To the Editor—Kabbani et alReference Kabbani, Palms and Bartoces 1 published an interesting report on the utility of pharmacy dispensing data to measure antibiotic days of therapy (DOT) and antibiotic starts in nursing homes. Their data analysis was limited by a lack of resident identifiers, which led to a reliance on the number of antibiotic transactions as a proxy for starts. The authors state that this is likely an overestimate because antibiotic courses in nursing homes are often dispensed incrementally. As part of a 5-year, quality improvement study conducted in several nursing homes in Rochester, New York, we developed a methodology for calculating antibiotic starts, inferring missing data and providing data feedback to help nursing homes monitor their antibiotic use over time. The primary goals of the project were to reduce C. difficile infections (CDI) and to implement antibiotic stewardship programs (ASPs) via a hospital–nursing home partnership.
We worked with pharmacists at 7 in-house and commercial dispensing pharmacies to obtain antibiotic data that included (1) drug name (2) date and quantity dispensed (3) directions for use (4) duration (5) resident location and unique identifier, and (6) ordering provider. In some cases, obtaining the data required sending the pharmacy a template spreadsheet to illustrate the data needed and/or having a conversation with the pharmacist to discuss the importance of the requested data elements. Data were often received on paper or in a format that was not conducive to manipulation so extensive manual data entry was conducted. We also performed substantial data cleaning to remove topical, ophthalmic, and otic agents; antivirals and antifungals; antibiotics given for noninfectious reasons (eg, gastroparesis); and prescriptions for emergency-box replacement. Drug names were standardized using their generic equivalents; indications were categorized into common syndromes including urinary tract and lower respiratory infections. Time variables (year, quarter, and month) were added to track data over time. If not included in the original data, DOT, defined as the aggregated days a resident received an antibiotic, was calculated manually using the quantity dispensed and directions for use. Using SAS version 9.3 software (SAS Institute, Cary NC), we collapsed observations of the same antibiotic prescribed to the same resident within 4 days of the preceding prescription to calculate antibiotic starts and duration and to infer the indication if it had not been carried over from the original observation.
From these data, we generated several measures of antibiotic use including (1) total DOT rate; (2) DOT rate by the most common antibiotics and indications; (3) DOT rate by the number of residents and unit; (4) antibiotic starts; and (5) length of treatment. Each metric has several pros and cons.Reference Kabbani, Palms and Bartoces 1 , Reference Mylotte 2 The specific summary measures we found useful are summarized in Table 1. In our experience, nursing homes are most familiar with antibiotic starts and number of residents treated. Although the DOT rate is useful to monitor the facility-wide antibiotic burden, it is a less tangible measure and can be easily skewed by residents on chronic, prophylactic antibiotics.Reference Mylotte 2 Other metrics that we found to be especially valuable to nursing homes are usage by unit to account for differences in resident populations and comparative DOT rates from long-term care units across several nursing homes to encourage friendly competition. We created a data dashboard to summarize these metrics and shared the dashboard with nursing home ASP teams at face-to-face, quarterly meetings. During these meetings, we also provided coaching on how to interpret the data and make it actionable. Examples of nursing home interventions based on the summarized antibiotic data include (1) determining where documentation breakdowns occurred in a nursing home with a large number of prescriptions missing indication; (2) monitoring drug selection, specifically fluoroquinolone use to reduce CDI riskReference Piacenti and Leuthner 3 , Reference Stevens, Dumyati, Fine, Fisher and van Wijngaarden 4 for common infections such as urinary tract infections; and (3) comparing length of treatment to treatment durations suggested by established guidelines.Reference Stone, Ashraf and Calder 5
Table 1. Description of Possible Antibiotic Summary Measures
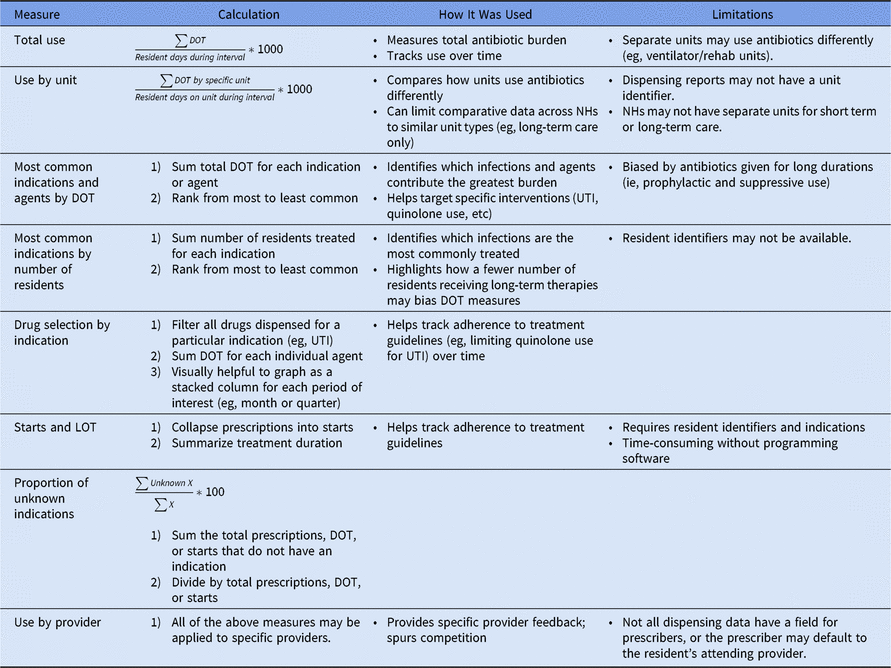
Note. DOT, days of therapy; LOT, length of treatment; NH, nursing home; UTI, urinary tract infection.
The main limitation of our analysis was the inability to verify that dispensed antibiotics were actually administered. However, in our experience, dispensing data are sufficient to guide nursing homes in the development of ASP interventions. Unlike the limitations faced by Kabbani et al,Reference Kabbani, Palms and Bartoces 1 collaboration with dispensing pharmacists allowed us to obtain data that included fields like resident identifier and location as well as antibiotic indication, allowing for more robust analyses. The in-depth evaluation of nursing home antibiotic data that we conducted was made possible by our hospital-based team’s expertise in stewardship and infectious diseases and our dedicated time to clean and summarize the data. We believe that it is important to share the lessons we have learned from this process because visualizing trends in a nursing home’s antibiotic data is the best way to identify areas for improvement and monitor progress over time. However, our methodology may not be possible for nursing home staff that have competing priorities and fewer resources. Therefore, we created a tool in collaboration with the Atlantic Quality Innovation Network/IPRO to help nursing homes monitor their antibiotic use. The tool requires manual data entry but automatically summarizes data by antibiotic, indication, unit, and prescriber. It is available on our website (http://www.rochesterpatientsafety.com/index.cfm?Page=For Nursing Homes) along with our guide to cleaning antibiotic data and our SAS code for collapsing data into antibiotic starts. In summary, we hope that our experiences and methods will be useful to the nursing home community in monitoring and improving antibiotic usage.
Author ORCIDs
Christina Felsen, 0000-0002-7677-6708
Acknowledgments
We thank David Johnson from the Atlantic Quality Innovation Network/IPRO for his role in the development of our antibiotic tracking tool. Additionally, we thank the staff and providers at all participating nursing homes and pharmacies.
Financial support
This project was supported by a grant from the New York State Department of Health.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



