concentrations also increased rapidly (Fig. 2). Another potential influence on the absorption results for the present study was the processing procedure. In order to remove cyanide, the cassava was chopped, lightly heated in water and cooled before being re-heated prior to serving. This may be relevant because the chopping and heating involved in this procedure effectively disrupts plant cell walls and carotenoid–protein complexes and results in increased bioavailability(Reference Khachik, Beecher and Goli39, Reference Dietz, Kantha and Erdman40).
The fractional absorption rates that we determined for the BFO and BF meals are somewhat difficult to compare with past human studies, because most of these studies had subjects consuming high-dose β-carotene supplements given in oil, which tend to be better absorbed than carotenoids from food(Reference van Vliet, Schreurs and van den Berg13, Reference O'Neill and Thurnham19, Reference Hu, Jandacek and White28). Nevertheless, fractional absorption rates seen in these studies ranged from 2·5 to 22·3 % for eccentric cleavage. The only human study measuring fractional absorption of β-carotene in food used red palm oil and had an absorption of 65–68 %(Reference van den Berg and van Vliet38).
Furthermore, even when pure supplements are given, absorption and conversion of β-carotene to vitamin A are variable(Reference Borel, Grolier and Mekki41–Reference Lin, Dueker and Burri43), in part because of polymorphisms in the β,β-carotene-15,15′-monoxygenase gene(Reference von Lintig44). Although the β-carotene and retinyl palmitate AUC values in the present study were low, they are similar to other studies using food-derived β-carotene(Reference Li, Nugroho and Rocheford16, Reference Muzhingi, Gadaga and Siwela45).
The vitamin A equivalence of the biofortified cassava was 4·2:1 for the BFO meal and 4·5:1 for the BF meal. This is comparable to a study that estimated vitamin A equivalence to be 3·7:1 in vitamin A-depleted Mongolian gerbils(Reference Howe, Maziya-Dixon and Tanumihardjo12), an appropriate small animal model for human provitamin A absorption and metabolism(Reference Lee, Boileau and Boileau46). In addition, a human study that measured β-carotene and retinyl palmitate response in the TRL plasma fraction estimated biofortified cassava to have a vitamin A equivalence of 2·8:1 (W. Liu, unpublished results). This efficient conversion is also similar to that seen in other β-carotene-biofortified foods, which appear to have higher bioconversion rates than natural sources of this compound(Reference Tanumihardjo, Palacios and Pixley47). For example, studies in human subjects, mostly involving stable isotopes, have estimated the vitamin A equivalence of β-carotene in biofortified maize and rice to range from 3:1 to 6·5:1(Reference Li, Nugroho and Rocheford16, Reference Muzhingi, Gadaga and Siwela45, Reference Tang, Qin and Dolnikowski48), while natural sources of β-carotene have ranged from 10:1 to 28:1(Reference Tang, Qin and Dolnikowski25, Reference de Pee, West and Permaesih49–Reference Tang53). These results indicate a more efficient conversion than the present estimate (12 μg β-carotene:1 μg retinol from food)(Reference Yeum and Russell11).
The efficiency of β-carotene bioconversion appears to be influenced by vitamin A status(Reference Tanumihardjo, Palacios and Pixley47). The subjects in the present study were well-nourished and probably had more than adequate reserves of vitamin A. The absorption of β-carotene from biofortified cassava might be greater in healthy people with marginal vitamin A status. Furthermore, studies in target populations of Africans or Asians with marginal vitamin A status might produce even stronger results than those seen in the present study (Figs. 3 and 4).

Fig. 3 β-Carotene concentration in plasma TAG-rich lipoproteins after subjects ingested either 2 mg β-carotene from biofortified cassava with oil (BFO, ![]() ) or biofortified cassava without oil (BF,
) or biofortified cassava without oil (BF, ![]() ). The unfortified white cassava, containing a negligible amount of β-carotene, was used as a control and its concentrations at each time point were subtracted from the BFO and BF groups. Values are means, with their standard errors represented by vertical bars.
). The unfortified white cassava, containing a negligible amount of β-carotene, was used as a control and its concentrations at each time point were subtracted from the BFO and BF groups. Values are means, with their standard errors represented by vertical bars.

Fig. 4 Change in retinyl palmitate content in plasma TAG-rich lipoproteins after subjects ingested 2 mg β-carotene from biofortified cassava with oil (![]() ), biofortified cassava without oil (
), biofortified cassava without oil (![]() ) or an unfortified white cassava meal low in β-carotene with oil but containing a 0·3 mg retinyl palmitate (
) or an unfortified white cassava meal low in β-carotene with oil but containing a 0·3 mg retinyl palmitate (![]() ) reference dose. Values are means, with their standard errors represented by vertical bars.
) reference dose. Values are means, with their standard errors represented by vertical bars.
The biofortified cassava used in the present study contained β-carotene concentrations similar to those observed in other varieties of biofortified cassava, which have concentrations as high as 2·55 mg/100 g fresh weight(Reference Iglesias, Mayer and Chavez54). Therefore, the present results may be extrapolated to other biofortified cassava varieties. Given its substantial β-carotene concentrations, efficient bioconversion and the high dietary intake of cassava in many African countries (310–820 g/d)(Reference Montagnac, Davis and Tanumihardjo3), current varieties of biofortified cassava may be suitable for food-based interventions(Reference Iglesias, Mayer and Chavez54).
That being said, there remain issues with biofortified cassava that should be addressed before it can be promoted as a vitamin A food source and incorporated into food-based interventions. For example, cyanide concentrations in cassava can potentially reach toxic concentrations, typically ranging from 10 to 500 mg cyanide equivalents/kg dry weight(Reference Montagnac, Davis and Tanumihardjo55). Nevertheless, our observation of a 50-fold variation in cyanogenic glycoside concentration is surprising, given that the two lots were from within the same cultivar. Variations may be due to differences in the plants' age and variety or environmental factors(Reference Ubalua56). This is important information, because biofortified cassava is likely to be grown in a variety of conditions and climates, which might influence both the carotenoid and cyanide concentrations of these plants. The present results indicate that concentrations of cyanogenic glycosides can vary significantly even in the same variety of biofortified cassava, suggesting that great care should be taken during its preparation.
The cassava processing method that we used to remove cyanide was developed so that we could control the cyanide removal process while retaining carotenoids. It is more elaborate than most methods used by consumers of cassava and requires a large amount of water. Furthermore, although the cyanide content of the biofortified cassava from lot 2 probably could have been removed by simple soaking, roasting or drying, the high cyanide concentration of lot 1 would have required more extensive processing, such as prolonged soaking in running water or garification. Eating the cassava from lot 2 with minimal preparation would be safe, but consuming the cassava from lot 1 would have been inadvisable.
Unfortunately, these procedures may negatively affect β-carotene concentrations. Although the mild processing techniques used in the present study resulted in a relatively low loss of β-carotene (4 %), studies testing more common processing procedures, such as sun-drying, boiling, frying and gari preparation, show decreases in β-carotene concentration ranging from 5·5 to 78·5 %(Reference Chavez, Sánchez and Ceballos9, Reference Vimala, Thushara and Nambisan10). Therefore, when attempting to lower cyanide content, the effects of processing on the β-carotene content of biofortified cassava must also be considered.
Even so, the present results are promising. These results indicate that present cultivars of biofortified cassava may be an effective component of food-based interventions in vitamin A deficiency in populations who consume this food as a staple part of their diet.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512005004
Acknowledgements
We thank the Cassava Study team of the Western Human Nutrition Research Center, especially Ellen Bonnel, Evelyn Jenner, Emma White, Debra Standridge, Joe Domek, Jerome Crawford, Sara Stoffel, Julie Edwards, Bill Horn and Delphine La Porte, as well as Marjorie Haskell, Jan Peerson, Lacey Baldiviez and Charles Stephensen. The US department of Agriculture (USDA) is an equal opportunity provider and employer. We also thank Hernan Ceballos of CIAT for providing the biofortified cassava. The present work was supported by HarvestPlus grant 8227; and was made possible by grant no. 2UL1RR024146 from the National Center for Research Resources, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research. Additional support was provided by the Western Human Nutrition Research Center in-house funds, CRIS project no. 5306-51530-018–23T. The authors have no conflicts of interest. B. J. B., L. R. W., D. J. B. and M. R. L. F. designed the research; L. R. W., D. J. B. and M. R. L. F. conducted the research and analysed data; B. J. B. and M. R. L. F. wrote the paper; B. J. B. had primary responsibility for final content. All authors read and approved the final manuscript.














