Energy density is defined as the amount of energy in a given weight of food consumed. Given that people tend to consume a fairly consistent weight of food, rather than a consistent energy intake (EI)( Reference Stubbs, Johnstone and O’Reilly 1 – Reference Bell and Rolls 3 ), dietary energy density may play an important role in regulating energy balance and thus in body weight and adiposity( Reference Rolls, Drewnowski and Ledikwe 4 ). There is strong evidence from epidemiological studies that diets high in energy density are associated with increasing adiposity( Reference Karl and Roberts 5 – Reference Murakami, Sasaki and Takahashi 14 ). In addition, energy-dense diets are generally associated with unfavourable dietary intake patterns and lower diet quality, including lower intake of fruits and vegetables( Reference Kant and Graubard 7 , Reference Bes-Rastrollo, van Dam and Martinez-Gonzalez 11 , Reference Murakami, Sasaki and Takahashi 14 – Reference O’Connor, Walton and Flynn 17 ). However, most of the studies have been conducted in Western countries( Reference Kant and Graubard 7 – Reference Savage, Marini and Birch 12 , Reference Ledikwe, Blanck and Khan 15 – Reference O’Connor, Walton and Flynn 17 ), and the associations of dietary energy density with dietary intake and obesity have been largely unexplored in non-Western countries, including Japan.
Traditional dietary cultures and patterns of the Japanese have long been of interest worldwide because of, for example, the low prevalence of CHD and the long-life expectancy in Japan( Reference Nakaji, MacAuley and O’Neill 18 – Reference Yoshinaga and Une 20 ). The Japanese dietary pattern has several characteristics, including high intakes of white rice, soyabean products, fish, seaweeds and green tea and low intakes of animal fat and soft drinks( 21 ). White rice (boiled) contributes most to total daily EI in the Japanese diet (29 %)( 21 ) and has a low energy density (7·03 kJ/g)( 22 ) mainly because of high water concentration (60 %)( 22 ). Furthermore, fat intake in Japanese people is low (overall mean 26·2 % of energy)( 21 ) mainly because of low consumption of fats and oils (accounting for 4·9 % of EI)( 21 ). In a non-representative population (Japanese female dietetic students) dietary energy density was associated with both unfavourable dietary intakes (e.g. lower intakes of fruit and vegetables and dietary fibre and higher intakes of sugar and confectionery) and higher BMI and waist circumference (WC)( Reference Murakami, Sasaki and Takahashi 14 ), which is generally consistent with previous findings from Western countries( Reference Karl and Roberts 5 – Reference Savage, Marini and Birch 12 , Reference Ledikwe, Blanck and Khan 15 – Reference O’Connor, Walton and Flynn 17 ). Further research in a more representative sample of Japanese is merited.
Therefore, the aim of the present cross-sectional study was to examine the associations between energy density of the diets of Japanese adults and food and nutrient intake and general and abdominal obesity, using data from the 2012 National Health and Nutrition Survey, Japan (NHNSJ).
Methods
National Health and Nutrition Survey, Japan
Data source
The NHNSJ, which has been running since 1945, is an annual nationwide nutrition survey conducted by local public health centres under the supervision of the Japanese Ministry of Health, Labour and Welfare on the basis of the Health Promotion Law. The present cross-sectional study was based on the data from the 2012 NHNSJ, with permission from the Ministry of Health, Labour and Welfare, Japan. Full details of the 2012 NHNSJ have been described elsewhere( 21 ). In brief, 475 (out of about one million) census units were randomly sampled as survey areas on the basis of population census. All the non-institutionalised Japanese people aged ≥1 years living in these areas (approximately n 61 000) were invited to participate. The survey was conducted from 25 October to 7 December 2012. A total of 12 750 out of 24 555 eligible households (52 %) took part in the survey( 21 ).
This survey was conducted according to the guidelines laid down in the Declaration of Helsinki, and verbal informed consent was obtained from all the individual participants. Under the Statistics Act, the Ministry of Health, Labour and Welfare anonymised individual-level data collected from the NHNSJ and provided the first author with the data sets for this study. In accordance with the Ethical Guidelines of Epidemiological Research established by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare( 23 ), Institutional Review Board approval was not required for this analysis.
Anthropometric measurements
Anthropometric measurements were performed on approximately 90 % of the participants by trained fieldworkers using standardised procedures. Height (to the nearest 0·1 cm) and weight (to the nearest 0·1 kg) were measured while the participants were barefoot and wearing light clothes only. WC was measured at the level of the umbilicus (to the nearest 0·5 cm) at the end of a normal respiration while the participant was standing erect and with the arms at the side and the feet together. Standardisation on the instruments for height, weight and WC measurement was not feasible in this survey( 21 ). Otherwise, height, weight and WC were measured either by other household members at home or self-reported.
Dietary assessment
Dietary intake data were collected using a 1-d semi-weighed household dietary record, the procedure of which has been described in detail elsewhere( 21 , Reference Iwaoka, Yoshiike and Date 24 ). Thus, in NHNSJ, dietary intake data were collected at the household level (whereas all other data were collected at the individual level). In brief, the participants and the main record-keeper were given both the written and verbal instructions by trained fieldworkers (registered dietitians) on the purpose of the dietary record and how to weigh and record food items consumed by household members in the diary. The main record-keeper was asked to record and weigh all foods and drinks consumed by household members on the recording day. When household members shared foods from the same dish, the record-keeper was also asked to record approximate proportions of the food taken by each of members so that dietary intake of each individual could be calculated. When weighing was not possible (e.g. eating out), the record-keeper was asked to record as much information as possible, including the portion size consumed and details of any leftovers. The recording day was freely selected by each household from any day except for Sundays, national holidays and days with some special events (e.g. wedding party or funeral). Although information on who was the main record-keeper was not formally collected in the survey( 21 ), it was assumed that the main cook in the household (mainly women in Japanese households) is responsible for diet recording. Trained fieldworkers visited the household and checked the completeness of food recording, and if necessary, additional information was added.
Assessment of non-dietary variables
Using a questionnaire, information on basic characteristics including sex, age, smoking status, alcohol drinking and habitual exercise was collected.
Data handling
Analytic sample
The number of participants aged ≥20 years was 30 639 in the 2012 NHNSJ. Of these, the number of participants with missing information on dietary intake, anthropometric measurements and lifestyle variables was 3913, 8593 and 14 044, respectively (some had more than one missing information)( 21 ). After excluding 246 lactating and 136 pregnant women, the final sample used in this analysis comprised 15 618 male and non-lactating and non-pregnant female participants aged ≥20 years with complete information on the variables of interest, except for the analysis on the basis of WC, where 344 participants with missing information on WC were further excluded. The subjects included in the present analysis (n 15 618 except for WC where n 15 274) differed somewhat from those excluded from the analysis (n 1032–15021 depending on variables). The excluded subjects were more likely to be men, be younger, be current smokers, be physically inactive and had lower mean EI (kJ/d), BMI (kg/m2) and WC (cm) (all P<0·0001).
Adiposity measures
BMI (kg/m2) was calculated as weight (kg) divided by height squared (m2). General obesity was defined as BMI≥25 kg/m2 whereas abdominal obesity was defined as WC≥90 cm in men and ≥80 cm in women, on the basis of cut-off points for Asian adults according to the WHO( 25 ).
Dietary variables
Estimates of daily intake for foods, energy and selected nutrients in each individual were calculated from the record of household food consumption and, for shared dishes or foods, approximate proportions consumed by each household member, based on the Standard Tables of Food Composition in Japan, 2005( 22 ). Values of food and nutrient intake were energy-adjusted using the density method (i.e. percentage of energy for energy-providing nutrients and amount per 4184 kJ of energy for foods and other nutrients). The nutrients examined in the present study included protein, fat, SFA, MUFA, PUFA, n-3 and n-6 PUFA, linoleic acid, α-linolenic acid, EPA, DHA, carbohydrate, alcohol, dietary fibre, water-soluble dietary fibre, and selected minerals (including Na, K, Ca, Mg and Fe) and vitamins (including vitamins A, E, C and folate). These were selected mainly for assessing dietary intakes comprehensively while considering the current dietary intake patterns in Japanese( 21 ).
The utility of this household dietary record for estimating dietary intake at the individual level in the Japanese population has previously been examined( Reference Iwaoka, Yoshiike and Date 24 ). In brief, dietary intakes among young women (about 20 years of age) estimated by this 1-d household dietary record by mothers (mean age: 49 years) were compared with those estimated by a 1-d weighed dietary record, which was independently conducted by the young women themselves (n 32). Mean difference between intakes estimated by the two methods were 6·2 % for energy, 5·7 % for protein, 6·7 % for fat and 6·3 % for carbohydrate, whereas the Pearson correlation coefficients were 0·90 for energy, 0·89 for protein, 0·91 for fat and 0·90 for carbohydrate.
Dietary energy density (kJ/g) was calculated as total EI from foods divided by total grams of foods consumed (i.e. on the basis of foods only), excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk), because EI from beverages is regulated differently from EI from foods( Reference Mattes 26 ). In addition, this calculation method has been shown to provide the best correlations with measurements of obesity in several previous analyses( Reference Kant and Graubard 7 , Reference Mendoza, Drewnowski and Christakis 8 , Reference Bes-Rastrollo, van Dam and Martinez-Gonzalez 11 ).
Other variables
In accordance with the NHNSJ report( 21 ), six age categories were defined (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70 years). The following variables were also created: smoking status (never, past or current), habitual alcohol drinking (no or yes) and habitual exercise (no or yes). Dietary reporting status was evaluated based on the ratio of EI:BMR (the Goldberg cut-off)( Reference Black 27 ). Participants were identified as plausible-, under- and over-reporters of EI according to whether the individual’s ratio was within, below or above the 95 % confidence limits for agreement between EI:BMR and the respective physical activity level (PAL). For this purpose, the PAL for sedentary lifestyle (i.e. 1·55)( Reference Black 27 ) was assumed for all subjects, because of a lack of information on physical activity in the present study. BMR was estimated using sex-specific equations developed for Japanese, on the basis of age, body height and body weight( Reference Ganpule, Tanaka and Ishikawa-Takata 28 , Reference Miyake, Tanaka and Ohkawara 29 ). The 95 % confidence limits for agreement (upper and lower cut-off values) between EI:BMR and the PAL were calculated, taking into account CV in intakes and other components of energy balance (i.e. the within-subject variation in EI: 23 %; the precision of the estimated BMR relative to the measured BMR: 8·5 %; the between-subject variation in PAL: 15 %)( Reference Black 27 ). Consequently, under-, plausible- and over-reporters were defined as having EI:BMR<0·87, 0·87–2·75 and >2·75, respectively.
Statistical analysis
All statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute Inc.). Sex-specific analysis was conducted not only taking into account the natural differences in body composition and energy needs between men and women but also because prior analyses indicated a suggestion of interactions between sex and the relation of dietary energy density with BMI and WC (P for interaction=0·13 and 0·06, respectively). There were also significant differences in dietary intakes and adiposity measures between men and women (using independent t test), as shown in Table 1. Differences in dietary energy density across categories of each of the characteristics were examined on the basis of independent t test or ANOVA. When the overall P value from ANOVA was <0·05, a Bonferroni’s post hoc test was performed. Associations of dietary energy density with dietary variables, BMI and WC were investigated by linear regression analyses using the PROC REG procedure, with adjustment for potential confounding factors listed below. Further, using log-binomial regression (PROC GENMOD procedure)( Reference Greenland 30 – Reference Spiegelman and Hertzmark 32 ), multivariate-adjusted prevalence ratio (PR) (95 % CI) for general and abdominal obesity was calculated for each tertile of dietary energy density, with the lowest category used as the reference. We tested for linear trends with the levels of dietary energy density by assigning each participant the median value for the category and modelling this value as a continuous variable. The potential confounding factors considered were age, smoking status, habitual alcohol drinking, habitual exercise and dietary reporting status. For the analysis on adiposity measures and obesity, EI from beverages was further included as a potential confounding factor. All reported P values are two-tailed, and P values<0·05 were considered statistically significant.
Table 1 Dietary characteristics and adiposity measures of the participants: the 2012 National Health and Nutrition Survey, Japan (Mean values with their standard errors)

* P values for differences between men and women based on independent t test.
† Based on foods.
‡ Based on all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk).
§ Calculated on the basis of solid foods only; excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk).
|| Retinol equivalent.
¶ α-Tocopherol.
** n 6395 for men and 8879 for women.
Data have not been weighted to take into account unequal probabilities of selection resulting from the sample design and non-response, because of a lack of information for doing so (i.e. sampling weights)( 21 ).
Results
Means of all EI variables (total, from food and from beverage), food weight consumed and dietary energy density were higher in men than in women (Table 1). Conversely, mean energy-adjusted intakes of all food groups and nutrients examined were higher in women than in men, with the exception of higher intakes of white rice, noodles, meat and alcohol in men and no sex difference in fish and shellfish, EPA and DHA. Mean BMI was higher in men than in women, with a higher prevalence of general obesity (BMI≥25 kg/m2) in men than in women (31·0 v. 21·9 %; P<0·0001). Mean WC was also higher in men than in women, but the prevalence of abdominal obesity was lower in men than in women (30·5 % v. 52·8 %; P<0·0001) mainly because of the sex-specific cut-off values (WC≥90 cm for men and ≥80 cm for women). Dietary energy density was inversely associated with age groups in both sexes (Table 2). Current smokers and participants without habitual exercise had higher mean dietary energy density compared with each of the corresponding groups in both sexes. Although non-alcohol drinkers had higher mean dietary energy density in men, the opposite was observed in women. Over-reporters of EI had higher mean dietary energy density only in men.
Table 2 Dietary energy density according to categories of participants’ characteristics: the 2012 National Health and Nutrition Survey, JapanFootnote * (Mean values with their standard errors)

* Dietary energy density was calculated on the basis foods only; excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk).
† On the basis of independent t test for habitual alcohol drinking and habitual exercise and ANOVA for other variables. When the overall P from ANOVA was <0·05, a Bonferroni’s post hoc test was performed; values within each variable with unlike superscript letters are significantly different (P <0·05).
‡ Under-reporters were defined as participants with the ratio of energy intake (EI):BMR<0·87; plausible reporters as participants with EI:BMR 0·87–2·75; over-reporters as participants with EI:BMR>2·75.
The associations between dietary energy density and dietary characteristics were generally similar in both sexes (Table 3). After adjustment for potential confounding factors including age, smoking status, alcohol drinking, habitual exercise and dietary reporting status, dietary energy density was positively associated with total EI and EI from food, and inversely with food weight consumed (all P<0·0001). There was an inverse association between dietary energy density and EI from beverage in women only (P=0·008). For food intake, dietary energy density was positively associated with bread, noodles, meat (only men), fats and oils, and sugar and confectionery but inversely associated with white rice, potatoes, pulses, vegetables, fruit, and fish and shellfish (all P≤0·02). For nutrient intake, dietary energy density showed positive associations with total fat, SFA, MUFA, PUFA, n-3 and n-6 PUFA, linoleic acid and α-linolenic acid but inverse associations with all other nutrients examined, including protein, carbohydrate, alcohol (only women), dietary fibre, Na, K, Ca, Mg, Fe, vitamins A, E, and C and folate (all P≤0·01), except for no associations with EPA and DHA.
Table 3 Associations between dietary energy density (in kJ/g) and dietary intakes: the 2012 National Health and Nutrition Survey, JapanFootnote * (Regression coefficients (β) and 95% confidence intervals)

* Dietary energy density was calculated on the basis of foods only; excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk). Adjustment was made for age (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70 years), smoking status (never, past or current), habitual alcohol drinking (no or yes), habitual exercise (no or yes) and dietary reporting status (under-, plausible- or over-reporting).
† Regression coefficients mean the change of dietary variables with 1-unit increase of dietary energy density (kJ/g).
‡ Based on foods.
§ Based on all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk).
|| Retinol equivalent.
¶ α-Tocopherol.
After adjustment for potential confounding factors including EI from beverages, dietary energy density showed positive associations with BMI and WC in women (Table 4; P=0·04 and 0·0001, respectively). However, the strength of the associations was relatively small (β=0·07; 95 % CI 0·002, 0·14 and β=0·37; 95 % CI 0·18, 0·55, respectively). In men, there was a week inverse association between dietary energy density and BMI (β=−0·08; 95 % CI −0·15, −0·006; P=0·03). Table 5 shows the associations between dietary energy density and general and abdominal obesity. After multivariate adjustment, dietary energy density was positively associated with abdominal obesity in women (adjusted PR between the extreme tertiles 1·07; 95 % CI 1·02, 1·12; P fortrend=0·003). Dietary energy density was also positively but non-significantly associated with general obesity in women (adjusted PR between the extreme tertiles 1·09; 95 % CI 0·99, 1·21; P fortrend=0·08). There were no associations between dietary energy density and general and abdominal obesity in men. A repeated analysis including only participants with body height and weight measured by trained fieldworkers (n 13 829 for general obesity and 13 722 for abdominal obesity) provided essentially same results (data not shown).
Table 4 Associations between dietary energy density (kJ/g) and adiposity measures: the 2012 National Health and Nutrition Survey, JapanFootnote * (Regression coefficients and 95 % confidence intervals)
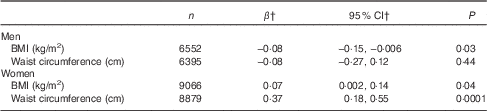
* Dietary energy density was calculated on the basis of foods only; excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk). Adjustment was made for age (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70 years), smoking status (never, past or current), habitual alcohol drinking (no or yes), habitual exercise (no or yes), dietary reporting status (under-, plausible- or over-reporting) and energy intake from beverages (kJ/d, continuous).
† Regression coefficients mean the change of adiposity measures with 1-unit increase of dietary energy density (kJ/g).
Table 5 Prevalence ratios (PR) for general and abdominal obesity according to tertile (T) of dietary energy density: the 2012 National Health and Nutrition Survey, JapanFootnote * (Prevalence ratios and 95 % confidence intervals)

Ref., referent value.
* Dietary energy density was calculated on the basis of foods only; excluding all energy-containing and non-energy-containing beverages (tea, coffee, water, alcoholic beverages, soft drinks, diet drinks, fruit and vegetable juice and milk). General obesity was defined as BMI≥25 kg/m2 for both sexes; abdominal obesity was defined as waist circumference ≥90 cm for men and ≥80 cm for women( Reference Iwaoka, Yoshiike and Date 24 ). PR (95 % CI) was calculated using log-binomial regression.
† Median values for each quartile category of dietary energy density were entered as a continuous variable in the model. The median value in the first, second and third tertile of dietary energy density was 4·90, 5·85, and 7·01 kJ/g in men and 4·64, 5·60 and 6·77 kJ/g in women, respectively.
‡ Adjusted for age (20–29, 30–39, 40–49, 50–59, 60–69 or ≥70 years), smoking status (never, past or current), habitual alcohol drinking (no or yes), habitual exercise (no or yes), dietary reporting status (under-, plausible- or over-reporting) and energy intake from beverages (kJ/d, continuous).
Discussion
To our knowledge, this is the first study to examine the associations of dietary energy density with food and nutrient intake and general and abdominal obesity among Japanese adults, on the basis of data from a national nutrition survey. Lower dietary energy density was associated with favourable dietary intake patterns, in both men and women, including lower intakes of meat, fats and oils, sugar and confectionery, total fat and SFA and higher intakes of pulses, vegetables, fruits, fish and shellfish, dietary fibre, and several minerals and vitamins, but also higher intake of Na. Lower dietary energy density was also associated with a lower prevalence of abdominal obesity in women. Thus, while lowering dietary energy density may generally be a good strategy for improving diet quality and, at least for women, lowering the risk of abdominal obesity, the advice to increase energy-dilute foods could inadvertently be pushing up Na intake.
When comparing with estimates of dietary energy density on the basis of same method (based on foods only), our mean (5·98 kJ/g in men and 5·72 kJ/g in women) was comparable with that observed in 1136 Japanese female dietetic students (5·90 kJ/g)( Reference Murakami, Sasaki and Takahashi 14 ), but considerably lower than that observed in Western countries (7·49–8·28 kJ/g)( Reference Kant and Graubard 7 , Reference Mendoza, Drewnowski and Christakis 8 , Reference Vernarelli, Mitchell and Rolls 10 , Reference Ledikwe, Blanck and Khan 15 , Reference O’Connor, Walton and Flynn 17 ). This may be due to higher consumption of foods high in water content (such as white rice, noodles, and fish and shellfish) accompanied by lower intake of energy-dense foods (such as fats and oils, and sugar and confectionery) in Japanese than in Western people( Reference Kant and Graubard 7 , Reference Murakami, Sasaki and Takahashi 14 , Reference Ledikwe, Blanck and Khan 15 ). Lower dietary energy density was associated with more favourable dietary intake patterns in this study of Japanese adults, including higher intakes of pulses, vegetables, fruits, fish and shellfish, dietary fibre, and several minerals and vitamins and lower intakes of meat, fats and oils, sugar and confectionery, total fat and SFA. This is generally consistent with the observation in Japanese female dietetic students( Reference Murakami, Sasaki and Takahashi 14 ) but also with the findings from Western countries despite the large difference in mean estimate of dietary energy density between Japan and Western countries( Reference Kant and Graubard 7 , Reference Bes-Rastrollo, van Dam and Martinez-Gonzalez 11 , Reference Murakami, Sasaki and Takahashi 14 – Reference O’Connor, Walton and Flynn 17 ). However, lower dietary energy density was also associated with higher Na intake in the present Japanese population. The major source of Na in Japanese diet is seasonings such as salt and soya sauce (about 62 %)( Reference Asakura, Uechi and Masayasu 33 ), which make negligible contribution to dietary energy density, and they are traditionally consumed with energy-dilute foods such as vegetables, pulses, and fish and shellfish. Thus, while reducing dietary energy density may be important for improving diet quality in Japanese as is the case in Western populations, an effort to reduce Na intake should also be considered.
Evidence from epidemiological studies in Western countries has generally supported the positive association between dietary energy density and adiposity( Reference Karl and Roberts 5 – Reference Savage, Marini and Birch 12 ). We also found that dietary energy density was positively associated with BMI (P=0·04), WC (P=0·0001), general obesity (P fortrend=0·08) and abdominal obesity (P fortrend=0·003) in women although no such associations were observed in men, except for unexpected inverse associations with BMI (P fortrend=0·03) and general obesity (P fortrend=0·16). Although several previous studies have also observed similar associations in women( Reference Mendoza, Drewnowski and Christakis 8 – Reference Vernarelli, Mitchell and Rolls 10 ), it is unclear why no such associations were observed in men. A possible explanation may be that given that women (the main cook in many Japanese households) are usually responsible for diet recording, dietary data for men may be less accurate than those for women in this study( 21 ), producing null associations between dietary energy density and adiposity in men.
Several limitations of the present study warrant mention. First, the cross-sectional nature of the study does not permit the assessment of causality owing to the uncertain temporality of the association. Only a prospective study would provide better understanding of the relationship between dietary energy density and obesity. In addition, although NHNSJ intends to represent a national representative sample of the non-institutionalised population of Japan, only 52 % of households sampled took part in the survey. Further, information on the basic characteristics of households that did not participate is unfortunately unavailable( 21 ). Moreover, the exact response rate at the individual level is not known. Thus selection bias cannot be ruled out.
All self-reported dietary assessment methods are subject to both random and systematic measurement errors( Reference Livingstone and Black 34 ). Given the day-to-day variability in dietary intakes of free-living individuals, estimates of dietary intakes derived from a 1-d semi-weight household dietary record used here unlikely represent the usual intakes of individual respondents. As this kind of random error would tend to result in bias towards attenuating the relationship, the associations observed here would have been underestimated. Moreover, the days of the week were not proportionately selected for dietary assessment and Sundays were intentionally excluded as a survey day (based on the survey protocol), which should produce some bias to estimate an average intake, although it is unknown what type of bias it is or how this possible bias affects the present findings, because of a lack of information on differences in dietary intake among day of the week in Japanese. Unfortunately, information on the days that were selected for dietary recording is not available( 21 ). Further, as the survey was conducted between 25 October and 7 December 2012, any seasonal variation has not been considered, which could produce some additional bias to estimate an average intake. In any case, several days of dietary assessment or the use of a validated dietary assessment questionnaire would have been preferable to estimate usual dietary intake, the feasibility of which should be considered in future NHNSJ.
In addition, misreporting of dietary intake, particularly by obese individuals, is a serious problem associated with self-reported dietary assessment methods( Reference Livingstone and Black 34 ). To minimise possible influence of dietary misreporting, we included EI:BMR as a confounding factor in our analysis. Although consistent misreporting across all types of foods would likely have little influence on dietary energy density estimates( Reference Ledikwe, Blanck and Khan 9 ), studies have indicated that overweight or obese persons may selectively under-report their intake of fatty or sugary foods( Reference Heitmann and Lissner 35 , Reference Heitmann, Lissner and Osler 36 ), which could cause dietary energy density estimates to be lower than actual values. In the present study, the potential shared error created by under-reporting of dietary measures by participants with a high BMI (and WC) would likely have weakened the associations of dietary energy density with measures of obesity and could possibly have led to a null finding. Finally, although we adjusted for a variety of potential confounding variables, residual confounding could not be ruled out.
In conclusion, in this cross-sectional study based on data from a national nutrition survey, dietary energy density was associated positively with unfavourable dietary intake patterns in Japanese men and women, including higher intakes of meat, fats and oils, sugar and confectionery, total fat and SFA and lower intakes of pluses, vegetables, fruits, fish and shellfish, dietary fibre, and several minerals and vitamins, but also lower intake of Na. Dietary energy density was also associated positively with a prevalence of abdominal obesity in women. Thus, while lowering dietary energy density may generally be a good strategy for improving diet quality and, at least for women, lowering the risk of abdominal obesity, the advice to increase energy-dilute foods could inadvertently be pushing up Na intake. Prospective studies are needed to confirm the associations observed in this study.
Acknowledgements
This study was supported in part by the Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K. M., grant number 15K16213). The Ministry of Education, Culture, Sports, Science and Technology of Japan had no role in the design, analysis or writing of this article.
K. M. designed the study, analysed and interpreted the data and wrote the manuscript. M. B. E. L. designed the study, interpreted the data and helped in the writing of the manuscript. H. O. and S. S. helped in the writing of the manuscript. All the authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








