Introduction
Foetal growth rate and subsequent birth weight (BWT) of lambs are determined by the genetic background of the foetus which governs its drive for growth, by its paternal genotype which determines placental size, morphology and efficiency of nutrient transfer to the growing foetus, by external environmental conditions such as nutritional status, which affect maternal physiology, and by specific uterine environment-related conditions such as the number of foetuses in the litter (Dickinson et al., Reference Dickinson, Hancock, Hovell, Taylor and Wiener1962; Anthony et al., Reference Anthony, Scheaffer, Wright and Regnault2003; Redmer et al., Reference Redmer, Wallace and Reynolds2004; Safari et al., Reference Safari, Fogarty and Gilmour2005; Reynolds et al., Reference Reynolds, Caton, Redmer, Grazul-Bilska, Vonnahme, Borowicz, Luther, Wallace, Wu and Spencer2006). Intrauterine growth restriction (IUGR), which leads to the birth of smaller lambs with reduced survival ability, occurs in sheep naturally and can be induced by several means.
Naturally occurring IUGR
IUGR occurs in ewes carrying multiple foetuses. Increasing prolificacy by genetic and managerial means is associated with an increase in the number of ewes producing large litters. Prolificacy is an important trait in semi-intensive and intensive sheep-production systems. Nevertheless, the economic advantages of high prolificacy are often not fully exploited because pregnancies with multiple foetuses are associated with IUGR of those foetuses, and lower BWTs and pre- and postnatal survival rates of the lambs (Figure 1) (Hinch et al., Reference Hinch, Crosbie, Kelly, Owens and Davis1985; Gama et al., Reference Gama, Dickerson, Young and Leymaster1991; Fogarty et al., Reference Fogarty, Hopkins and van der Ven2000; Holst et al., Reference Holst, Fogarty and Stanley2002; Vallet et al., Reference Vallet, Leymaster and Christenson2002; Christley et al., Reference Christley, Morgan, Parkin and French2003; Kleemann and Walker, Reference Kleemann and Walker2005).

Figure 1 Birth weights and perinatal survival rates for lambs (n = 4 781) born to Afec-Assaf ewes (Volcani Center, Israel), according to litter size (after Gootwine and Rozov (Reference Gootwine and Rozov2006)).
Lamb BWT is also affected naturally by maternal age, with lambs born to relatively young ewes (first and second parities), and to relatively old ewes (ninth and above parities), being smaller than lambs born to ewes in their third to the eighth parities (Al-Shorepy, Reference Al-Shorepy2001; Gootwine et al., Reference Gootwine, Rozov, Bor and Reicher2006b). Seasonality also affects lambs' BWT, those born in the summer and autumn being smaller than those born in the winter and spring (McCoard et al., Reference McCoard, Peterson, Jenkinson, Campbell and McCutcheon1996; Al-Shorepy and Notter, Reference Al-Shorepy and Notter1998; Gootwine and Rozov, Reference Gootwine and Rozov2006). The seasonal effect on BWT is due to both a direct seasonal effect on foetal growth, possibly mediated through variations in melatonin secretion, and an indirect seasonal effect on gestation length (Gootwine and Rozov, Reference Gootwine and Rozov2006).
Altitude is another natural factor that affects BWT of lambs, with those born to ewes at high altitude and subjected to hypobaric hypoxia being lighter than those born to ewes at low altitude (Parraguez et al., Reference Parraguez, Atlagich, Diaz, Bruzzone, Behn and Raggi2005). Thus, foetal growth is affected by a number of intrinsic and extrinsic factors that can negatively impact on lamb BWT.
Experimental IUGR
IUGR can be induced in ewes by various means, including manipulation of maternal nutrition, administration of glucocorticoids, artificial restriction of placental size, creation of uteroplacental embolisms, and exposure of pregnant ewes to extreme environmental conditions, such as high temperature and low oxygen pressure (McMillen et al., Reference McMillen, Adams, Ross, Coulter, Simonetta, Owens, Robinson and Edwards2001; Regnault et al., Reference Regnault, Galan, Parker and Anthony2002; Redmer et al., Reference Redmer, Wallace and Reynolds2004; Fowden et al., Reference Fowden, Ward, Wooding, Forhead and Constancia2006c; Wallace et al., Reference Wallace, Luther, Milne, Aitken, Redmer, Reynolds and Hay2006). Interestingly, such simple practices as shearing ewes during mid or late pregnancy can increase lamb BWTs (Symonds et al., Reference Symonds, Bryant, Clarke, Darby and Lomax1992; Revell et al., Reference Revell, Main, Breier, Cottam, Hennies and McCutcheon2000; Kenyon et al., Reference Kenyon, Morrison, Revell and McCutcheon2002). In most cases, changes in foetal weight due to either natural or induced IUGR are associated with a reduction in placental size and potentially, functionality.
The enigma of litter-size-dependent IUGR
The literature clearly indicates that foetal growth is regulated by the placental transport of nutrients, oxygen and water to the foetus from the maternal compartment through the uteroplacental complex (Regnault et al., Reference Regnault, Friedman, Wilkening, Anthony and Hay2005; Fowden et al., Reference Fowden, Ward, Wooding, Forhead and Constancia2006c). Therefore, litter-size-dependent IUGR may result from failure of the uteroplacental complex fully to support growth of the multiple foetuses within the same uterus. Indeed, available evidence from studies of both sheep (Edwards and McMillen, Reference Edwards and McMillen2002; Gardner et al., Reference Gardner, Jamall, Fletcher, Fowden and Giussani2004; MacLaughlin et al., Reference MacLaughlin, Walker, Roberts, Kleemann and McMillen2005) and humans (De Boo and Harding, Reference De Boo and Harding2006) suggests that reduced BWTs of individual lambs born in litters with more than one lamb have features in common with IUGR in lambs resulting from undernutrition, such as reduction in uterine blood flow per foetus and lower metabolite concentration in the maternal and foetal circulations. However, as ewes can clearly maintain a litter to term for which total weight approaches three times that of a normal single lamb (Dickinson et al., Reference Dickinson, Hancock, Hovell, Taylor and Wiener1962), it is unclear why the individual BWTs of lambs born as twins or triplets are lower than those of lambs born as singletons. This observation suggests that sheep foetuses that gestate in multi-foetal pregnancies have unique features that make their growth trajectory different from that of singletons.
Foetal growth and development have been investigated mainly in ewes pregnant with singleton lambs. Information on foetal development in pregnancies with multiple foetuses is therefore relatively limited. The present review focuses on foetal growth in ewes with multiple foetuses during pregnancy, aiming to better understand the nature of litter-size-dependent IUGR, a biological phenomenon that has major economic implications in sheep-production systems, as well as in other livestock-producing enterprises such as pig production (Wu et al., Reference Wu, Wallace, Bazer and Spencer2006).
General description of foetal and placental growth during pregnancy in sheep
Uterine anatomy
In sheep, the uterine endometrium has two distinct areas: aglandular caruncular and glandular intercaruncular. The intercaruncular area contains large numbers of endometrial glands that synthesise and secrete a complex array of proteins termed histotroph, which include enzymes, growth factors, cytokines, lymphokines, hormones, transport proteins and other substances (Wimsatt, Reference Wimsatt1950; Roberts and Bazer, Reference Roberts and Bazer1988). During pregnancy, histotroph is transported via placental areolae from the uterine glands into the foetal circulation and then much is cleared via the kidney and urachus into the allantoic fluids which serves as a nutrient reservoir to nourish the growing conceptus (the foetus and its extraembryonic membranes) (Renegar et al., Reference Renegar, Bazer and Roberts1982). Histotroph is particularly important during the early stages of pregnancy (Spencer and Bazer, Reference Spencer and Bazer2004), before hematotrophic nutrition is established. Hematotrophic nutrition relies on the exchange of nutrients and gases between vasculature of the maternal uterus and placenta of the conceptus (Gray et al., Reference Gray, Bartol, Tarleton, Wiley, Johnson, Bazer and Spencer2001). The relative contribution of histotrophic and hematotrophic nutrition to conceptus development during the late stages of pregnancy is not established. However, it can be assumed that while placental transport of O2, amino acids, glucose and micronutrients is mostly hematotrophic, delivery of key regulatory molecules such as vitamins, hormones and growth factors is mediated by uptake of histotroph from uterine glands via the placental areolae.
The aglandular areas of the endometrium are termed caruncles. On average, the sheep uterus has about 100 caruncles, with numbers ranging from 50 to 150 per uterus (Alexander, Reference Alexander1964b). Caruncles are features of the neonatal uterus of lambs (Wiley et al., Reference Wiley, Bartol and Barron1987) and their number in primiparous ewes is related to uterine size (Figure 2). Little information is available on what initiates placentome formation and how it develops during gestation.

Figure 2 Association between number of caruncles and uterine weight in primiparous crossbred ewes (n = 22, Texas A&M University, Texas, USA).
Placental formation
Sheep have a non-invasive, synepitheliochorial-type placenta (Björkman, Reference Björkman1970). Following implantation, the chorioallantoic membranes of the conceptus elongate and through an unknown mechanism, form cotyledons that interdigitate with the caruncles to form placentomes (Wimsatt, Reference Wimsatt1950), where the placenta becomes syndesmochorial in nature. Placentomes are structures containing the uteroplacental complexes responsible for exchange of gases and micronutrients. The placenta is an autocrine, paracrine and endocrine organ that synthesises and secretes a broad range of steroid and peptide hormones which regulate foetal development, and directs maternal physiology to support this process (Anthony et al., Reference Anthony, Limesand, Fanning, Liang and Bazer1998; Gootwine, Reference Gootwine2004; Murphy et al., Reference Murphy, Smith, Giles and Clifton2006).
Placenta of a single foetus appears to be programmed to involve only about 70% of the total available caruncles in placentome formation. Variation among ewes in the numbers of caruncles on the endometrial surface may account for the variation in placentome numbers (Alexander, Reference Alexander1964a). On average, placentae of singleton conceptuses have 70 placentomes that vary in size and morphological appearance (Ward et al., Reference Ward, Forhead, Wooding and Fowden2006).
Foetal and placental growth
Average gestation length in sheep is 147 days. Throughout gestation, growth patterns for the placenta and foetus differ, as main placental growth takes place before the period of rapid foetal growth (Figure 3). Foetal growth in sheep is best described by the Gompertz equation (Robinson et al., Reference Robinson, McDonald, Fraser and Crofts1977). During the first half of gestation, foetal growth is slow: by the end of that period the foetus has achieved only about 10% of its BWT. During the second half of pregnancy, foetal growth is exponential and near the time of parturition, daily foetal weight gain ranges from 70 to 150 g/day, which is reflected directly in the BWT of the foetus (Figures 3 and 4).

Figure 3 Growth of the foetus and placenta during pregnancy in Florida Native ewes (n = 4 per age group; FW Bazer, TE Spencer, WW Thatcher and DC Barron, unpublished results).

Figure 4 Association between foetal weight at about 140 days of gestation and its daily growth rate in different experiments (FW Bazer, TE Spencer, WW Thatcher and DC Barron, unpublished results form study on Florida Native ewes; Rattray et al., Reference Rattray, Garrett, East and Hinman1974; Robinson et al., Reference Robinson, McDonald, Fraser and Crofts1977; Sinclair et al., Reference Sinclair, McEvoy, Carolan, Maxfield, Maltin, Young, Wilmut, Robinson and Broadbent1998; Corner et al., Reference Corner, Kenyon, Stafford, West and Morris2006; Gootwine and Rozov, Reference Gootwine and Rozov2006).
In contrast to the pattern of foetal growth, placental development, in terms of weight, length and surface area, increases progressively from the peri-implantation period (16 to 30 days of gestation) to its maximum size at 75 to 80 days of pregnancy. Then, indices of placental size either stabilise or decline slightly near the end of the pregnancy (Figure 3). Placental weight and foetal weight are poorly correlated during early gestation; however, they are highly correlated late in gestation and at parturition (Naaktgeboren and Stegeman, Reference Naaktgeboren and Stegeman1969; Greenwood et al., Reference Greenwood, Slepetis and Bell2000).
Placentome restructuring during pregnancy
During the last two-thirds of gestation, both the maternal (caruncular) and foetal (cotyledonary) portions of the placentome undergo structural changes which include vascular development and angiogenesis. Angiogenesis and vasodilation within placentomes are mediated by various angiogenic factors, including vascular endothelial growth factor (VEGF) and nitric oxide (NO). While the cotyledonary capillary beds grow primarily by branching (angiogenesis) to provide a very high density of small capillaries, the caruncular capillary beds grow mainly by increasing the size of the capillaries (vasodilation), with smaller increases in capillary density (Reynolds and Redmer, Reference Reynolds and Redmer2001; Reynolds et al., Reference Reynolds, Borowicz, Vonnahme, Johnson, Grazul-Bilska, Redmer and Caton2005a and Reference Reynolds, Borowicz, Vonnahme, Johnson, Grazul-Bilska, Wallace, Caton and Redmer2005b).
Placental weight may decrease slightly during the second half of gestation; however, the amount of DNA in the placentomes changes little during that period, suggesting that the reduction in placental weight is due mainly to structural modifications and/or the tissues' state of hydration, rather than changes in cell number (Ott et al., Reference Ott, Wiley and Bartol1997; Reynolds et al., Reference Reynolds, Borowicz, Vonnahme, Johnson, Grazul-Bilska, Redmer and Caton2005a).
Uterine blood flow
Increases in vascularisation of the placentomes during gestation are followed by increases in uterine blood flow to accommodate the requirements for nutrients and gases that support rapid foetal growth (Reynolds et al., Reference Reynolds, Caton, Redmer, Grazul-Bilska, Vonnahme, Borowicz, Luther, Wallace, Wu and Spencer2006). Thus, while the rate of total uterine blood flow is about 0.4 l/min at 71 days of pregnancy, it increases some threefold by day 130 of gestation, to about 1.2 l/min. Similarly, total umbilical blood flow increases throughout gestation (Reynolds et al., Reference Reynolds, Caton, Redmer, Grazul-Bilska, Vonnahme, Borowicz, Luther, Wallace, Wu and Spencer2006). It is estimated that by the end of gestation, 20% of the maternal cardiac output is targeted to the uterus to provide nutrients and oxygen to support the growing conceptus, primarily the foetus.
Placental oxygen and glucose consumption
The placenta has high metabolic demands for its own growth and for the endocrine and transport functions that support the growing foetus. The placenta consumes approximately two-thirds of the oxygen and one-half of the glucose transferred from the uterine circulation to the conceptus (Harding and Johnston, Reference Harding and Johnston1995). Part of the placental glucose is converted to fructose in the placenta, which is an important form of stored energy for the foetus (Moores et al., Reference Moores, Rietberg, Battaglia, Fennessey and Meschia1993).
In general, the size of the placenta is correlated with its glucose and amino acid transfer capacity, which is determined by transporter abundance (reviewed by Regnault et al. (Reference Regnault, Friedman, Wilkening, Anthony and Hay2005) and Fowden et al. (Reference Fowden, Ward, Wooding, Forhead and Constancia2006c)). Interruptions in the normal placental growth trajectory caused by carunclectomy (Owens, et al., Reference Owens, Falconer and Robinson1987), heat stress (Thureen et al., Reference Thureen, Trembler, Meschia, Makowski and Wilkening1992), nutrition manipulation (Wallace et al., Reference Wallace, Bourke, Aitken, Milne and Hay2002), or prolonged hypoglycemia (Carver and Hay, Reference Carver and Hay1995) may either increase or decrease glucose and amino acid transporter abundance and hence, the efficiency of placental nutrient-transfer capacity.
Roles of the conceptus and ewe in foetal growth
The understanding that both the foetus and ewe determine the foetal growth trajectory comes from crossbreeding trials and embryo-transfer experiments between large and small breeds, as well as from within-breed genetic analyses of source BWT variations.
Between-breed analyses
Lambs of large breeds have higher BWTs than those of small breeds (Donald and Russell, Reference Donald and Russell1970), reflecting genetic differences in foetal growth, maternal uterine functions, uteroplacental development, and likely a combination of all of these factors. Results of embryo-transfer experiments demonstrated that Welsh Mountain (small breed) lambs gestated in Lincoln (large breed) surrogate ewes are heavier at birth than Welsh Mountain lambs born to Welsh Mountain ewes. In addition, Lincoln lambs born to Welsh Mountain ewes had lower BWTs than Lincoln lambs born to Lincoln ewes (Dickinson et al., Reference Dickinson, Hancock, Hovell, Taylor and Wiener1962). Thus, there is an interaction between foetal and maternal factors which determines the extent of foetal growth, and the maternal environment does not, in and of itself, determine or support maximal foetal growth.
Similar conclusions were reached following experiments in which Romanov embryos (small breed with average ewes' body weight of 45 kg) were transferred into Columbia ewes (large breed with average ewe weight of 104 kg) (Scheaffer et al., Reference Scheaffer, Caton, Redmer and Reynolds2004). At 130 days of gestation, Romanov foetuses gestated in Columbia ewes were 22% heavier than control Romanov foetuses gestated in Romanov ewes. The remarkable plasticity of foetal and placental growth and the ability of the foetus to respond to permissive or restrictive aspects of the maternal environment have also been observed following embryo-transfer experiments involving small and large breeds of horses (Allen et al., Reference Allen, Wilsher, Turnbull, Stewart, Ousey, Rossdale and Fowden2002), pigs (Wilson et al., Reference Wilson, Biensen, Youngs and Ford1998) and cattle (Ferrell, Reference Ferrell1991).
Maternal genetic effects on foetal growth were also demonstrated in crossbreeding experiments between large and small breeds. For example, crossbreeding between Assaf (large) and Booroola Merino (small) breeds (Gootwine et al., Reference Gootwine, Bor, Braw-Tal and Zenou1993) increased BWTs of lambs in response to contributions of the large breed to the foetal genome, and to contributions of the maternal genome. A difference in BWT of about 0.5 kg resulted when lambs of similar genetic backgrounds were gestated in ewes with different genetic backgrounds in terms of body size.
Within-breed genetic analysis
BWT can be considered both a foetal-related trait (direct effect) and a maternal-related trait (maternal effect). Within-breed genetic variation in BWT has been estimated for several breeds of sheep (see reviews by Fogarty (Reference Fogarty1995) and Safari et al. (Reference Safari, Fogarty and Gilmour2005)). Both direct and maternal effects have been found to have moderate heritability values and in most cases, the genetic correlation between direct and maternal effects was negative (Table 1). These findings suggest that some genetic and physiological factors that enhance foetal growth also have negative effects later in life on the maternal ability to support foetal growth. Antagonistic or negative genetic correlations between direct and maternal effects on BWT have also been reported for beef cattle and pigs (Robinson, Reference Robinson1981; Meyer, Reference Meyer1992).
Table 1 Estimates of direct heritability (h2a), maternal heritability (h2m) and coefficients of correlation between direct and maternal genetic effects (ram) for birth weight of lambs born to ewes with different genotypes

† Age of ewes, where known (y = year).
Genetic imprinting and foetal and placental growth
An antagonistic relationship between the expression of genes supporting foetal growth and those supporting placental development and function is observed in imprinted genes. Genomic imprinting (Tilghman, Reference Tilghman1999) is an epigenetic phenomenon in which the expression of certain genes is dependent on whether they are inherited from the mother or father. A substantial proportion of known imprinted genes are involved in the control of foetal growth and placental development (Reik and Walter, Reference Reik and Walter2001). In general, paternally expressed genes enhance placental growth, while maternally expressed genes reduce placental size and its nutrient-transport ability (Isles and Holland, Reference Isles and Holland2005; Angiolini et al., Reference Angiolini, Fowden, Coan, Sandovici, Smith, Burton, Tycko, Reik, Sibley and Constância2006; Cattanach et al., Reference Cattanach, Beechey and Peters2006; Fowden et al., Reference Fowden, Sibley, Reik and Constância2006b).
Most of the research on genomic imprinting has been conducted with mice. Imprinting of genes controlling foetal and placental growth has been suggested to occur in sheep as well following the observation that parthenogenetic development of embryos is associated with retardation of foetal growth (Feil et al., Reference Feil, Khosla, Cappai and Loi1998; Hagemann et al., Reference Hagemann, Peterson, Weilert, Lee and Tervit1998). A detailed study on the genomic imprinting status of the IGF2 gene in sheep (McLaren and Montgomery, Reference McLaren and Montgomery1999) revealed that ovine IGF2 is imprinted in the foetal kidney, liver, and spleen but not in the brain. As in humans, but not in mice, ovine IGF2 imprinting in the liver is switched off postnatally and in the adult sheep liver IGF2 has a bi-alleleic mode of expression.
As the interaction between paternally and maternally expressed genes is still under investigation, it is not yet clear how the expression of imprinted genes in the control of foetal growth-drive and placental structure and function is associated with the genetic response to selection for BWT. Nevertheless, in multi-foetal pregnancies, polymorphism in genetic imprinted genes can contribute to within-litter-size variations in BWT (Gootwine et al., Reference Gootwine, Rozov and Weller2006c), even when all foetuses have a similar genetic make-up.
Major genes affecting BWT
Foetal growth rate and BWT of lambs are quantitative traits that are controlled by many loci with small effects of each locus on variations in the trait. Recently, the FecB gene which controls ovulation rate in ewes (Piper et al., Reference Piper, Bindon, Davis, Land and Robinson1985) was found to have a major effect on BWT of lambs (Gootwine et al., Reference Gootwine, Rozov, Bor and Reicher2006b). When the Booroola mutation was carried by either the lamb or the ewe, it negatively affected the lambs' BWT. Differences in foetal growth rates between carriers and non-carriers of the Booroola mutation were detected as early as day 40 of gestation (Smith et al., Reference Smith, O, Hudson, Shaw, Heath, Condell, Phillips and McNatty1993 and Reference Smith, Hudson, Corrigan, Shaw, Smith, Phillips and McNatty1996).
Foetal and placental growth in pregnancies with multiple conceptuses
BWT and body composition
Individual foetuses in pregnancies with multiple conceptuses have lower BWTs than a lamb gestated as a singleton. On average, BWTs for lambs born as twins, triplets and quadruplets are 0.83, 0.70 and 0.63 that of singleton lambs, respectively (Gootwine, Reference Gootwine2005). Furthermore, in a study in which average BWTs of singletons, twins, triplets and quadruplets were 5.4, 4.4, 3.6 and 3.1 kg, respectively, gestation lengths for ewes carrying twins, triplets and quadruplets were reduced by an average of 0.5, 1.0 and 1.5 days, respectively, compared with that for ewes carrying a single foetus (Gootwine and Rozov, Reference Gootwine and Rozov2006). Thus, only a minor part of the reduction in BWT of foetuses born in litters can be attributed to reduced gestation length, as foetal growth rate during the last part of the gestation is estimated to be 101 g/day for twins compared with 199 g/day for singletons (Rattray et al., Reference Rattray, Garrett, East and Hinman1974).
The average increase in total litter weight for twins in sheep is about 1.6 times that for a single lamb (Freetly and Leymaster, Reference Freetly and Leymaster2004). As illustrated in Figure 5, divergence in foetal weight gain as the number of foetuses increases can be detected before day 100 of gestation (Winters and Feuffel, Reference Winters and Feuffel1936; Naaktgeboren and Stegeman, Reference Naaktgeboren and Stegeman1969; Rattray et al., Reference Rattray, Garrett, East and Hinman1974), when placental size is almost maximal but overall foetal mass is less than one-half that of a singleton foetus.

Figure 5 Foetal and placental growth in ewes with single, twin and triplet lambs (after Rattray et al. (Reference Rattray, Garrett, East and Hinman1974)).
The degree of litter-size-dependent IUGR—the rate at which BWT declines as litter size increases, was found to be highly variable among sheep populations (Gootwine, Reference Gootwine2005). The significance of that variability, which may result from both genetic and environmental factors, can be demonstrated by calculating the expected BWT of triplets in a population of ewes in which the average BWT of singleton lambs is 4.5 kg. Using the extreme values for litter-size-dependent IUGR, BWT of individual lambs born as triplets can vary between 1.7 and 3.6 kg, that is 0.38 to 0.80 of the BWT of lambs born as singletons, respectively.
The reduction in BWT of individual lambs as litter size increases is closely associated with a reduction in total energy and total protein content in the lamb's body (Rattray et al., Reference Rattray, Garrett, East and Hinman1974). Lower foetal weights in pregnancies with twin lambs is associated with reduced weights of muscle and, for some muscles, differences were found between singleton and twin lambs in fiber number and cross-sectional area (McCoard et al., Reference McCoard, Peterson, McNabb, Harris and McCutcheon1997 and Reference McCoard, McNabb, Peterson, McCutcheon and Harris2000).
Within-litter variation in BWT
Sibling foetuses may differ in their BWTs. Within-litter variability in BWT increases with litter size (Gootwine et al., Reference Gootwine, Rozov and Weller2006c) (Table 2). The degree of within-litter variability in lamb BWT may be defined by the maternal uterine environment or by segregation of genes among sibling foetuses that affect placental development and function, as well as foetal growth. Interestingly, a low but significant estimate of heritability was found for within-litter variation in BWT in pigs, suggesting that this trait can be improved in those animals by genetic selection (Damgaard et al., Reference Damgaard, Rydhmer, Lovendahl and Grandinson2003). In contrast, within-litter variation in lamb BWT has a very low heritability value, suggesting that most of that variation is non-genetic in nature (Gootwine et al., Reference Gootwine, Rozov and Weller2006c).
Table 2 Least-squares means±s.e. for birth weight (BWT) of Afec-Assaf lambs, within-litter coefficient of variation (CV) of BWT, and within-litter range of BWTs according to litter size (Gootwine et al., Reference Gootwine, Rozov and Weller2006c)
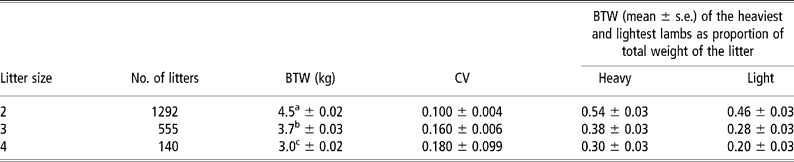
a,b,c Within a column, means with different letters differ significantly (P < 0.05).
Placentome number and size
Increasing the number of foetuses in a litter leads to higher occupancy of uterine caruncles and greater aggregate placental weight. However, there is an overall reduction in placental size and fewer placentomes per conceptus. Placentome number per conceptus decreases from about 70 for singletons to about 40 in twins, and 28 and 22 in triplets and quadruplets, respectively (Alexander, Reference Alexander1964b; Rhind et al., Reference Rhind, Robinson and McDonald1980; Greenwood et al., Reference Greenwood, Slepetis and Bell2000; Kaulfuss et al., Reference Kaulfuss, Schramm and Berttram2000; Pant et al., Reference Pant, Choi, Luther, Borowicz, Weigl, Kirsch, Kraft, Redmer, Reynolds and Grazul-Bilska2003; Dwyer et al., Reference Dwyer, Calvert, Farish, Donbavand and Pickup2005; Grazul-Bilska et al., Reference Grazul-Bilska, Pant, Luther, Borowicz, Navanukraw, Caton, Ward, Redmer and Reynolds2006). In studies in which total placentome numbers were relatively high, maximum occupancy of caruncles was observed in pregnancies with twins, while for litters in which total placentome numbers were relatively low, maximal occupancy of caruncles was found to be highest in ewes with triplets (Figure 6).

Figure 6 Association between litter size and number of placentomes per foetus according to different studies.
The decrease in placentome number per foetus with increases in litter size is associated with an increase in the average size of the placentomes (Figure 7), followed by an increase in the vascular density of cotyledons (Virrenga et al., Reference Virrenga, Borowicz, Luther, Pant, Redmer, Grazul-Bilska and Reynolds2004). However, despite the increase in placentome size, placentae of twins and triplets do not achieve the total placentome mass of single conceptuses.

Figure 7 Association between litter size and average weight of cotyledons according to different studies.
Manifestations of compensatory growth of the placenta when the number of placentomes is reduced have been demonstrated using three experimental models.
Model 1: uterine ligation
The ligation of one uterine horn and removal of the ipsilateral ovary restricts conceptus development throughout pregnancy by reducing the endometrial surface available for placental development (Bazer et al., Reference Bazer, Roberts, Basha, Zavy, Caton and Barron1979). The average number of caruncles occupied by the placenta of a single foetus was reduced by 47% in ewes with ligated uterine horns, to a level characteristic of placentome numbers for each conceptus in a litter of quadruplets (Caton et al., Reference Caton, Bazer, Kalra and Moffatt1984) or by 22%, to the level characteristic of a pregnancy with twins (Ott et al., Reference Ott, Wiley and Bartol1997). However, average foetal and placental weights in ewes with singletons did not differ at 140 days of gestation between control ewes and those with a ligated uterine horn.
Model 2: carunclectomy
The surgical removal of caruncles from the uteri of non-pregnant ewes results in fewer placentomes during pregnancy (Alexander, Reference Alexander1964a). This experimental model has been used to study the foetus' physiological adaptation to placental restriction (Robinson et al., Reference Robinson, Kingston, Jones and Thorburn1979; Butler et al., Reference Butler, Schwartz and McMillen2002; Danielson et al., Reference Danielson, McMillen, Dyer and Morrison2005). However, the reduction in placentome numbers by carunclectomy did not affect placental or foetal size, even when 67 caruncles were removed. These results again demonstrate the amazing capacity for compensatory development of the placentomes to support conceptus development in ewes.
Model 3: foetectomy
Unlike the situation in rabbits, rats and monkeys (Taylor et al., Reference Taylor, Jenkin, Robinson and Thorburn1983; Albrecht and Pope, Reference Albrecht and Pope1985), removal of the foetus from the pregnant uterus of ewes results in degeneration or expulsion of the placenta. This is attributed to a lack of adequate circulation in which the foetal heart is the pump required to sustain perfusion of the entire conceptus with blood. In an experiment where one of two foetuses in the pregnant uterus was removed surgically at day 50 of pregnancy, the remaining foetus and placenta grew to the size characteristic of a singleton conceptus (Vatnick et al., Reference Vatnick, Schoknecht, Darrigrand and Bell1991). The number of placentomes of the remaining conceptus was similar to the average number of placentomes for twins, but significantly lower than the average number of placentomes for pregnancies with a singleton conceptus.
Angiogenic factors
The increase in the vascular density of cotyledons in pregnancies with twin compared with singleton conceptuses has been associated with increased expression of VEGF and VEGF receptors at 140 days of gestation (Johnson et al., Reference Johnson, Reynolds, Redmer and Grazul-Bilska2005). However, a further increase in VEGF and its receptors was not detected in cotyledons of ewes carrying triplet compared with twin conceptuses. Thus, twins and triplets appear to differ in their ability to induce mechanisms involved in stimulating angiogenesis in placentomes. Measurements of NOx, defined as total plasma NO2 and NO3 in pregnant ewes (Magness et al., Reference Magness, Sullivan, Li, Phernetton and Bird2001), revealed that ewes pregnant with twins and triplets had more NOx than ewes carrying a single foetus.
Cardiovascular and uterine blood flow
Alterations in the cardiovascular system during pregnancy in sheep include decreases in arterial blood pressure, systemic vascular resistance and uterine vascular resistance, but increases in heart rate, cardiac output and blood volume. In sheep, the decrease in systemic vascular resistance, and the increase in cardiac output and expansion of blood volume are greater for ewes with multiple foetuses than singleton foetuses (Magness, Reference Magness and Bazer1998). Uterine arterial blood flow in ewes carrying twins at 120 to 130 days of gestation is 1.30- to 1.53-fold higher than in ewes carrying single foetuses (Christenson and Prior, Reference Christenson and Prior1978). However, when blood flow per kg foetus per min was considered, blood flow in twin conceptuses was on average only 0.87 of that of singleton conceptuses. Similarly, total uterine blood flow in ewes carrying triplets was 1.46-fold higher than for ewes carrying twins at 105 days of gestation, but uterine blood flow per kg foetus per min was similar for ewes with twin and triplet foetuses. An approx. 1.2-fold increase in total uterine blood flow was found in ewes carrying twin conceptuses compared with a singleton conceptus at 120 to 140 days of gestation (Caton et al., Reference Caton, Crenshaw, Wilcox and Barron1979).
Experimental reduction of uterine blood flow in ewes has been achieved by arterial occlusion and by the induction of embolisms using microspheres (Gagnon, Reference Gagnon2003). Daily injection of microspheres for 21 days into the foetal abdominal aorta during the last trimester of pregnancy decreased foetal arterial oxygen content, which was followed by a 28% reduction in foetal weight (Murotsuki et al., Reference Murotsuki, Gagnon, Matthews and Challis1996). Extreme (45%) or moderate (30%) reductions in uterine blood flow following arterial occlusion from day 113 to day 138 of gestation decreased foetal weights by 33 and 15%, and placental weights by 34 and 27%, respectively (Lang et al., Reference Lang, Baker, Khoury and Clark2000). Interestingly, the degree of reduction in foetal and placental weights in the extreme and moderately restricted blood-flow groups resembled the decrease in BWTs and placental weights in twins and triplets, respectively, compared with BWTs and placental weights for singleton conceptuses (Figure 5). A reduction in uterine blood flow is associated with an increase in the efficiency of nutrient transport from maternal to foetal blood (Owens et al., Reference Owens, Falconer and Robinson1987), but with a significant decrease in oxygen delivery and foetal arterial oxygen content (Boyle et al., Reference Boyle, Lecklitner and Liechty1996).
Nutrient levels
Glucose is the main source of energy for the growing foetus. During the last trimester of pregnancy, concentrations of glucose are stable in both the maternal and foetal circulations. Increases in total foetal body mass in pregnancies with multiple foetuses raise the demand for glucose. Accordingly, plasma concentrations of glucose in the foetus and ewe in the case of twin pregnancies are 20% and 30% less, respectively, than for singleton pregnancies during late gestation (Edwards and McMillen, Reference Edwards and McMillen2002; Vonnahme et al., Reference Vonnahme, Hess, Hansen, McCormick, Rule, Moss, Murdoch, Nijland, Skinner, Nathanielsz and Ford2003). The decline in plasma glucose concentration may be associated with changes in glucose-transporter abundance or activity; however, this has never been investigated in multiple pregnancies. Concentrations of free fatty acids and urea in the maternal plasma are similar for ewes with single and twin foetuses (Budge et al., Reference Budge, Dandrea, Mostyn, Evens, Watkins, Sullivan, Ingleton, Stephenson and Symonds2003). Although recent reports indicate significant changes in amino acid metabolism in the sheep conceptus during gestation, these changes have been studied in nonprolific ewes (Wu et al., Reference Wu, Wallace, Bazer and Spencer2006).
Function of the hypothalamic-pituitary-adrenal (HPA) axis
It is well established that the foetal HPA axis is activated during the pre-partum period and that the increase in circulating levels of cortisol in the foetus is important for both organ development and normal timing of parturition (Liggins, Reference Liggins1994). However, HPA-axis function appears to be suppressed in twin compared with single foetuses based on lower concentrations of ACTH and cortisol in the plasma and delayed onset of the pre-partum cortisol surge that determines time of parturition (Edwards and McMillen, Reference Edwards and McMillen2002; Gardner et al., Reference Gardner, Jamall, Fletcher, Fowden and Giussani2004). Interestingly, activation of the foetal HPA axis, as indicated by the initial increase in cortisol over basal levels, does not occur simultaneously in twins, even when they have similar BWTs (Schwartz and Rose, Reference Schwartz and Rose1998).
Plasma levels of prolactin (PRL), placental lactogen (PL) and growth hormone (GH)
Concentrations of both PRL and PL in maternal blood during late gestation are directly related to litter size (Butler et al., Reference Butler, Fullenkamp, Cappiello and Handwerger1981; Leibovich et al., Reference Leibovich, Gertler, Bazer and Gootwine2000). Both PRL and PL bind to PRL receptors (PRLRs), which are more abundant in adipose tissues of twin than singleton foetuses. Interestingly, PRLR abundance in livers was not found to be related to litter size (Budge et al., Reference Budge, Dandrea, Mostyn, Evens, Watkins, Sullivan, Ingleton, Stephenson and Symonds2003).
GH is expressed in the sheep placenta mainly during the early stages of pregnancy and it is involved in regulating endometrial gland proliferation and function (Spencer et al., Reference Spencer, Gray, Johnson, Taylor, Gertler, Gootwine, Ott and Bazer1999). Interestingly, Valinsky et al. (Reference Valinsky, Shani and Gootwine1990), showing the occurrence of gene duplication in the ovine GH locus, found two alleles: the GH1 allele with a single GH copy, and the GH2 allele with two gene copies designated GH2-N and GH2-Z. The GH2 allele is found in wild sheep and is the more frequent allele in most of the domesticated sheep breeds studied (E. Gootwine, unpublished results).
The duplicated copy of the GH2 allele is expressed in the placenta but not in the pituitary and differs from the original gene copy by two non-synonymous substitutions in the open reading frame (Ofir and Gootwine, Reference Ofir and Gootwine1997). The recombinant product of the duplicated copy of the ovine GH gene manifests ca. 10-fold higher binding affinity for the GH receptor than the product of the original GH gene copy (Gootwine et al., Reference Gootwine, Reicher and Gertler2006a). Interestingly, not all sheep carry the gene duplication. Thus, in pregnancies with multiple foetuses, conceptuses carrying the duplicated gene copy may have a selective advantage in a competition with sibling foetuses.
Plasma levels of progesterone and oestrogen
In sheep, plasma concentrations of progesterone increase continually throughout most of gestation. By 90 days of gestation, one-half of the estimated circulating levels of progesterone are derived from the corpus luteum and the other half from the placenta. Sulphated oestrogens are the primary oestrogens present in both maternal blood and foetal fluids throughout gestation. There are two periods of high oestrogen production by the placenta: the first is between days 31 and 46 and the second is after mid gestation. By day 90 of pregnancy, it is estimated that 90% or more of the circulating oestradiol-17β has been secreted by the placenta. Interestingly, circulating levels of progesterone and oestrogens are higher in twin than singleton pregnancies (reviewed by Magness (Reference Magness and Bazer1998)).
Plasma levels of pregnancy-associated glycoproteins
Pregnancy-associated glycoproteins (PAGs) belong to a multigene protein family (Hughes et al., Reference Hughes, Green, Garbayo and Roberts2000) which is related to aspartic proteinases. The PAG I group is expressed throughout pregnancy by the binucleated cells of the trophectoderm, while the PAG II group is expressed throughout the trophectoderm. In sheep, PAGs are secreted in a relatively constant manner during pregnancy and can be detected in the maternal serum at as early as day 20 of pregnancy.
Maternal plasma PAG level increases with an increase in foetal number, and this foetal-number effect is evident at as early as 25 days of gestation (Willard et al., Reference Willard, White, Wesson, Stellflug and Sasser1995; Ranilla et al., Reference Ranilla, Sulon, Mantecon, Beckers and Carro1997; Vandaele et al., Reference Vandaele, Verberckmoes, El Amiri, Sulon, Duchateau, Van Soom, Beckers and de Kruif2005). Use of PAG serum levels to predict litter size based on PAG concentration in early gestation was found to not be useful (Vandaele et al., Reference Vandaele, Verberckmoes, El Amiri, Sulon, Duchateau, Van Soom, Beckers and de Kruif2005).
Foetal kidney development
The abundance of mRNAs for genes that regulate foetal kidney development, i.e., IGF1, IGF1 receptor, IGF2, IGF2 receptor, GH receptor and glucocorticoid receptor, is lower in kidneys of twin versus singleton foetuses and inadequate maternal nutrition enhances these differences (Brennan et al., Reference Brennan, Gopalakrishnan, Kurlak, Rhind, Kyle, Brooks, Rae, Olson, Stephenson and Symonds2005).
Foetal and placental growth in other natural IUGR models
Altitude effects
The BWTs of lambs born to ewes at high altitude and exposed to hypobaric hypoxia were 24% lower than those of lambs born to ewes at or near sea level (Parraguez et al., Reference Parraguez, Atlagich, Diaz, Bruzzone, Behn and Raggi2005). However, placental weights were about 30% higher for lambs born at high altitude compared with those for lambs born at low altitude (Parraguez et al., Reference Parraguez, Atlagich, Diaz, Cepeda, Gonzalez, De Los Reyes, Bruzzone, Behn and Raggi2006). The effect of oxygen pressure on BWT was demonstrated experimentally by exposing ewes to hypobaric concentrations of oxygen, which resulted in reduced BWTs, without any change in placental weight (Jacobs et al., Reference Jacobs, Robinson, Owens, Falconer and Webster1988).
Age effect
IUGR is evident in both primiparous and multiparous ewes. In Polypay ewes, lamb BWTs increased from 76% of maximum for ewes (lambing at 11 months of age to maximum for ewes lambing at 76 months of age, and then declined to 97% of maximum for ewes lambing at 105 months of age (Notter et al., Reference Notter, Borg and Kuehn2005). Similar results were obtained with Assaf ewes (Figure 8; Gootwine and Rozov, Reference Gootwine and Rozov2006), which produced the heaviest lambs at their third parity. Increasing lamb BWTs up to the third parity were associated with increases in placental weight (Dwyer et al., Reference Dwyer, Calvert, Farish, Donbavand and Pickup2005).

Figure 8 Least-squares means for birth weights of lambs relative to parity of ewes (after Gootwine and Rozov (Reference Gootwine and Rozov2006)).
Primiparous ewes have usually not reached their mature body weight and therefore, foetal nutritional demands may be in conflict with maternal nutritional needs. Indeed, it was found (E. Gootwine, unpublished data) that age at first lambing does not affect BWTs of singleton lambs born to primiparous Assaf ewes lambing at 11 to 20 months of age (Figure 9). Only at 21 months of age were BWTs of lambs born to primiparous ewes found to be similar to BWTs of lambs born to mature multiparous ewes.

Figure 9 Least-squares means for birth weights of lambs born as singletons to primiparous Afec-Assaf ewes, according to age of ewe at lambing (n = 361, at least 15 ewes per age group).
Age-dependent IUGR in primiparous ewes has been associated with reduced expression of a number of angiogenic factors at 135 days of pregnancy, including expression in placentomes of VEGF, angiopoietin (ANG1), the ANG receptor Tie-2, endothelial NO synthase (eNOS) and soluble guanylate cyclase (sGC). Interestingly, these reductions in gene expression occurred in the cotyledonary, but not caruncular components of the placentomes (Borowicz et al., Reference Borowicz, Vonnahme, Grazul-Bilska, Redmer, Johnson and Reynolds2005).
Breeding out of season
One environmental factor that has a significant effect on lamb BWT is season. Lambs born in the autumn (short days) are, on average, 0.7 kg lighter than those born in spring (long days), regardless of litter size (Gootwine and Rozov, Reference Gootwine and Rozov2006). The reduction in foetal growth rate due to seasonal effects is first manifested at mid gestation, and gradually progresses as pregnancy advances, with reductions in the weights of cotyledons, but not caruncles, within placentomes (McCoard et al., Reference McCoard, Peterson, Jenkinson, Campbell and McCutcheon1996).
Litter-size-dependent IUGR and nutritional insult
Maternal undernutrition
Maternal undernutrition may lead to lower BWTs of foetuses and their placentae at term, depending on the severity and timing of the nutritional insult, either before or during pregnancy, and on the ewes' body condition during pregnancy. Moderate or severe undernutrition during mid or late gestation reduces lamb BWT (Heasman et al., Reference Heasman, Clarke, Stephenson and Symonds1999; Anthony et al., Reference Anthony, Scheaffer, Wright and Regnault2003; Redmer et al., Reference Redmer, Wallace and Reynolds2004; Luther et al., Reference Luther, Redmer, Reynolds and Wallace2005). Interestingly, undernutrition can also cause relative increases in the weights of specific foetal organs, such as the heart and lungs (Harding and Johnston, Reference Harding and Johnston1995).
Complete fasting in ewes carrying singeltons results in lower concentrations of glucose and amino acids in the maternal blood, a marked reduction in uterine blood flow, and decreases in uterine uptake of glucose and amino acids (Morriss et al., Reference Morriss, Rosenfeld, Carandell and Adcock1980). Undernutrition to 70% of nutrient requirements (National Research Council (NRC), 1985) throughout gestation also reduced maternal and foetal concentrations of blood glucose in singeltons' pregnancies (Edwards and McMillen, Reference Edwards and McMillen2002). Similarly, restriction to 50% of nutrient requirements (NRC, 1985) for pregnant ewes carrying singeltons between days 28 and 78 of gestation significantly reduced foetal and placental weights, as well as concentrations of total amino acids and polyamines in both the maternal and foetal plasma (Vonnahme et al., Reference Vonnahme, Hess, Hansen, McCormick, Rule, Moss, Murdoch, Nijland, Skinner, Nathanielsz and Ford2003; Kwon et al., Reference Kwon, Ford, Bazer, Spencer, Nathanielsz, Nijland, Hess and Wu2004).
Litter-size-dependent IUGR is similar to IUGR resulting from maternal undernutrition. Both cases are associated with an inadequate supply of nutrients to the growing foetus, due to reduction in uterine blood flow and lower concentrations of metabolites in the maternal and foetal circulations. It can therefore be anticipated that undernutrition of twin-bearing ewes will enhance physiological symptoms that are already observed in singleton foetuses gestated in undernourished mothers. Surprisingly, several studies (Table 3) indicate that twin foetuses respond differently from singleton foetuses to maternal undernutrition (Table 3).
Table 3 Response of sheep singleton and twin foetuses to various maternal-undernutrition treatments. Ewes' diet is expressed as percentage of normal requirements (National Research Council, 1985)

Maternal overnutrition
Re-alimentation of ewes following a period of undernutrition restores normal foetal growth (Kwon et al., Reference Kwon, Ford, Bazer, Spencer, Nathanielsz, Nijland, Hess and Wu2004). On the other hand, overfeeding ewes carrying multiple foetuses does not promote foetal growth to individual BWTs that are similar to those for singleton lambs. In an experiment in which gestating Afec-Assaf ewes were offered a diet calculated to meet the nutritional requirements of ewes carrying triplets (NRC, 1985), ewes that conceived naturally following oestrus synchronisation and carried triplets did not experience a loss in body weight during gestation and their pre-mating and post-partum body weights were similar (E. Gootwine, unpublished results; Figure 10). However, feeding the same diet to ewes with twins increased post-partum body weight of the ewes, but not the BWTs of the lambs. Ewes carrying four, five or six foetuses lost weight during pregnancy when fed the same diet.

Figure 10 Birth weights of lambs and deviation of maternal body weight post partum from pre-mating in an experiment where Afec-Assaf ewes (Volcani Center, Israel) were fed to meet the nutritional requirements of ewes carrying triplets (n = 7, 25, 77, 37,11 and 3 for litter sizes of 1, 2, 3, 4, 5 and 6, respectively).
Overnourishing adolescent ewes throughout gestation results in significant restriction in placental and foetal growth (Wallace et al., Reference Wallace, Luther, Milne, Aitken, Redmer, Reynolds and Hay2006). Similar to litter-size-dependent IUGR, maternal overnutrition does not increase BWT of foetuses gestated in adolescent ewes that manifest age-dependent IUGR (Wallace et al., Reference Wallace, Milne and Aitken2005).
Short- and long-term effects of large litter size on lamb health and performance
Short-term effects
Lower BWT for individual lambs born in large litters affects prenatal and neonatal survival (Fogarty et al., Reference Fogarty, Hopkins and van der Ven2000; Kleemann and Walker, Reference Kleemann and Walker2005). The survival rate of lambs (n = 31) whose BWT was 1.5 or less was as low as 0.42, although all were raised artificially (E. Gootwine, unpublished results). The lowest BWT for a lamb from that group that survived until 5 months of age was 1.0 kg. The BWT of lambs also affects health (Ross et al., Reference Ross, Desai, Guerra and Wang2005), behaviour (Dwyer et al., Reference Dwyer, Calvert, Farish, Donbavand and Pickup2005) and postnatal body composition.
Lower BWT is associated with reduced postnatal growth rate (Greenwood and Bell, Reference Greenwood and Bell2003; Gootwine et al., Reference Gootwine, Rozov, Bor and Reicher2006b). Even after BWT is included in a statistical model analysing lamb growth rate up to 5 months of age (Table 4), growth rate of lambs born as triplets and quadruplets is significantly lower than that of lambs born as singletons, suggesting that litter-size-dependent factors besides BWT are responsible for the relatively low postnatal growth ability of those lambs.
Table 4 Least-squares mean values for birth weight (BWT) and growth rate (GR) up to 5 months of age of Afec-Assaf lambs (following Gootwine et al., Reference Gootwine, Rozov, Bor and Reicher2006b). Main effects included in the statistical model for BWT and GR (model 1) were: sire, lambing group (crop), parity number, sex of lamb and litter size. BWT of the lambs was included in model 2

a,b,c,d Within a column, means with different letters differ significantly (P < 0.05).
Long-term effects
IUGR and low BWT of human infants have received special attention because of their long-term effects on adult onset of various diseases (Gluckman and Pinal, Reference Gluckman and Pinal2003; Schwartz and Morrison, Reference Schwartz and Morrison2005; Bloomfield et al., Reference Bloomfield, Oliver and Harding2006b; De Boo and Harding, Reference De Boo and Harding2006; Fowden et al., Reference Fowden, Giussani and Forhead2006a; Murphy et al., Reference Murphy, Smith, Giles and Clifton2006). Similarly, low BWT was found to have long-term effects in sheep on glucose tolerance and blood pressure at 5 months but not at 30 months of age, and long-term effects at 30 months but not at 5 months of age on insulin tolerance and circulating levels of IGF1 (Oliver et al., Reference Oliver, Breier, Gluckman and Harding2002). BWT also affects HPA-axis function as studied in 10-month-old ewes (Bloomfield et al., Reference Bloomfield, Oliver and Harding2006a) and this effect was similar for both singleton- and twin-born ewes.
Information on possible long-term effects of litter-size-dependent IUGR on sheep health, longevity, and reproduction and production performance is almost unavailable in the literature. The common practice of selecting only a portion of ewe lambs and only a few ram lambs as replacements, interferes with analyses of field records for long-term effects of litter size on performance as adults. Taking this into consideration, it was reported (Gootwine et al., Reference Gootwine, Rozov, Bor and Reicher2006b) that mature BWs of Assaf ewes are not affected by litter size and, therefore, by their BWTs.
Undernutrition of ewes during gestation may have long-term effects on the health of their progeny. Indeed, undernutrition effects on ewes were observed for up to 3 years after birth as their offspring had higher resting blood pressures and heart rates than control progeny, and decreased heart rates in response to norepinephrine-induced increases in blood pressure (Gopalakrishnan et al., Reference Gopalakrishnan, Gardner, Rhind, Rae, Kyle, Brooks, Walker, Ramsay, Keisler, Stephenson and Symonds2004). Undernutrition to 50% of requirements (NRC, 1985) during early to mid gestation impaired renal nephrogenesis, increased arterial blood pressure and increased expression of angiotensin-converting enzyme (ACE) in the renal cortex and expression of angiotensin II receptor (AT2) in the renal medulla of 9-month-old lambs, independent of effects on BWT (Gilbert et al., Reference Gilbert, Lang, Grant and Nijland2005). In addition, the consequences of undernutrition of ewes with single and twin foetuses were tested using the intravenous glucose tolerance test in their progeny at 1 year of age. It was found that glucose-insulin homeostasis is indeed affected by undernutrition of the dams, but is independent of foetus number, despite the fact that BWTs of lambs born as singletons and twins differed (Gardner et al., Reference Gardner, Tingey, Van Bon, Ozanne, Wilson, Dandrea, Keisler, Stephenson and Symonds2005).
Undernutrition during the prenatal period has also been shown to reduce reproductive capacity throughout the adult life of female offspring: ewes from mothers that experienced undernutrition to 50% of estimated metabolisable energy requirements for pregnancy, from mating until day 95 of gestation, had reduced ovulation rates at 20 months of age (Rae et al., Reference Rae, Kyle, Miller, Hammond, Brooks and Rhind2002). On the other hand, the reproductive function of male offspring in the same study was unaffected by prenatal undernutrition.
New paradigm for litter-size-dependent IUGR
Reduced numbers of placentomes leading to smaller placenta has been associated with relatively low BWTs of individual lambs born in large litters. However, results of several studies indicate that the placenta can manifest compensatory growth under conditions of reduced numbers of placentomes and limited uterine space, such that foetal development and BWTs are normal for single lambs (Alexander, Reference Alexander1964a; Caton et al., Reference Caton, Bazer, Kalra and Moffatt1984). Such compensatory growth of the conceptus does not normally occur in pregnancies with multiple foetuses. In contrast, compensatory growth of the conceptus did occur when one of two twin foetuses was removed at day 50 of gestation, to allow for enhanced growth of the remaining conceptus to that characteristic of a singleton (Vatnick et al., Reference Vatnick, Schoknecht, Darrigrand and Bell1991). These results clearly suggest that in multi-foetal pregnancies, reduced placentome number does not account for foetal IUGR.
Possible explanations for litter-size-dependent IUGR might be the inability of the maternal system fully to meet nutrient demands of multiple conceptuses, mutual growth interference among sibling foetuses, and maternal inhibition of placental and foetal growth in the case of multiple conceptuses by downregulation genes controlling placental growth and nutrient transport, as was noted in the case of maternal genomic imprinting (Isles and Holland, Reference Isles and Holland2005; Angiolini et al., Reference Angiolini, Fowden, Coan, Sandovici, Smith, Burton, Tycko, Reik, Sibley and Constância2006; Cattanach et al., Reference Cattanach, Beechey and Peters2006; Fowden et al., Reference Fowden, Sibley, Reik and Constância2006b).
Litter-size-dependent IUGR and nutrition insult
The observations that blood flow per foetus in twin pregnancies is less than that in singleton pregnancies (Christenson and Prior, Reference Christenson and Prior1978), and that concentrations of glucose in the maternal and foetal circulations are lower in twin than in singleton pregnancies (Vonnahme et al., Reference Vonnahme, Hess, Hansen, McCormick, Rule, Moss, Murdoch, Nijland, Skinner, Nathanielsz and Ford2003), support the notion that litter-size-dependent IUGR results from the maternal system's inability fully to support growth of multiple conceptuses. However, three lines of evidence suggest that nutrient supply from the dam to the conceptus may not be the sole rate-limiting factor for foetal growth in pregnancies with multiple foetuses, or at least for twin pregnancies.
● Litter-size-dependent IUGR is evident at about day 100 of pregnancy (Winters and Feuffel, Reference Winters and Feuffel1936; Naaktgeboren and Stegeman, Reference Naaktgeboren and Stegeman1969; Rattray et al., Reference Rattray, Garrett, East and Hinman1974), when foetal size and growth rate are minimal, but placental size is maximal (Figure 5). Interestingly, the conclusion that the foetal growth trajectory in twins is determined early in gestation can also be drawn from studies of women with multiple conceptuses (Alexander et al., Reference Alexander, Hammond and Steinkampf1995), in which foetal number was reduced early in pregnancy. In those studies, foetal size and gestation length were related to the initial number of foetuses and not to the number of foetuses present at delivery.
● Total weight of foetuses in a litter increases as litter size increases (Freetly and Leymaster, Reference Freetly and Leymaster2004). Thus, total weight of triplets and quadruplets at birth is more than twice that of the BWT of single lambs, indicating that the ewe could support growth of each twin to the size of a singleton lamb.
● Overnutrition of ewes with twin conceptuses increases maternal body weight rather than enhancing intrauterine growth of the twin foetuses (Figure 10).
Litter-size-dependent IUGR and mutual foetal growth inhibition
Mutual growth interference among sibling foetuses can be mediated by direct interactions between placentae of conceptuses in the same uterus. However, to date, there is no experimental evidence in sheep to support this hypothesis.
Litter-size-dependent IUGR and placental reprogramming
An alternative explanation for litter-size-dependent IUGR is that the patterns of placental and foetal growth are reprogrammed in pregnancies with multiple foetuses such that they do not follow their normal course of intrauterine development as determined by their genetic potential. This reprogramming event may take place at mid gestation, after day 50 of pregnancy, as it was shown that conceptuses at that stage retain their full growth potential (Vatnick et al., Reference Vatnick, Schoknecht, Darrigrand and Bell1991).
Regulation of foetal growth involves multidirectional interactions between the mother, the placenta and the foetus (Murphy et al., Reference Murphy, Smith, Giles and Clifton2006). Reprogramming patterns of conceptus growth by attenuating placental growth and function, i.e. nutrient transport and hormone production (Spencer et al., Reference Spencer, Johnson, Bazer and Burghardt2004), may limit foetal growth to term, as foetal size is clearly correlated with size of the placenta (Greenwood et al., Reference Greenwood, Slepetis and Bell2000). The reduction in size and function of individual placenta in pregnancies with multiple conceptuses may result from both reduced placental cell proliferation and, possibly, increases in apoptosis early in gestation, as observed in a well-fed adolescent IUGR experimental model (Lea et al., Reference Lea, Hannah, Redmer, Aitken, Milne, Fowler, Murray and Wallace2005).
The mechanism(s) that induces the proposed litter-size-dependent IUGR is not yet clear. However, it may involve endocrine signals from the maternal compartment. Indeed, a mechanism whereby maternal hormones affect conceptus growth has been proposed for season-dependent IUGR (Gootwine and Rozov, Reference Gootwine and Rozov2006), where seasonal variation in BWT was proposed to be related to the pattern of maternal melatonin secretion.
Adaptation of placental and foetal growth to a multi-foetal pregnancy situation can be mediated through alterations in the expression of placental and foetal imprinted genes, shown to be involved in the control of foetal growth and nutrient-transport efficiency through the placenta (Angiolini et al., Reference Angiolini, Fowden, Coan, Sandovici, Smith, Burton, Tycko, Reik, Sibley and Constância2006). As maternal imprinted genes are more involved in restraining placental and foetal growth, their down-regulation in multi-foetal pregnancies can be suggested as a mechanism underlying litter-size-dependent IUGR.
Reprogramming foetal growth during litter-size-dependent IUGR relies on the recognition of pregnancies involving multiple conceptuses. The signal for the presence of more than one foetus can be circulating levels of PAGs (Hughes et al., Reference Hughes, Green, Garbayo and Roberts2000) or circulating levels of hormones produced by the placenta during mid gestation, i.e. progesterone and oestrogens (Magness, Reference Magness and Bazer1998), and members of the GH-PRL family (Anthony et al., Reference Anthony, Liang, Kayl and Pratt1995). The plasma concentrations of all of these factors are positively correlated with numbers of conceptuses.
Litter-size-dependent IUGR as a foetal protective mechanism
The relatively low BWTs of individual lambs born in large litters may negatively affect their neonatal and postnatal survival rates (Kleemann and Walker, Reference Kleemann and Walker2005). However, the adaptive mechanisms in pregnancies with multiple conceptuses that delay placental and foetal growth at mid-gestation may have the advantage of protecting foetuses from nutritional insults during advanced stages of gestation, when their daily weight gains are maximal. Indeed, Harding and Johnston (Reference Harding and Johnston1995) reported that foetuses growing slowly before the onset of undernutrition of their dams do not further reduce their growth rates. In addition, restricted foetal growth rates reduce the risk of pregnancy toxemia which, in sheep, may result in death of both the mother and its foetuses.
Further studies are needed to understand the mechanisms that regulate foetal growth in multi-foetal pregnancies and how those mechanisms may differ from mechanisms that control growth and development of singleton foetuses. The same signals that may restrict foetal growth in multi-foetus pregnancies may, under pathological conditions, induce IUGR in singleton pregnancies.


















