Article contents
Deep in the systematics of Camallanidae (Nematoda): using integrative taxonomy to better understand the phylogeny and consistency of diagnostic traits
Published online by Cambridge University Press: 03 May 2021
Abstract
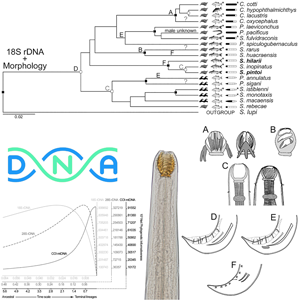
Due to conflicts between classic and molecular systematics of Camallanidae, different data types were used for the first time, to better understand the evolutionary history and taxa consistency within this family. Genetic [18S and 28S rDNA; cytochrome c oxidase subunit I (COI) mtDNA], morphological and life history traits were used to infer phylogenies using Bayesian inference, reconstructed from separated and concatenated datasets. The consistency of tree and morphological traits was evaluated using the consistency index. Characters were mapped on the trees and the phylogenetic informativeness of genetic markers was estimated. Phylogenetic informativeness of 18S provided better resolution for outer nodes, COI for inners and 28S had an intermediate profile. New sequences for two camallanid species were obtained. Phylogenies of genetic and concatenated data largely agreed, showing more divergence in the COI dataset, due to its higher mutation rate vs stable morphology for diagnosing higher taxa. No genus sustained monophyly. The lack of autapomorphy and phylogenetic proximity supported the partition of Batrachocamallanus as synonym of Procamallanus and Spirocamallanus, which should not be considered as subgenera. Although traits of buccal capsule, male tail, habitat, host and biogeographic were highly consistent, intrinsic patterns varied according to different taxa assemblages. Morphological systematics of Camallanidae, based on buccal capsule, is artificial for certain taxa.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 8
- Cited by





