Introduction
Nuisance flies, especially Calliphoridae (Diptera), are often associated with decaying organic materials, including animal waste and carrion. Despite their ecological importance and beneficial aspects for humans (Heath Reference Heath1982), the flies are proven to transmit, or at least are implicated in the transmission of, numerous infectious agents of disease in humans and domestic animals (Greenberg Reference Greenberg1973) and are responsible for serious cases of myiasis (Walker Reference Walker1920; Hall and Townsend Reference Hall and Townsend1977; Hall Reference Hall1995; Hall and Wall Reference Hall and Wall1995). Because of this, their presence around human habitation, food preparation areas, and livestock facilities is generally discouraged.
This study was conducted during 1978, 1979, and 1980 in Winnipeg, Manitoba, Canada and vicinity in response to complaints from area residents about large numbers of filth flies during warm summer periods. The objective of the study was to determine species composition and seasonal abundance of blow flies, in particular, to provide the City of Winnipeg Insect Control Branch with better information regarding timing of spray programmes for fly control at sanitary landfill sites. The only similar survey conducted near Winnipeg is that of Bruce (Reference Bruce1936) at Fargo, North Dakota, United States of America. Similar trap design and bait were used in the present study.
Mechanical transmission of disease agents by calliphorids is considered less of a problem today than it was at the time of these studies. However, the emergence of multiresistant bacterial strains, together with the re-emergence of diseases commonly transmitted by calliphorids, such as poliomyelitis (Nuorteva and Skaren Reference Nuorteva and Skaren1960; Link-Gelles et al. Reference Link-Gelles, Lutterloh, Ruppert, Backenson, St. George and Rosenbeerg2022), shows that the risk of future outbreaks and calliphorid transmission are still present. However, much of the interest today around carrion-feeding Calliphoridae relates to their value in criminal investigations. Forensic entomology is a well-accepted tool in criminal investigations (Byrd and Tomberlin Reference Byrd and Tomberlin2020), and it is important to understand habitat and seasonality of species of flies involved, which aids in knowing what species to expect in certain areas and in establishing in what season a body became exposed.
Materials and methods
Conical hoop fly traps, following the design of Bishopp (Reference Bishopp1925), were constructed and placed on a wooden platform 1 m above the soil surface (Fig. 1). Bait consisted of approximately 450 g coarsely chopped fresh beef liver and two overly ripe bananas covered with water in a rectangular aluminum cake pan (33 × 23 × 5 cm). The beef liver was obtained fresh from Canada Packers (Winnipeg, Manitoba, Canada) and frozen in aliquots until required. The bananas were sourced from grocery stores when they were too ripe to be sold. They were also frozen until required. The ripe bananas were added because they were used in a seminal study in blow fly trapping in North Dakota (Bruce Reference Bruce1936) and in other studies. The cake pan was placed beneath the trap, where it remained for one week, at which time old bait was removed and replaced with fresh bait. Traps were occasionally damaged by equipment in sanitary landfill sites or by vertebrate scavengers and had to be replaced. At the end of each week, traps were returned to the lab, where they were placed in a chest freezer (–20 °C) until all flies were killed. Where total trap catch was fewer than 1000 flies, all flies were counted and identified using Hall (Reference Hall1948) and Hall and Townsend (Reference Hall and Townsend1977). Voucher specimens were deposited in the J.B. Wallis/R.E. Roughley Museum of Entomology (WRME), Department of Entomology, University of Manitoba (Winnipeg, Manitoba). When total trap catch exceeded 1000 flies, the catch was weighed and then spread onto a large sheet of craft paper and gently distributed into an even layer. The entire catch was inspected visually for remarkable or rare individuals before a plastic Petri dish bottom (9 cm diameter) was dropped blindly onto the flies, and all flies contained were retrieved, weighed, counted, and identified. This process was repeated five times. The proportions of each species were determined for the mean among the subsamples and extrapolated to estimate the totals of each species in the entire sample.

Figure 1. Conical hoop fly traps used in the research, following the design of Bishopp (Reference Bishopp1925): A, full trap (left) and new trap ready to be deployed (right), Assiniboine Park Zoo; B, full trap deployed on stand and new trap ready to be deployed, Brady Sanitary Landfill.
Eight trapping locations were selected as representative of possible sources of blow flies, including one rural location further removed from the other sites. Seven sites were located within the bounds of the Perimeter Highway, which surrounds the City of Winnipeg (Fig. 2), and are considered urban, although some sites were located near confined domestic and zoo animals (University of Manitoba, Assiniboia Downs, and Assiniboine Park Zoo), which had more rural-like characteristics. One trapping location was established at the Glenlea Research Farm of University of Manitoba, Faculty of Agricultural and Food Sciences, approximately 20 km south of the University of Manitoba campus (49° 39′ 06″ N, 97° 07′ 09″ W). Swine, cattle, and horses were present on the research farm at the time of the study. The sites within city boundaries were as follows: (1) Assiniboia Downs (49° 53′ 48″ N, 97° 19′ 21″ W), (2) Summit Road Sanitary Landfill Site (49º 55′ 04″ N; 97º 18′ 04″ W), (3) Assiniboine Park Zoo (49° 52′ 26″ N, 97° 14′ 13″ W), (4) Springfield Road Sanitary Landfill Site (49° 56′ 01″ N, 97° 02′ 25″ W), (5) Burns/Canada Packers Stockyard (49° 52′ 43″ N, 97° 04′ 36″ W), (6) University of Manitoba campus (49° 48′ 20″ N, 97° 08′ 15″ W), and (7) Brady Sanitary Landfill Site (49° 45′ 47″ N; 97° 11′ 47″ W; Fig. 2). Traps were operated on the schedule indicated in Table 1. All sites were operated in 1978, but the first traps were not set until after 9 May 1978, after blow fly activity had already begun. Although the number of sites operated in 1979 was reduced, traps were run from the time of earliest blow fly activity. Two traps were maintained until the end of October, when flies were no longer active. Only one trap was operated in 1980, but it was set at the time of earliest blow fly activity. Trapping continued until about the date of the first trap set in 1979, effectively providing two complete years of trapping.

Figure 2. Map of Winnipeg showing trapping sites. Dotted line represents the Perimeter Highway. 1, Assiniboia Downs; 2, Summit Road Sanitary Landfill; 3, Assiniboine Park Zoo; 4, Springfield Road Sanitary Landfill; 5, Burns/Canada Packers; 6, University of Manitoba; 7, Brady Sanitary Landfill. One additional site, Glenlea, is located 20 km south of 6.
Table 1. Dates of operation for baited blow fly traps (Calliphoridae) within Winnipeg, Manitoba, Canada and surrounding area, 1978–1980. Numbers of weekly sampling periods are in parentheses.

Daily temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222) for all sites except Glenlea, which had its own weather station (Climate ID 5021054). Averages for maximum, minimum, and mean temperatures were determined for each trapping period.
Results
The species of calliphorids collected were consistent among sites and over 2–3 years, although abundance varied with season and site. Cynomya cadaverina (Robineau-Desvoidy), Protophormia terraenovae (Robineau-Desvoidy), Phormia regina (Meigen), Lucilia illustris (Meigen), and L. sericata (Meigen) were regularly collected in large numbers, with Calliphora vomitoria (Linnaeus), C. terraenovae MacQuart, C. vicina Robineau-Desvoidy, and C. livida Hall (Diptera: Calliphoridae) collected only in very small numbers.
Figures 3–10 show the species and abundance of calliphorids collected from all eight sites and the temperature data over each season. Figures 11–16 compare trap captures for Cy. cadaverina, P. regina, Pr. terraenovae, L. illustris, L. sericata, and Calliphora spp. over 2–3 years at the four sites at which repeat trapping occurred. As the numbers of Calliphora spp. were so much lower than those of other species, they were grouped together and included C. vomitoria, C. terraenovae, C. vicina, and C. livida. To facilitate comparison, trap dates have been transformed to weeks. Numbers of each species varied greatly between years at each site, showing that abundance was not consistent over the years or among sites.

Figure 3. Trap collections of Calliphoridae at University of Manitoba, 1978, 1979, and 1980. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 4. Trap collections of Calliphoridae at Brady Landfill, 1978 and 1979. No collections were made during the weeks of 27 June–5 July 1978 and 26 July–9 August 1978. In 1979, aerial insecticides were sprayed on 19 and 26 June and on 4, 6, and 16 July. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 5. Trap collections of Calliphoridae at Assiniboine Park Zoo, 1978 and 1979. From 17 to 24 May 1979, the trap lid was partially off, and some flies likely escaped. From 16 September to 3 October 1979, approximately one-third of the sample was lost to moisture. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 6. Trap collections of Calliphoridae at Burns/Canada Packers Stockyard, 1978 and 1979. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 7. Trap collections of Calliphoridae at Springfield Road Sanitary Landfill, 1978. No collections were made during the weeks of 25–31 May, 20–27 July, and 17–24 August. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 8. Trap collections of Calliphoridae at Summit Road Sanitary Landfill, 1978. No collection was made during the week of 7–14 July. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 9. Trap collections of Calliphoridae at Assiniboia Downs Stable, 1978. Temperature data were downloaded from https://climate.weather.gc.ca/ from Richardson International Airport Station (Climate ID 503222). Note: log scale on y axis.

Figure 10. Trap collections of Calliphoridae at Glenlea Research Station, 1978. No collections were made during the week of 9–16 August. Temperature data were downloaded from https://climate.weather.gc.ca/ from Glenlea Station (Climate ID 5021054). Note: log scale on y axis.

Figure 11. Trap capture of Cynomya cadaverina (Robineau-Desvoidy) (Diptera: Calliphoridae) over two and three years at four sites. Dates of trapping listed in Table 1. Note at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978, and pesticides were sprayed by the city on 19 and 26 May 79 and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, some flies were lost due to escape and moisture 17–24 May 1979 and 26 September 1979. Note: log scale on y axis.

Figure 12. Trap capture of Phormia regina (Meigen) (Diptera: Calliphoridae) over two and three years at four sites. Dates of trapping listed in Table 1. Note: at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978, and pesticides were sprayed by the city on 19 and 26 May 1979 and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, some flies were lost due to escape and moisture 17–24 May 1979 and 26 September–3 October 1979. Note: log scale on y axis.

Figure 13. Trap capture of Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae) over two and three years at four sites. Dates of trapping listed in Table 1. Note at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978, and pesticides were sprayed by the city on 19 and 26 May 1979, and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, some flies were lost due to escape and moisture 17–24 May 1979 and 26 September–3 October 1979. Note: log scale on y axis.

Figure 14. Trap capture of Lucilia illustris (Meigen) (Diptera: Calliphoridae) over two and three years at four sites. Dates of trapping listed in Table 1. Note at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978 and pesticides were sprayed by the city on 19 and 26 May 1979 and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, flies were lost due to escape and moisture 17–24 May 1979 and 26 September–3 October 1979. Note: log scale on y axis.

Figure 15. Trap capture of Lucilia sericata (Meigen) (Diptera: Calliphoridae) over two and three years at four sites. Dates of trapping listed in Table 1. Note at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978, and pesticides were sprayed by the city on 19 and 26 May 1979 and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, some flies were lost due to escape and moisture 17–24 May 1979 and 26 September–3 October 1979. Note: log scale on y axis.
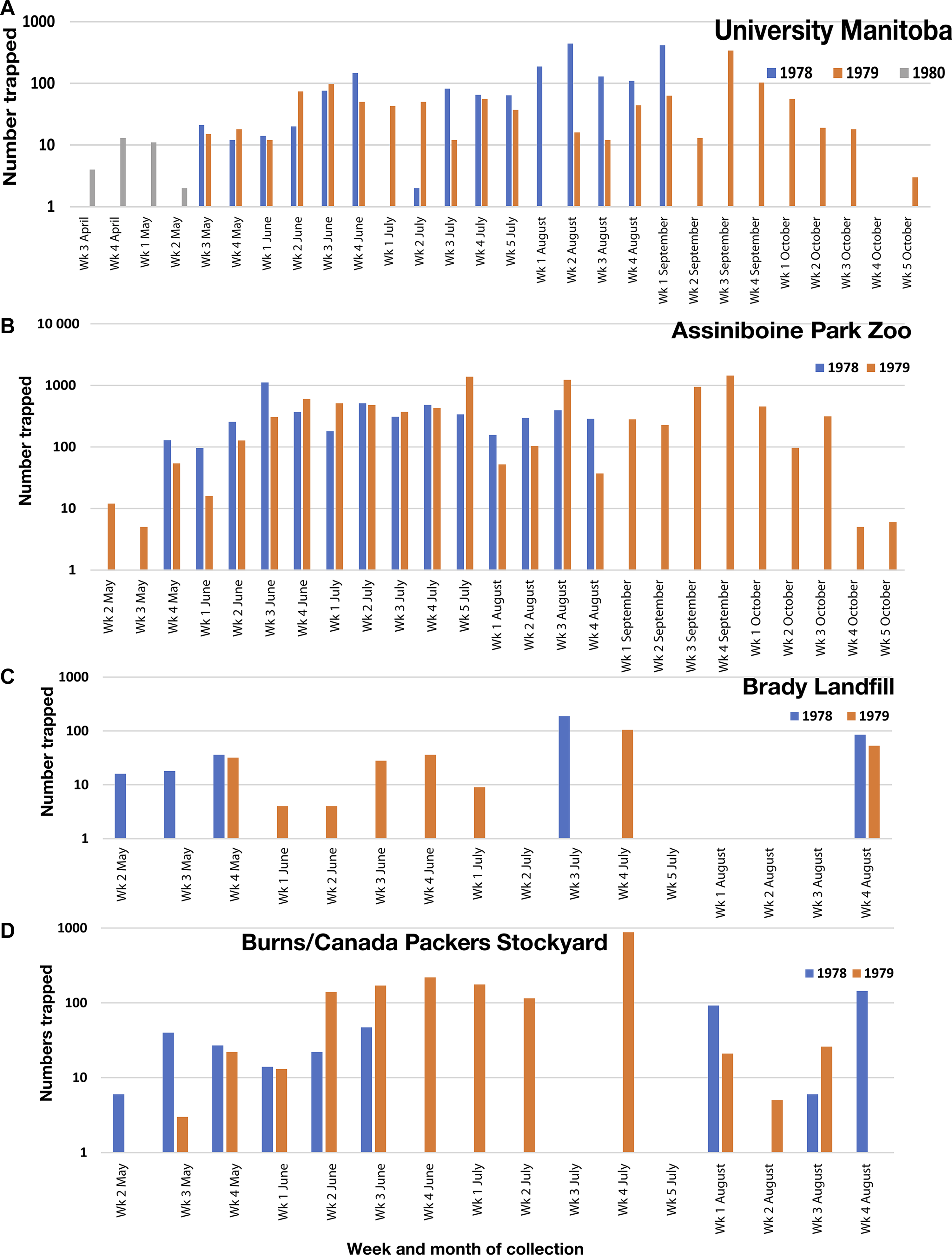
Figure 16. Trap capture of Calliphora Robineau-Desvoidy species over two and three years at four sites. Dates of trapping listed in Table 1. Species included Calliphora vomitoria (Linnaeus), C. terraenovae MacQuart, C. vicina Robineau-Desvoidy, and C. livida Hall (Diptera: Calliphoridae). Note at Brady, no trapping occurred from 27 June to 5 July 1978 or from 26 July 1978 to 9 August 1978, and pesticides were sprayed by the city on 19 and 26 May 1979 and on 4, 6, and 16 July 1979. At the Assiniboine Park Zoo, some flies were lost due to escape and moisture 17–24 May 1979 and 26 September–3 October 1979. Note: log scale on y axis.
Very rarely, other species of Calliphoridae were collected. These included Cochliomyia macellaria (Fabricius), the secondary screwworm, and Pollenia sp. (Fabricius). Single specimens of C. macellaria were collected on 13 July 1978 in Winnipeg and 5 September 1978 at Glenlea. At the time of this study, Pollenia was still classified as a calliphorid, although they are now in their own family, Polleniidae (Cerretti et al. Reference Cerretti, Stireman, Badano, Gisondi, Rognes, Giudice and Pape2019).
Scavenger species of yellowjackets were also collected in these traps, sometimes in large numbers. This resulted in early records for the German yellowjacket, Vespula germanica (Fabricius), and Vespula flavopilosa Jacobson (Hymenoptera: Vespidae) in Manitoba (Galloway and Preston Reference Galloway and Preston2012).
Discussion
Five species of Calliphoridae – Cy. cadaverina, P. regina, Pr. terraenovae, L. illustris, and L. sericata – were found at all sites, often in extremely high numbers, in the tens of thousands, although abundance varied seasonally, between sites, and over sequential years. Other species were collected, including C. vomitoria, C. terraenovae, C. vicina, and C. livida, but in much lower numbers. Bruce (Reference Bruce1936) recorded these species, among others, in his survey conducted at Fargo, North Dakota, in 1930, with some interesting exceptions. Among the Calliphora spp., he recorded C. vomitoria and C. vicina (as C. erythrocephala Meigen) but not C. terraenovae or C. livida. However, he recorded the presence of Calliphora coloradensis Hough. Unfortunately, Bruce (Reference Bruce1936) did not report the numerical data on each species of fly in the same ways we have, so direct comparisons in abundance are not possible. However, he did report that the black blow fly, P. regina, was the most abundant species trapped during the peak season in mid-summer. Of particular interest, Bruce (Reference Bruce1936) found only “a few Cynomyia [sic] cadaverina” (p. 12) in 1931, not the year in which the majority of his trapping efforts had been focused. This species was relatively abundant in the present study, perhaps related to differences of local source populations. The predominant species in the present survey varied in different locations within the same year and in the same location from year to year. This might be because the nature of larval sites differed over time and location, and potential for competition for oviposition sites and larval substrates may vary. Potential sources of flies were completely uncontrolled. This may be reflected in the relative abundance of different species at different times of the year. It is intuitive that the landfill sites might have the greatest diversity and quantity of larval sources, but all the major species were present at all sites in all years, which may reflect the dispersal capacity of blow flies as much as the proximity of blow fly sources to trap locations. In addition, variations between years in landfill sites may relate to management practices, including insecticide applications.
Species of blow flies that overwinter as adults, such as P. regina (Wallis Reference Wallis1962; Norris Reference Norris1965; Siverly Reference Siverly1972), Pr. terraenovae (Nuorteva Reference Nuorteva1959), and Cy. cadaverina (Mail and Schoof Reference Mail and Schoof1954; Webb and Graham Reference Webb and Graham1956; Galloway, unpublished data), generally appeared earlier in the season, whereas those that overwinter as larvae or pupae, such as Lucilia spp. (Holdaway and Evans Reference Holdaway and Evans1930; Hagemann and Barber Reference Hagemann and Barber1948), appeared later and tended to dominate in the trap catch during summer. Lucilia species are usually considered warm-weather and summer species (Faucherre et al. Reference Faucherre, Cherix and Wyss1999; Lutz et al. Reference Lutz, Verhoff, Rosenbaum and Amendt2022).
No other surveys of Calliphoridae have been conducted specifically in the Winnipeg area of Manitoba, although Pr. terraenovae and Cy. cadaverina, with small numbers of L. illustris, were collected in a 1956 trap survey of filth flies in Manitoba’s far north, near Fort Churchill, on the western shore of the Hudson Bay (Webb and Graham Reference Webb and Graham1956). A cross-country survey conducted in 2011–2013, unfortunately, did not include Manitoba, although it did show that Cy. cadaverina and P. regina were by far the most common species across Canada (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019), albeit being caught in much lower numbers than in the present study.
Calliphorid surveys are rare in Canada, but the few baited trap studies conducted in other regions of Canada have shown similar overall species composition. That the dominant species collected vary across the country highlights the need for such studies. Baited traps in British Columbia collected primarily C. vicina, P. regina, L. sericata, L. illustris, and C. latifrons in the Metro Vancouver area (Anderson Reference Anderson2000), with a recent study that compared rural, urban, and semi-urban habitats collecting 12 species of Calliphoridae, with C. latifrons, C. vicina, L. illustris, and L. sericata being the most common (Smith et al. Reference Smith, Poirier and Anderson2023). In a trap study in British Columbia overall, including trapping sites in Metro Vancouver, interior and northern British Columbia, and Vancouver Island, Cy. cadaverina, P. regina, C. vomitoria, and C. terraenovae were most common (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019). In other provinces, baited traps collected P. regina most commonly in Alberta, New Brunswick, Nova Scotia, and Ontario, with Cy. cadaverina being the second most common species overall across the country and the most common in Saskatchewan (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019). In a trapping study in New Brunswick comparing forest, periurban, and urban sites, L. illustris was by far the most common species across all habitats, followed by P. regina, although this latter species was much more abundant in urban and periurban sites (Boudreau et al. Reference Boudreau, Hammami and Moreau2021). Lucilia illustris was common but found in much greater numbers in the urban traps, followed by C. livida, which was found in all habitats but most commonly in forested areas. Other species were collected in low numbers, including Cy. cadaverina (Boudreau et al. Reference Boudreau, Hammami and Moreau2021); this differs from the present study, in which Cy. cadaverina was extremely common.
The relatively low numbers of Calliphora spp. in comparison with the much higher numbers of Luciliini, Phormiini, and the Calliphorini, Cy. cadaverina, may have been influenced by the survey design used in the present study. Some Calliphora spp., such as C. vomitoria, have been shown to prefer larger carcasses (Davies Reference Davies1990, Reference Davies1999; Greenberg and Tantawi Reference Greenberg and Tantawi1993) and may simply not be attracted to smaller baits as were used in our study. However, in a later colonisation study of 70-kg pig carcasses, which included the Glenlea site, adult L. illustris and P. regina were collected at the carcasses, with C. vicina collected very rarely and only at exposed sites and with no other Calliphora spp. collected (Gill Reference Gill2005). In human casework from the Winnipeg area, we have collected primarily P. regina, L. illustris, and L. sericata, with rare cases including Pr. terraenovae, C. vicina, and C. vomitoria, suggesting Calliphora spp. are less abundant in this area. In New Brunswick, although bait traps worked well to determine the make-up of blow fly communities, LeBlanc et al. (Reference LeBlanc, Boudreau and Moreau2021) noted that there may be differences between trap captures versus flies collected at carrion.
Some species were collected very rarely in the present study. Two individual specimens of C. macellaria were collected at Glenlea and Winnipeg. Cochliomyia macellaria is considered a Neotropical species, found in South and Central America, the Caribbean, and the southern two-thirds of the United States of America (Jones et al. Reference Jones, Whitworth and Marshall2019). Bruce (Reference Bruce1936) reported it in North Dakota in small numbers (0.08%), so it is a rare occurrence this far north. Anecdotally, one of us (TDG) noted a few specimens at Whitewater Lake, Manitoba, Canada on 27 August 1996, when very large numbers of waterfowl and shore birds died during a botulism outbreak. This species had not been found in Canada at the time of trapping, so the collection here is the first report of this species in Manitoba and, at the time, in Canada. It remains a very rare species in Canada, not being collected in several other Canadian surveys (Smith et al. Reference Smith, Poirier and Anderson2023; Cormier, personal communication), including a trapping survey conducted across Canada from British Columbia to Newfoundland and Labrador (Langer et al. Reference Langer, Kyle, Illes, Larkin and Beresford2019). Cochliomyia macellaria has been collected in only a few studies in Canada. Four specimens out of a total trap capture of 20 575 were collected in a trapping survey in New Brunswick (Boudreau et al. Reference Boudreau, Hammami and Moreau2021), and the first report of C. macellaria in Québec, Canada involved an adult collected by sweep net on only one occasion on a human cadaver donor in Canada’s first human decomposition facility in the industrial park and Port of Bécancour (Maisonhaute and Forbes Reference Maisonhaute and Forbes2022). Therefore, this is a very rare species in Canada. Pollenia sp. were also only very rarely collected, similar to other studies (Smith et al. Reference Smith, Poirier and Anderson2023).
Studies such as these are important as they provide baseline data for assessing later changes in species range. As global warming continues, changes in species abundance and range are likely to occur. This is illustrated by the collection of C. macellaria in Canada, typically a much more southern, warm-weather species (Jones et al. Reference Jones, Whitworth and Marshall2019). The introduction of invasive species can have major ecological, genetic, and economic impacts on local ecosystems (Lee Reference Lee2002; Rosati and VanLaerhoven Reference Rosati and VanLaerhoven2007) and affect analyses of forensic determinations in medico-legal cases, so it is important that such species are monitored. Cochliomyia macellaria is forensically important because it is one of the most common early colonisers of human and animal remains in the southern United States of America (Bauer et al. Reference Bauer, Bauer and Tomberlin2020). However, it is also known as the secondary screwworm, a facultative parasite, which causes secondary myiasis (Boatright and Tomberlin Reference Boatright and Tomberlin2010), so it can become a major pest. Similarly, an Old World Chrysomyinae, Chrysomya rufifacies (Macquart), was first introduced into North America in the 1980s (Wells Reference Wells1991), spread further north (Cammack and Nelder Reference Cammack and Nelder2010), and was first collected in Canada in 2004 (Rosati and VanLaerhoven Reference Rosati and VanLaerhoven2007).
The present study speaks to the suitability of the trap design and trapping protocols used and to the appropriateness of the study design for future studies. The traps collected the same species over the region, over several years, and in very large numbers and therefore appear to have collected all relevant species present in the area. The findings reflect the universality and abundance of these important synanthropic blow fly species and underscore their importance to associated public health risks and forensic investigations. The trap design and bait selection appear to have been suitable choices for this study, given the number and diversity of flies collected. During peak trapping periods, total weekly trap catch for calliphorids often exceeded 250 000 flies per trap. However, even though the bait was standardised in content, it is likely this bait varied in attraction from day to day during the week the trap was set and according to changes in temperature, precipitation, and relative humidity. In Australia, baits aged for four days were shown to be as attractive as fresh baits to adult blow flies, but baits aged for eight days were less attractive (George et al. Reference George, Archer and Toop2012). However, in an earlier Australian study, although no difference in effectiveness was noted between fresh and three-day-old baits, seven-day-old baits were up to five times more attractive than fresh baits (Vogt and Woodburn Reference Vogt and Woodburn1994). Higher temperatures would accelerate decomposition of the bait and the evaporation rate of the water, whereas relative humidity affected the volatility of the attractants from the bait. It is also likely that wind speed and direction affect flight activity and ability of blow flies to detect and reach bait sources. This was of particular concern in sanitary landfill sites, where traps were placed in exposed locations.
Understanding species ranges is important in forensic entomology because they indicate the likelihood of species that could be expected at a scene. This can be used to focus research efforts on key species, such as developing local developmental databases. Geographically differing populations of the same species may develop at different rates (Tarone et al. Reference Tarone, Picard, Spiegelman and Foran2011). In addition, the presence of certain insects on remains may indicate that a corpse has been moved, but this can be ascertained only when regional databases have been established (Babcock et al. Reference Babcock, Pechal and Benbow2019).
Although intense nuisance pressure generated by large numbers of adult blow flies in urban neighbourhoods prompted the present study, blow flies are important mechanical vectors for a variety of bacterial and other human diseases worldwide, including polio (Picornaviridae) (Nuorteva Reference Nuorteva1959; Link-Gelles et al. Reference Link-Gelles, Lutterloh, Ruppert, Backenson, St. George and Rosenbeerg2022), anthrax (Bacillaceae) (Basson et al. Reference Basson, Hassim, Dekker, Gilbert, Beyer, Rossouw and Van Heerden2018), Salmonella spp. (Enterobacteriaceae) – including S. typhi – Streptococcus group D (Streptococcaceae), Shigella spp. (Enterobacteriaceae), Escherichia coli (Enterobacteriaceae), Bacillus sp. (Bacillaceae), and Enterococcus sp. (Enterococcaceae), Staphylococcus aureus (Staphylococcaceae), and Pseudomonas aeruginosa (Pseudomonadaceae) (Chaiwong et al. Reference Chaiwong, Srivoramas, Sueabsamran, Sukontason, Danford and Sukontason2014), and may be involved in the spread of bovine paratuberculosis, Mycobacterium avium subsp. paratuberculosis (Mycobacteriaceae) (Fischer et al. Reference Fischer, Matlova, Svastova, Bartl, Weston and Pavlik2004). Perhaps of greater concern is that Pr. terraenovae has been shown to carry mobile resistance genes to colistin, an antibiotic drug used as the last recourse against bacteria that are multidrug resistant (Zhang et al. Reference Zhang, Wang, Chen, Yassin, Kelly and Butaye2018).
Blow flies are an integral component of the decomposition process and present a potential seasonal threat to human and animal health in most regions in Canada. Detailed studies on species composition and their spatial and temporal distribution can provide important insights for ecological, medical, veterinary, and forensic investigations. These needs, in the face of projected climatic change, will benefit from continued research.
Acknowledgements
The authors thank David G. Delf for his dedicated assistance in the field and laboratory. Funding and permission for trap locations in sanitary landfill sites and elsewhere for this project were provided by the City of Winnipeg, Assiniboia Downs, Assiniboine Park Zoo, Burns Foods, Canada Packers, and the Department of Entomology. Canada Packers generously donated beef liver for this study, and numerous grocery stores donated overly ripe bananas. The authors also thank the Department of Entomology, University of Manitoba, for their continued support and the Faculty of Agricultural and Food Sciences, University of Manitoba, for permission to locate a trap at the Glenlea Research Station. The authors gratefully acknowledge the Assiniboine Park Zoo, Burns/Canada Packers meat processors, and staff at Assiniboia Downs for their support during this project.
Funding
This project was funded by a grant from the City of Winnipeg to T.D. Galloway.
Competing interests
The authors declare that they have no competing interests.




















