There is growing awareness that gut microbiotas have a substantial influence on human health and disease. Both animal and human studies have shown that gut microbial metabolism of dietary trimethylamines produces trimethylamine-N-oxide (TMAO)( Reference Koeth, Wang and Levison 1 – Reference Koeth, Levison and Culley 3 ), a metabolite associated with risk of CVD, independent of traditional CVD risk factors( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 , Reference Tang, Wang and Levison 4 ). Studies from this group have also established that elevated plasma levels of carnitine, choline and betaine are associated with CVD risk because of their role in formation of TMAO( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 , Reference Wang, Tang and Buffa 5 ). More recently, another precursor of TMAO, γ-butyrobetaine, was shown to be associated with the development of atherosclerosis in a susceptible mouse model( Reference Koeth, Levison and Culley 3 ). Together, these studies have fuelled interest in the potential for dietary modification to alter TMAO production( Reference Boutagy, Neilson and Osterberg 6 – Reference Rohrmann, Linseisen and Allenspach 8 ). Given the obligatory role of gut microbes in the conversion of trimethylamine-containing nutrients to TMAO( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 ), it is of interest to determine whether dietary components associated with changes in gut microbial communities affect plasma concentrations of TMAO.
Dietary starches differ in their rates of digestion and absorption. Compared with most starches, resistant starches (RS) undergo limited digestion by α-amylases in the small intestine, but may be converted by amylolytic bacterial species in the colon to a range of metabolites including SCFA( Reference Birt, Boylston and Hendrich 9 ). Differing forms of RS have been shown to rapidly alter the composition of the human gut microbiota( Reference Abell, Cooke and Bennett 10 – Reference Walker, Ince and Duncan 12 ). In view of this, and because production of TMAO is dependent on gut microbes( Reference Wang, Roberts and Buffa 13 ), we undertook a study to determine whether diets that differed in RS content affected plasma concentrations of TMAO, and to test whether any such effect was modified by total dietary CHO. In addition, we sought to confirm the attenuation in postprandial glucose and insulin responses by high RS intake( Reference Behall, Scholfield and Canary 14 – Reference Sands, Leidy and Hamaker 18 ), and to examine changes in plasma lipids and lipoproteins whose associations with RS intake are less well established( Reference Behall and Howe 15 , Reference Behall, Scholfield and Yuhaniak 16 ).
Methods
Study participants
In all, fifty-two individuals (thirty-two women, twenty men) were recruited from participants of our previous dietary intervention studies and respondents to advertisements on the Internet. The sample included men (>20 years) and post-menopausal women (defined as ≥43 years of age and amenorrhoea for ≥3 years or amenorrhoea for ≥1 but <3 years and plasma follicle-stimulating hormone concentrations elevated to the postmenopausal range) with BMI ≥20 and ≤35 kg/m2. All of them were non-smokers, had no history of CVD or other chronic diseases, and were not taking lipid- or glucose-lowering medications, blood thinning agents or hormones. Moreover, to permit testing of the insulin-lowering effects of RS, we also excluded individuals with relatively high insulin sensitivity as assessed by the homoeostatic model assessment of insulin resistance (HOMA-IR) <50th percentile( Reference Bravata, Wells and Concato 19 ) (based on HOMA-IR distributions of a comparable group of men and women screened for a previous study( Reference Chiu, Williams and Dawson 20 ); median: 2·1). Additional selection criteria included fasting glucose <7 mmol/l, total and LDL-cholesterol ≤90th percentile for age and sex, fasting TAG<5·65 mmol/l, blood pressure (BP) <150/90 mmHg, stable weight (<3 % change) for at least 3 months before study onset and willingness to refrain from alcohol and dietary supplements during the study period.
The study protocol was approved by the Institutional Review Board of Children’s Hospital and Research Center of Oakland. All participants gave their written informed consent to take part in the study. Participants were provided with a list of clinical staff to contact should they need to discuss study procedures or report adverse events. This trial was registered at clinicaltrials.gov as NCT01027325.
Study design and diets
The present study was a controlled, randomised, cross-over dietary intervention conducted in an outpatient setting with weekly visits to our clinic located in Berkeley, CA. The logistical constraints of creating twenty-five different diets (five experimental diets, at five energy levels) required that the first twenty-six subjects enrolled into the study be assigned to the higher-CHO study arm and the second twenty-six to the lower-CHO study arm. Within each diet, a uniform random number generator was used to determine block sizes (two, four, six or eight subjects) and the sequence of the high- v. low-RS diets within each block, which were supplied to the project coordinator in sealed, numbered envelopes. The project statistician was the only person aware of the treatment assignment before subject enrolment.
The study design consisted of two arms: higher and lower total CHO intake with comparison of high RS v. low RS intake in random order in each arm (Fig. 1). All study participants (n 52) first consumed the lower-CHO baseline diet for 2 weeks, after which they followed the higher-CHO diet (the first twenty-six subjects recruited) or the lower-CHO diet (the second twenty-six subjects recruited). The high- and low-RS diets were each consumed for 2 weeks, separated by a 2-week washout, during which they were instructed to consume their habitual diet for 7 d, followed by repeating the baseline diet for an additional 7 d (Fig. 1). Clinic staff met with participants weekly to review and reinforce dietary patterns and ensure that body weight remained within ±3 % of initial weight. Investigators, laboratory staff and study participants were blinded to the dietary assignment, whereas staff responsible for provision of food and monitoring of dietary compliance (nutritionist, study coordinator and nurse) was not.
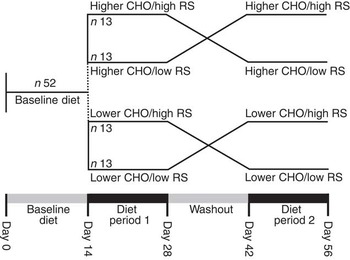
Fig. 1 Study design. CHO, carbohydrate; RS, resistant starch.
The lower-CHO baseline diet was designed to match the macronutrient distribution of the lower-CHO study arm, but to be low in foods containing naturally occurring RS in order to facilitate limitation of RS intake. High- and low-RS contents of the diets (Table 1) were achieved by incorporating, respectively, a high-amylose maize starch (41·5 g RS/100 g starch, Hi-Maize 260; Ingredion Inc.) or a conventional, high-amylopectin maize starch (2·3 g RS/100 g starch, Melojel; Ingredion Inc.) into recipes. The resulting high-RS diets provided 19 g RS/4184 kJ (1000 kcal) for the lower-CHO study arm and 26 g RS/4184 kJ (1000 kcal) for the higher-CHO study arm, for an average daily intake of 48–66 g RS. These amounts are within ranges previously shown to affect human faecal microbiota composition( Reference Abell, Cooke and Bennett 10 – Reference Walker, Ince and Duncan 12 ) as well as glycaemic control( Reference Behall and Hallfrisch 22 ).
Table 1 Composition of baseline and experimental dietsFootnote †
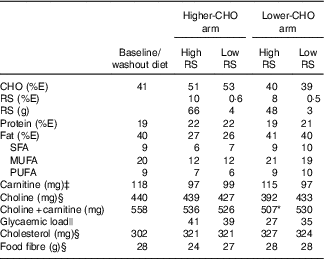
CHO, carbohydrate; RS, resistant starch; %E, percentage of energy.
* P<0·05 compared with all other diets.
† Values shown are for 10 460 kJ (2500 kcal) menus.
‡ Estimated values, based on published carnitine content of commonly consumed foodsReference Demarquoy, Georges and Rigault (21) .
§ Calculated values, Nutrition Data System for Research (University of Minnesota).
|| Calculated values, ProNutra software (Viocare Technologies Inc.). Data were analysed by ANOVA for a cross-over design. %E for macronutrients is based on compositional analysis (Covance Laboratories) and represents an average for 3-d cycle menus (days 1, 3 were consumed twice per week; day 2 was consumed three times per week).
Although rapidly digested maize starch was consumed mostly cooked, in baked goods and entrees, approximately 50 % of the high-RS maize starch was consumed raw, mixed into beverages, fruit purees and soups (online Supplementary Table S1). Hi-Maize 260 maize starch was chosen on the basis of its high RS content and because such starches resist losing their granular structure under the range of processing conditions typically used to prepare conventional food products. Maintenance of granular structure and starch polymer association during cooking make high-amylose starch resistant to enzymatic degradation( Reference Ratnayake and Jackson 23 ). Melojel maize starch was chosen on the basis of its low RS content and because it is widely used in conventional food products.
Dietary control was achieved by provision of standard entrées for home consumption and by having weekly meetings with nutritionists and clinic staff to ensure compliance with the study protocol. Specifically, the clinic provided two standardised entrées (lunch and dinner) during the baseline diet and three standardised meals (entrée, side dish, beverage and occasional dessert) and one to three snacks per day, contributing to approximately 80 % of daily energy content. Detailed menus and checklists were provided for the remaining food items that participants were required to purchase (dairy products, fresh produce, fruit juice), mostly during the baseline and low-RS diets. Participants were instructed on procedures to store and, where applicable, thaw and reheat study foods to minimise changes in starch digestibility due to processing. Diets and menus were developed and prepared by the Bionutrition Core of the University of California, San Francisco Clinical and Translational Science Institute. The nutrient composition of the diets was assessed using Nutrition Data System for Research Software (NDSR 2010; Nutrition Coordinating Center, University of Minnesota) and ProNutra software (version 3.3; Viocare Technologies Inc.). Compositional analysis of the menus was validated by Covance Laboratories. Body weight was measured weekly, and energy intake was adjusted when weight fluctuated by more than ±3 % of baseline. Participants were required to abstain from alcohol and dietary supplements during the study. The staff nutritionist used menu checklists, grocery receipts and information gathered from weekly interactions to assign a compliance score (1–5-point scale, where 5 is indicative of high compliance) for each study participant.
Fasting blood samples were collected on two consecutive days following completion of the baseline diet (days 13, 14) and at the end of each diet period (days 27, 28 and days 55, 56). A 3-h meal tolerance test was administered on days 28 and 56 (the concluding day of each experimental diet) with blood samples collected in the fasting state and 0·5, 1, 2 and 3 h after consumption of a meal representative in macronutrient composition to the assigned experimental diet. The different high- and low-RS meals were designed to correspond to the CHO content of the lower- or higher-CHO study arms (40 % v. 52 percentage of energy (%E) as CHO), and to provide one-third of the daily allotted energy and starch (e.g. 16 and 22 g of RS/meal for the high-RS diet for a subject consuming 10 460 kJ/d (2500 kcal/d); and 0·8 and 1·1 g RS/meal for the low-RS diet for a subject consuming 10 460 kJ/d (2500 kcal/d)).
Laboratory measures
Quantification of resistant starch
The RS content of the test starches was analysed by a modified AOAC method 2009.01, using a Megazyme K-INTDF assay kit (Megazyme International Ireland Ltd).
Plasma measurements
Plasma was prepared from blood samples obtained by venepuncture after an overnight fast, and collected in tubes containing Na2EDTA (1·4 g/l) and a preservative cocktail containing sodium azide, chloramphenicol succinate, gentamicin sulphate, PPACK dihydrochloride and aprotinin. Blood and plasma samples were maintained at 4°C until further processing.
Trimethylamine-N-oxide, choline, betaine, γ-butyrobetaine and carnitine
Analyses were performed in plasma samples stored at −80°C using a stable-isotope dilution HPLC with online electrospray ionisation tandem MS (LC/ESI/MS/MS)( Reference Koeth, Wang and Levison 1 , Reference Wang, Levison and Hazen 24 ). In brief, four volumes of methanol containing 10 μm-TMAO-trimethyl-d9 (d9-TMAO), betaine-trimethyl-d9 (d9-betaine), choline-trimethyl-d9 (d9-choline), γ-butyrobetaine-trimethyl-d9 (d9-γ-butyrobetaine) and carnitine-trimethyl-d9 (d9-carnitine) were added to plasma as internal standard to precipitate protein. Following centrifugation, the supernatant was collected for LC/MS/MS assay. Supernatants (10 μl) were analysed by injection into a silica column (4·6×250 mm, 5 µm Luna silica; cat. no. 00G-4274-E0; Phenomenex) at a flow rate of 0·8 ml/min interfaced with an API 5000 MS (AB SCIEX). Precursor–product ion transitions at m/z 76→58, m/z 104→60, m/z 118→59, m/z 146→60, m/z 162→60, m/z 85→66, m/z 113→69, m/z 127→68, m/z 155→69 and m/z 171→69 were used for TMAO, choline, betaine, γ-butyrobetaine, carnitine, d9-TMAO, d9-choline, d9-betaine, d9-γ-butyrobetaine and d9-carnitine, respectively. Increasing concentrations of TMAO, choline, betaine, γ-butyrobetaine and carnitine standards were spiked to control plasma to generate calibration curves with the y-axis as the peak area ratio to their respective internal standards for determining plasma concentrations of TMAO, betaine, choline, γ-butyrobetaine and carnitine, respectively.
Glucose, insulin and lipids
Plasma insulin concentrations were measured by an ELISA (EZHI-14K Human Insulin ELISA kit; Millipore). HOMA-IR was calculated from plasma insulin and glucose concentrations (insulin (mU/l)×glucose (nmol/l)/22·5)( Reference Bravata, Wells and Concato 19 ).
Total plasma cholesterol, TAG, HDL-cholesterol and glucose concentrations were measured enzymatically on a Liasys 330 Clinical Chemistry System (AMS Diagnostics), and LDL-cholesterol was calculated using the Friedewald formula( Reference Friedewald, Levy and Fredrickson 25 ). Quality control of lipid measurements was maintained through the standardisation programme of the Centers for Disease Control-National Heart, Lung and Blood Institute. Plasma apo B and apo AI were analysed on the same machine by immunoturbidimetric assays using the ITA reagent kit (Bacton Assay Systems)( Reference Rifai and King 26 , Reference Smith, Cooper and Henderson 27 ).
Particle concentrations of VLDL, intermediate-density lipoprotein and LDL subfractions in plasma were determined by ion mobility (IM) as described previously( Reference Caulfield, Li and Lee 28 ). This method uniquely allows for direct particle quantification following brief ultracentrifugation in D2O to remove albumin. The IM instrument uses an electrospray to create an aerosol of particles that pass through a dynamic mobility analyzer coupled to a particle counter. Particle numbers are measured in pre-specified particle diameter intervals and converted to plasma particle concentrations (nmol). LDL diameter is also measured at the peak of LDL particle distribution( Reference Caulfield, Li and Lee 28 ).
Faecal DNA extraction and sequencing
Faecal samples were collected at the end of each dietary intervention (high RS and low RS) from sixteen participants assigned to the higher-CHO study arm (2×16=32 faecal samples) and from twenty-three participants assigned to the lower-CHO study arm (2×23=46 faecal samples). From these samples, total genomic DNA was extracted in duplicate using the MoBio PowerSoil DNA extraction kit with additional heat lysis for 5 min at 60°C (MoBio Laboratories). PCR were used to amplify DNA, using the F515/R806 primer to target the V3/V4 region of the 16 S rRNA gene, and the reverse primer construct also contained a twelve-base error-correcting Golay code( Reference Caporaso, Lauber and Walters 29 ). 16 S rRNA was sequenced as described in the online Supplementary Methods. Sequence data were analysed using the Quantitative Insights into Microbial Ecology pipeline version 1.7( Reference Caporaso, Kuczynski and Stombaugh 30 ), as described in the online Supplementary Material.
Statistical analysis
On the basis of published data comparing high- v. low-RS diets( Reference Behall and Howe 15 , Reference Behall, Scholfield and Yuhaniak 16 ), a sample size of fifty-two participants was estimated to provide 80 % power (5 % significance) to detect a significant metabolic effect of RS, as manifest by 15 and 50 % changes in postprandial insulin and glucose responses (AUC), respectively, a 19 % change in plasma TAG, and a 13 % change in small dense LDL between the high- v. low-RS diets.
Statistical analyses were performed using ANOVA and cross-over experiments procedure of Stata 11.1 (StataCorp LP). The effects of high v. low RS were estimated by ANOVA for a cross-over design that involved the random assignment of subjects to high and low RS, and included effects due to RS, CHO and their interaction. These analyses also tested effects of dietary sequence (i.e. high- following low-RS diets v. low- following high-RS diets), and no significant diet order effects were observed for any of the measures of response (data not shown). The analyses were repeated within each CHO condition for a simple cross-over design that included only RS effects. Log-transformation of data that were not normally distributed (carnitine, choline, insulin, HOMA-IR, TAG and HDL-cholesterol) did not affect the results.
Results
Participant retention and baseline characteristics
A total of fifty-two participants (twenty men and thirty-two women) completed the study. The flow diagram of participant recruitment and withdrawal is illustrated in Fig. 2. On average, these individuals were middle aged (mean 44 (sd 14) years), normotensive (systolic BP: 119 (sd 14) mmHg, diastolic BP: 70 (sd 8) mmHg), and overweight or obese as characterised by their BMI (31 (sd 2) kg/m2), body fat (38 (sd 7) %) and /or waist circumference (107 (sd 8) cm, men; 101 (sd 7) cm, women). HOMA-IR ranged from 2·1 to 20·4 at screening (mean 3·87 (sd 2·90); median: 3·0).

Fig. 2 Participant enrolment and withdrawal. CHO, carbohydrate; RS, resistant starch; GI, gastrointestinal; PI, principal investigator.
With the exception of baseline plasma carnitine levels, which were lower in those randomised to the lower-carbohydrate diet, baseline characteristics of participants did not differ significantly between those in the lower- and higher-CHO study arms (Table 2). Adjustment for differences in baseline plasma carnitine levels between high- and low-CHO groups did not affect microbiome metabolite responses to diets high v. low in RS.
Table 2 Baseline characteristicsFootnote * (Mean values and standard deviations; n 10 males and 16 females in each diet group)
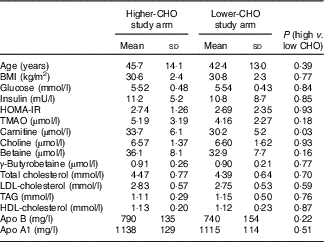
CHO, carbohydrate; HOMA-IR, homoeostatic model assessment of insulin resistance (insulin (mU/l)×glucose (nmol/l)/22·5).
* Data were analysed by ANOVA for a cross-over design.
Compliance with dietary protocol
Participants were highly compliant with the dietary protocol, with nutritionist-reported mean compliance scores of 4·7 (sd 0·7) (on a scale of 1–5). Self-reported gastrointestinal symptoms and perceived satiety during high and low RS intake were consistent with high dietary adherence, with significantly increased frequency of flatulence, fullness, loss of appetite and burping and significantly increased intensity of abdominal cramps, flatulence and fullness after the high-RS diets (online Supplementary Fig. S1). High RS intake also increased the reported number of weekly bowel movements in participants assigned to the higher-CHO diets (12 (sd 7) bowel movements/week with high RS and 9 (sd 5) bowel movements/week with low RS, P=0·005).
Documentation of adverse events
No serious adverse events were reported (online Supplementary Table S2).
Gut microbiome derived metabolites
Fasting plasma carnitine, betaine, γ-butyrobetaine and TMAO concentrations were significantly higher after the high- v. low-RS diet in the lower-CHO treatment arm (Table 3), but not the higher-CHO treatment arm (P>0·38 for all metabolites), resulting in a significant CHO by RS interaction for these metabolites. Plasma choline concentration was not significantly affected by starch digestibility. Additional analyses showed that plasma TMAO levels were not correlated with the sum of plasma choline and carnitine, both dietary precursors of TMAO (P=0·53).
Table 3 Plasma concentrations of carnitine, choline, betaine, γ-butyrobetaine and trimethylamine-N-oxide (TMAO) after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)Footnote * (Mean values and standard deviations)

* Data were analysed by ANOVA for a cross-over design.
Gut microbial taxa associated with plasma trimethylamine-N-oxide concentrations
Faecal samples were collected in a subgroup of thirty-nine participants for microbial community analysis. Consistent with findings in the group as a whole (n 52), plasma TMAO concentrations were significantly higher after the high- v. low-RS diets (4·35 (sd 2·35) and 3·14 (sd 1·74) μm, respectively; P=0·0008) in these thirty-nine participants. Microbial community analysis of these samples identified significant positive and negative correlations between multiple taxa and plasma TMAO levels (Fig. 3, P<0·05 for all taxa).

Fig. 3 Pearson’s correlations between relative abundance of taxa and plasma trimethylamine-N-oxide (TMAO) concentrations. An OTU table was filtered at a minimum depth of 5000 sequences per sample, summarised at the genus level, and filtered to exclude genera less than 0·05 % abundant. Relative abundances of taxa were correlated to TMAO values for each sample.
Fasting and postprandial insulin and glucose
Although high- and low-RS diets did not affect fasting concentrations of insulin and glucose (Table 4), the high-RS test meals produced significantly lower postprandial insulin and glucose responses, expressed as incremental AUC (IAUC), compared with low-RS test meals (Table 4 and online Supplementary Fig. S2). These differences were largely due to the differential effect of RS on the 0·5-h postprandial glucose response (P=0·0001) and the 1-h postprandial insulin response (P=0·007).
Table 4 Body weight and plasma insulin, glucose and lipid concentrations after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)Footnote * (Mean values and standard deviations)

IAUC, incremental AUC.
* Data were analysed by ANOVA for a cross-over design.
Consuming higher-CHO compared with lower-CHO test meals acutely did not affect the IAUC for glucose, but resulted in significantly higher postprandial insulin responses at all time points after the meal (online Supplementary Fig. S2), expressed as IAUC (P=0·001, Table 4). There were no significant CHO by RS interactions for postprandial glucose or insulin responses (P=0·49 and P=0·66, respectively).
Fasting lipids and lipoproteins
With the exception of plasma TAG and large VLDL particles, which were increased by high- v. low-CHO diets (P=0·02 and P=0·002, respectively), fasting plasma lipids, lipoproteins and apoproteins were not affected by starch digestibility or the amount of CHO in the diet (Tables 4 and 5).
Table 5 Total mass concentrations of plasma lipoprotein subfractions after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)Footnote * (Mean values and standard deviations)

IDL, intermediate-density lipoprotein; LDL ppd, LDL peak particle diameter.
* Data were analysed by ANOVA for a cross-over design.
Body weight
Changes in body weight were minimal (Table 4), although a reduction with low v. high RS in the low-CHO arm (87·7 (sd 12·8) v. 88·3 (sd 12·8) kg) was significant at P<0·05. Adjustment for change in body weight did not significantly affect the gut microbiome-derived metabolite or glycaemic and lipoprotein responses to the high- and low-RS diets (data not shown).
Discussion
We report that intake of dietary RS can modulate circulating levels of TMAO, a metabolite that is associated with increased future risk of major cardiovascular events( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 , Reference Tang, Wang and Levison 4 ). The production of TMAO is dependent on gut microbes and arises from dietary precursors such as choline, carnitine, phosphatidylcholine and γ-butyrobetaine, which are first converted by colonic bacteria to trimethylamine( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 ). Trimethylamine is then absorbed and rapidly oxidised to TMAO by hepatic flavin mono-oxygenases( Reference Bennett, de Aguiar Vallim and Wang 31 ).
The association of TMAO with CVD has been ascribed in part to inhibition of reverse cholesterol transport, changes in cholesterol and bile acid metabolism, and increased macrophage foam cell formation( Reference Koeth, Wang and Levison 1 , Reference Wang, Klipfell and Bennett 2 ). In more recent studies, TMAO has also been linked to development of vulnerable plaque, both through activation of arterial endothelial cells( Reference Seldin, Meng and Qi 32 ), and through a direct effect on intracellular Ca signalling in platelets, promoting a prothrombotic phenotype( Reference Zhu, Gregory and Org 33 ). Notably, in a susceptible mouse model, inhibition of microbial trimethylamine production from choline was recently shown to reduce plasma levels of TMAO and to inhibit the development of atherosclerotic lesions( Reference Wang, Roberts and Buffa 13 ). Moreover, in two different mouse models of atherosclerosis, anti-sense oligonucleotide targeting of hepatic flavin mono-oxygenase 3 has been shown to similarly inhibit TMAO formation and development of atherosclerosis( Reference Shih, Wang and Lee 34 , Reference Miao, Ling and Manthena 35 ).
Resident gut micro-organisms are rapidly modulated by variation in intake of starches, and these changes may vary in conjunction with differences in starch digestibility( Reference Abell, Cooke and Bennett 10 – Reference Walker, Ince and Duncan 12 ). It is known that starches that are relatively resistant to intestinal digestion are subject to fermentation by amylolytic bacterial species that reside in the colon( Reference Ze, Duncan and Louis 36 , Reference Macfarlane and Englyst 37 ). Consistent with this property of RS and the fact that the bacterial production of trimethylamine occurs primarily in the colon( Reference Koeth, Wang and Levison 1 ), we found that plasma levels of TMAO were significantly increased by high v. low RS intake, although this effect was dependent on total dietary CHO. Specifically, the TMAO-raising effect of RS was observed with a CHO intake of 39–40 %E, whereas with CHO intake >50 %E levels of TMAO were yet higher and independent of RS content. This finding suggests that both higher CHO intake alone and high RS intake alone are sufficient to promote the production of trimethylamine by the colonic microbiota, and that both must be reduced in order to attenuate this process.
It is unlikely that the increase in plasma TMAO with the high-RS diet was due to higher dietary intake of carnitine and choline. In fact, the sum of dietary carnitine+choline, both dietary precursors of TMAO, was slightly lower for the high-RS diet v. low-RS diet in the lower-CHO study arm. Moreover, the sum of plasma choline+carnitine levels was not correlated with plasma TMAO levels. Rather, our findings suggest that differential effects of high v. low RS on gut microbial composition led to increased TMAO concentrations with high RS intake.
In keeping with the obligatory role of gut microbiota in producing TMAO( Reference Koeth, Wang and Levison 1 ), analysis of the microbial composition of faecal samples showed that the proportions of certain taxa were correlated with plasma TMAO levels. Although recent studies have delineated biochemical processes involved in the microbial conversion of choline and carnitine to TMAO( Reference Craciun and Balskus 38 , Reference Zhu, Jameson and Crosatti 39 ), little is known of the diversity of microbial taxa that can contribute to this process. One recent study examined seventy-nine human microbial isolates spanning several common phyla observed in the human gut and identified several human commensals with TMA-producing activity( Reference Romano, Vivas and Amador-Noguez 40 ). Although it is not possible from the present results to determine the contribution of specific microbial communities to the diet-induced changes in TMAO levels observed here, it is intriguing that some that were inversely correlated with TMAO change – namely, Lachnospiraceae and Clostridiales (Fig. 3) – were also recently found to be associated with lower plasma TMAO levels in mice( Reference Zhu, Gregory and Org 33 ). Finally, the extent to which products of RS fermentation (e.g. SCFA) may have influenced the associations of these microbial communities with TMAO requires further study – for example, by examination of these associations in conjunction with measurements of faecal fatty acids and other metabolites at multiple time points after RS feeding.
In agreement with earlier observations( Reference Behall, Scholfield and Canary 14 – Reference Sands, Leidy and Hamaker 18 , Reference Behall and Hallfrisch 22 ), we report significantly attenuated insulin and glucose responses to meals providing 16–22 g RS. A strength of our study is that this was observed in the context of physiological meals that were balanced and matched for fat, protein and food fibre, which can markedly affect the digestion and absorption of RS( Reference Singh, Dartois and Kaur 41 ). These results suggest a potential utility for RS in improving meal-to-meal regulation of blood glucose. SCFA, particularly acetate and propionate generated from colonic fermentation by resident bacteria, have also been implicated in the insulin sensitising effects of RS( Reference Robertson, Bickerton and Dennis 42 , Reference Maki, Pelkman and Finocchiaro 43 ).
Although earlier studies have suggested that the lipid-lowering effects of RS may be dependent upon high levels of intake( Reference Behall and Howe 15 , Reference Behall, Scholfield and Yuhaniak 16 ), this is not supported by our findings. In the present study, test starches were provided in amounts 1·9–2·6-fold higher than in earlier interventions( Reference Heijnen, van Amelsvoort and Deurenberg 44 – Reference Noakes, Clifton and Nestel 46 ), but we found no effect of RS on fasting plasma lipids or lipoproteins. The short-term nature of the current intervention may also be a factor, but earlier studies showing reductions in cholesterol and TAG with high- v. low-RS diets at 4 weeks, but not at 8 and 13 weeks( Reference Behall and Howe 15 , Reference Behall, Scholfield and Yuhaniak 16 ), suggest that the effects of RS on plasma lipids are transitory. Notably, we observed that, independent of starch digestibility, higher-CHO diets increased plasma TAG and large VLDL particle concentrations, and promoted a shift in LDL particle distribution towards more medium and small LDL (Supplementary Fig. S4), in keeping with the recognised effect of carbohydrates on features of atherogenic dyslipidemia( Reference Krauss, Blanche and Rawlings 47 – Reference LeCheminant, Smith and Westman 51 ), and in overall agreement with the recent OmniCarb study( Reference Sacks, Carey and Anderson 52 ), which found that plasma TAG were increased by higher CHO intake, but were not influenced by starch quality as assessed by the glycaemic index.
Strengths of our study include a design that, for the first time, allowed testing of high v. low RS in the context of both higher- and lower-CHO diets, lack of confounding effects from other nutrients that were matched across diets, and strict dietary control achieved by the preparation and provision of most study foods. This differs from previous studies in which test starches were provided in the form of supplements that individuals consumed with their self-selected diet( Reference Behall and Howe 15 , Reference Noakes, Clifton and Nestel 46 , Reference Heijnen, van den Berg and Beynen 53 , Reference Park, Kang and Chang 54 ), with only one intervention conducted in a controlled setting( Reference Behall, Scholfield and Yuhaniak 16 ).
A limitation of our study is that the protocol and randomisation scheme required that all foods be prepared in advance, flash-frozen and stored until consumed, typically within 1–2 months. Starch processing conditions have been shown to affect their functionality and susceptibility to enzymatic degradation. When heated in excess water, high-amylopectin starches become highly digestible as a result of gelatinisation, a process that results in disruption of starch crystalline structure and swelling of starch granules. Upon cooling and storage, gelatinised starch may undergo retrogradation during which amylose and, to a lesser extent, outer branches of amylopectin re-align into more ordered crystalline structures that are less susceptible to degradation by α-amylases( Reference Wang and Copeland 55 ). Therefore, we cannot rule out the possibility that in our study freezing and storage may have promoted retrogradation of gelatinised starch products in a manner that rendered them more resistant to digestion, thus attenuating differential metabolic effects of high- and low-RS diets. However, in an earlier study, storage time, freezing, thawing and re-heating did not affect the RS content of high- and low-amylose muffins( Reference Jenkins, Vuksan and Kendall 45 , Reference Noakes, Clifton and Nestel 46 ). Also of note, re-heating of starch-based foods may promote the re-dispersion of crystallised starch chains and restoration of starch digestibility( Reference Yadav, Sharma and Yadav 56 , Reference Englyst, Wiggins and Cummings 57 ). In the present study, regular maize starch in the low-RS diet was incorporated mostly into entrees and baked goods, which, after freezing, were thawed and re-heated before consumption. The diets were otherwise consumed in a manner consistent with how individuals eat on a day-to-day basis. Under these conditions, and despite a 16-fold difference in the RS content of the high- and low-RS diets at time of preparation, we found no differences in their effects on fasting plasma glucose, insulin, lipids and lipoproteins.
Another limitation of our study is the short-term duration of the dietary intervention. As was also shown in an acute feeding study with egg yolks( Reference Miller, Corbin and da Costa 7 ), our findings demonstrate that changes in TMAO levels in response to dietary modification can occur rapidly. Our findings are also consistent with earlier demonstrations of rapid alterations in microbial community structure with RS intake( Reference Abell, Cooke and Bennett 10 – Reference Walker, Ince and Duncan 12 ), and suggest that such changes in gut microbiota promote generation of TMAO in a setting of lower carbohydrate, higher fat intake. However, it remains to be determined whether these effects are sustained with longer-term dietary interventions.
In light of the health benefits generally ascribed to RS, and of epidemiological evidence linking high fibre intake to reduced CVD risk( Reference Wu, Qian and Pan 58 ), the increase in TMAO with RS is contrary to what might be expected. Earlier dietary intervention studies have also shown increased abundance of TMAO after diets high in soya( Reference Solanky, Bailey and Beckwith-Hall 59 ) or low glycaemic load carbohydrates( Reference Barton, Navarro and Buas 60 ), typically deemed beneficial to cardiometabolic health. Hence, although there is strong evidence for the relation of TMAO to atherosclerotic CVD, we cannot conclude that the dietary effects on TMAO observed here would translate into changes in risk for CVD. Furthermore, whether increases in TMAO are clinically relevant in the context of a concomitant improvement in glycaemic control, as is commonly observed with RS, remains to be established.
In conclusion, our study showed that, in the context of a lower-CHO diet, high RS intake resulted in significantly higher plasma concentrations of TMAO, a novel CVD risk biomarker. In keeping with earlier findings, RS blunted the postprandial glucose and insulin responses to meals consumed acutely, but average daily intake of 49–68 g RS did not affect fasting plasma lipids, lipoprotein particle concentrations, glucose or insulin. Together, these observations support the conclusion that at least in the short term high RS intake does not improve biomarkers of cardiometabolic health.
Acknowledgements
The authors thank the staff of the Cholesterol Research Center for their help with conducting the study. The authors also thank the staff of the Bionutrition Core of the University of California, San Francisco, Clinical and Translational Science Institute for their help in designing the diets and preparing study foods.
The authors received the following financial supports: National Institutes of Health (NIH) (DK086472); NIH National Center for Advancing Translational Sciences, University of California, San Francisco (UCSF) Clinical and Translational Science Unit (UL1 TR000004); Ingredion Inc.; NIH and Office of Dietary Supplements (HL103866 and DK106000); S. L. H. is also partially supported by funds from the Lenard Krieger Endowment.
The authors’ contributions were as follows: N. B., P. T. W. and R. M. K. designed the study, analysed the data, performed statistical analysis and wrote the manuscript; N. B., N. F. and R. M. K. conducted the human study; R. L., A. G., R. K. and J. K. J conducted the microbiome analyses; X. L. and S. L. H. conducted the choline, carnitine and TMAO analyses. N. B. and R. M. K. had primary responsibility for the final content. All the authors reviewed and accepted the final content of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516004165













