Infants with Down Syndrome and CHD have altered loading conditions due to elevated pulmonary vascular resistance which persists beyond the early neonatal period coupled with increased left ventricular pre-load due to the preponderance of left to right shunt lesions. Reference Hawkins, Langton-Hewer, Henderson and Tulloh1 Pulmonary hypertension is an important co-morbidity and adds to the complexity of their management in both the pre-operative and post-operative period. Reference Saji2 The impact of the altered loading conditions on systolic and diastolic performance of the right and left ventricles and on the interplay between the ventricles during the peri-operative period is poorly understood. The presence of structural CHD can impose significant volume and pressure loading burdens on the right or left ventricles, both of which may influence myocardial performance.
There is limited data on myocardial deformation assessed using speckle-tracking echocardiography in infants with Down syndrome during the peri-operative period. The most significant value of deformation analysis rests in its ability to detect subclinical myocardial dysfunction before the appearance of clinically significant global ventricular impairment. Our group and collaborators have recently demonstrated the feasibility and reproducibility of the use of speckle-tracking echocardiography in the infant population. Reference Levy, Machefsky and Sanchez3,Reference Nestaas, Schubert, de Boode and El-Khuffash4 Further evaluation of right and left ventricular function is essential to categorise the degree of impairment of myocardial performance in order to optimise peri-operative management. Failure of full recovery of myocardial function may have significant long-term implications for post-operative infants.
In this study, we aimed to characterise the impact of Down syndrome, the type of cardiac lesion, and intra-operative cross-clamp time on peri-operative myocardial performance and loading conditions in infants with CHD with and without a diagnosis of Down syndrome. We also aimed to assess the relationship between myocardial performance, the type of CHD and a diagnosis of Down syndrome on short-term post-operative clinical outcomes. We hypothesised that a diagnosis of Down syndrome is associated with a distinct pattern of myocardial performance over the peri-operative period affecting the right and left ventricle that may influence the post-operative clinical course.
Materials and methods
Study setting and patient population
This was a prospective observational study carried out in The Children’s Heart Centre of Children’s Health Ireland at Crumlin between July 2018 and April 2020. Infants with Down syndrome who had CHD with a systemic to pulmonary shunt of either ventricular septal defect or atrioventricular septal defect were recruited and matched with infants with similar systemic to pulmonary shunt lesions who had a normal chromosome complement. The two groups of infants were recruited prior to planned surgical intervention from the cardiac outpatient paediatric clinic. The non-Down syndrome group was matched as much as practical to the Down syndrome group by cardiac lesion, gender, age, and weight at surgery, while recognising the preponderance of atrioventricular septal defects in infants with Down syndrome.
The exclusion criteria for the study were infants with obstructive or cyanotic CHD (including tetralogy of Fallot), those with chromosomal or genetic abnormalities other than Down syndrome, and pre-term infants with a gestational age at birth less than 33 weeks. Ethical approval was granted by the Local Ethics Committee and informed parental written consent was obtained from all participants prior to enrolment.
Clinical data collection
Clinical data collected included gestation at birth, birth weight, sex, age, and weight at surgery. Post-operative inotrope use was captured using a vasoactive inotropic score calculated as follows: = Dopamine + Dobutamine + (100 × Adrenaline) + (100 × Noradrenaline) + (10 × Milrinone) + (10,000 × Vasopressin). Reference Gaies, Gurney and Yen5 The following post-operative clinical outcomes were recorded for each enrolled infant: presence or absence of sinus rhythm; chylothorax defined as evidence of chyle draining from the pleural space with confirmed biochemical parameters quantifying by triglyceride level; the use of nitric oxide; mortality; duration of inotrope therapy, invasive ventilation, ICU stay and total hospital stay.
Echocardiography assessment
We performed echocardiography assessments peri-operatively in the two groups of the study infants at three time points: pre-operatively, post-operatively (within the first 24 hours following the procedure), and pre-discharge (within 24 hours of planned discharge); the timing of the third scan in infants with Down syndrome occurred at 10 (7–15) days following surgery compared with 5 (4–8) days in the Control group. We used the General Electric Echocardiography system (Vivid E95; General Electric Healthcare, Milwaukee, Wisconsin). All studies were conducted using a standardised imaging protocol in accordance with published guidelines. Reference de Boode, Singh and Gupta6,Reference Groves, Singh and Dempsey7 Image acquisition was very feasible in the cohort with left and right functional measurements available in all infants during the first and second scans and in 53/55 infants with Down syndrome and 28/29 controls pre-hospital discharge. The images were stored on an archiving system in raw format for later offline analysis. All images were analysed using the General Electrics EchoPac system version 112). We obtained the following echocardiography measurements in accordance with established guidelines. Reference Nestaas, Schubert, de Boode and El-Khuffash4,Reference Groves, Singh and Dempsey7-Reference Lopez, Colan and Frommelt9
Myocardial anthropometric measurements
Myocardial anthropometric measurements included mitral valve annulus (mm), left ventricular length (mm), left atrial to aortic root ratio, left ventricular end-diastolic diameter (mm), tricuspid valve annulus (mm), right ventricular length (mm), and right ventricular mid cavity diameter (mm). Reference Kossaify10
Clinical cardiovascular and afterload measurements
Clinical cardiovascular measurements and afterload measurements included heart rate, blood pressure (mmHg), right ventricular systolic pressure (mmHg) derived from the tricuspid valve regurgitant jet, left ventricle end-systolic wall stress (dynes/cm2), which was estimated using the non-invasive echocardiography method and using the formula Reference Wilson, Reichek and Hirshfeld11 :
 $$ = {\matrix{ 0.98 \times (0.344 \times {\rm{systolic}}{\mkern 1mu} {\rm{blood}}{\mkern 1mu} {\rm{pressure}} \times \hfill \cr {\rm{Left}}{\mkern 1mu} {\rm{ventircular}}{\mkern 1mu} {\rm{end}}{\mkern 1mu} {\rm{systolic}}{\mkern 1mu} {\rm{diameter}}) \hfill \cr} \over \matrix{ {\rm{Left}}{\mkern 1mu} {\rm{ventricular}}{\mkern 1mu} {\rm{posterior}}{\mkern 1mu} {\rm{wall}}{\mkern 1mu} {\rm{diameter}}{\mkern 1mu} {\rm{in}}{\mkern 1mu} {\rm{systole}} \times \hfill \cr (1 + {\rm{Left}}{\mkern 1mu} {\rm{ventricular}}{\mkern 1mu} {\rm{posterior}}{\mkern 1mu} {\rm{wall}}{\mkern 1mu} {\rm{diameter}}{\mkern 1mu} {\rm{in}}{\mkern 1mu} {\rm{systole}} \div \hfill \cr {\rm{Left}}{\mkern 1mu} {\rm{ventircular}}{\mkern 1mu} {\rm{end}}{\mkern 1mu} {\rm{systolic}}{\mkern 1mu} {\rm{diameter}}) - 2 \hfill \cr} }$$
$$ = {\matrix{ 0.98 \times (0.344 \times {\rm{systolic}}{\mkern 1mu} {\rm{blood}}{\mkern 1mu} {\rm{pressure}} \times \hfill \cr {\rm{Left}}{\mkern 1mu} {\rm{ventircular}}{\mkern 1mu} {\rm{end}}{\mkern 1mu} {\rm{systolic}}{\mkern 1mu} {\rm{diameter}}) \hfill \cr} \over \matrix{ {\rm{Left}}{\mkern 1mu} {\rm{ventricular}}{\mkern 1mu} {\rm{posterior}}{\mkern 1mu} {\rm{wall}}{\mkern 1mu} {\rm{diameter}}{\mkern 1mu} {\rm{in}}{\mkern 1mu} {\rm{systole}} \times \hfill \cr (1 + {\rm{Left}}{\mkern 1mu} {\rm{ventricular}}{\mkern 1mu} {\rm{posterior}}{\mkern 1mu} {\rm{wall}}{\mkern 1mu} {\rm{diameter}}{\mkern 1mu} {\rm{in}}{\mkern 1mu} {\rm{systole}} \div \hfill \cr {\rm{Left}}{\mkern 1mu} {\rm{ventircular}}{\mkern 1mu} {\rm{end}}{\mkern 1mu} {\rm{systolic}}{\mkern 1mu} {\rm{diameter}}) - 2 \hfill \cr} }$$
Left ventricular deformation measurements
Left ventricular deformation measurements included left ventricular global longitudinal strain (%), basal longitudinal strain (%), and basal longitudinal systolic strain rate (1/s), apical longitudinal strain (%), and longitudinal systolic strain rate (%). Global longitudinal strain and strain rates were calculated by averaging all the values of the regional peak longitudinal strain and strain rates obtained from 18 segments in two-chamber, three-chamber, and four-chamber in apical long-axis views. 8
Left ventricular rotational mechanics
Left ventricular rotational mechanics included twist (degrees), torsion (twist indexed to length, degrees), apical rotation (degrees), basal rotation (degrees), twist rate (degrees/second), and untwist rate (degrees/second).
Right ventricular deformation measurements
Right ventricular measurements included free wall strain (%) and free wall systolic strain rate (1/s). Strain and strain rate were measured from the right ventricular-focused apical four-chamber view using speckle-tracking echocardiography in accordance with previously published image acquisition and data analysis protocols. Reference Levy, Holland, Sekarski, Hamvas and Singh12
Statistical analysis
Continuous data were tested for normality using the Shapiro–Wilk test and a histogram representation of data and summarised as means (standard deviation) or medians (interquartile range) as appropriate. Categorical data were summarised as counts (%). Two group analyses were conducted using the Student’s t-test, the Mann–Whitney U test as appropriate, or Chi-square test as appropriate. Correlations between continuous variables were conducted using either Pearsons’s or Spearman’s correlation coefficients as appropriate. Linear regression analysis was conducted to assess the association between important predictors and relevant outcomes. Two-way analysis of variance was used to assess the changes in the functional parameters between the groups overtime. We used SPSS version 26 to conduct the analyses. Statistical significance was achieved with a p-value <0.05.
Results
Eighty-four infants were included in the study. Fifty-five infants had a diagnosis of Down syndrome and these were compared with 29 infants who were matched for cardiac lesion with normal chromosomal complement (Control group). There was no difference in the age or weight at surgery between the Down syndrome and Control groups. Except for gestational age which was lower in the Down syndrome group, there were no differences in the other basic demographics between the groups (Table 1). There was a higher rate of post-operative heart block and chylothorax in the Down syndrome group; in addition, cross-clamp time, vasoactive inotrope duration and score, invasive ventilation days, and intensive care days were all higher in the Down syndrome group. There was no difference in mortality (Table 1). None of the infants were intubated or were on inotropes prior to the surgery. In infants with Down syndrome, all values for left ventricular dimensions including mitral valve annulus (11 ± 1 versus 12 ± 1 mm, p < 0.01), end-diastolic diameter (17 ± 4 versus 22 ± 6 mm, p < 0.01), and length (34 ± 3 versus 36 ± 3 mm, p < 0.01) were significantly smaller than the Control group (Table 2). Right ventricular length measurements in the Down syndrome group were also smaller than in the Control group (Table 2). Of note, none of the infants had any significant residual atrioventricular valve regurgitation or left ventricular outflow tract obstruction.
Table 1. Demographics and clinical outcomes of the study cohort

Values are presented as median [interquartile range, IQR], or count (%) as appropriate. p-Value of < 0.05 was considered significant (p-value only compared Down syndrome and Controls, healthy infants are a reference value). AVSD = atrioventricular septal defect, PICU = Pediatric Intensive Care Unit, VIS = vasoactive inotrope score, VSD = ventricular septal defect.
Table 2. Myocardial anthropometric measurements in the study cohort
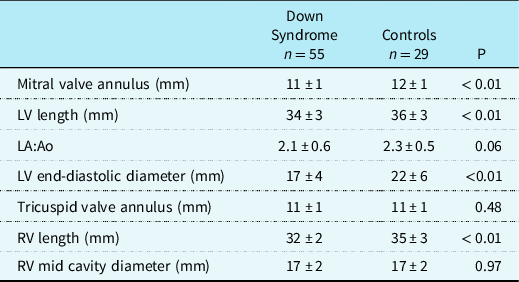
Values are presented as means ± SD. p-Value of < 0.05 was considered significant. LV = left ventricle, LA:Ao = left atrial to aortic root ratio, RV: right ventricle.
There were no differences in heart rate between the groups throughout the study period (Fig 1). Infants with Down syndrome had significantly lower systolic blood pressure at all three time points and lower diastolic blood pressure in the post-operative period when compared to Controls (Fig 1). Left ventricular wall stress values were significantly lower in the Down syndrome group throughout the study period and this persisted at discharge (79 ± 17 versus 99 ± 27 dynes/cm2, p < 0.01; Fig 1). Right ventricular systolic pressure remained higher in the Down syndrome group throughout the study period (27 ± 14 versus 12 ± 6 mmHg at discharge, p < 0.01; Fig 1).

Figure 1. Clinical cardiovascular measurements and afterload measurements. Values are presented as means (diamonds) and one standard error (Whiskers). * p < 0.05 comparing Down syndrome group (red) to Control group (blue), † p < 0.05 change over time in the entire cohort. ESWS = end-systolic wall stress, RVSp = right ventricle systolic pressure, Pre-op = pre-operatively, Post-op = post-operatively.
Left ventricular global longitudinal strain and apex longitudinal strain values were not significantly different between the groups on the first pre-operative Echo (Fig 2). Post-operatively, both study groups had lower values recorded with significantly higher global (18 ± 3 versus 16 ± 3%, p = 0.01) and apical strains (22 ± 5 versus 19 ± 4 %, p = 0.01) in the Down syndrome group pre-discharge. The measured values did not return to the pre-operative values at the point of hospital discharge for either group (Fig 2). Longitudinal strain rate was reduced in both study groups to a similar degree post-operatively and pre-discharge (Fig 2). Basal longitudinal strain was similar in both study groups on the first scan, and the post-operative reduced values persisted in both study groups until hospital discharge (Fig 2).
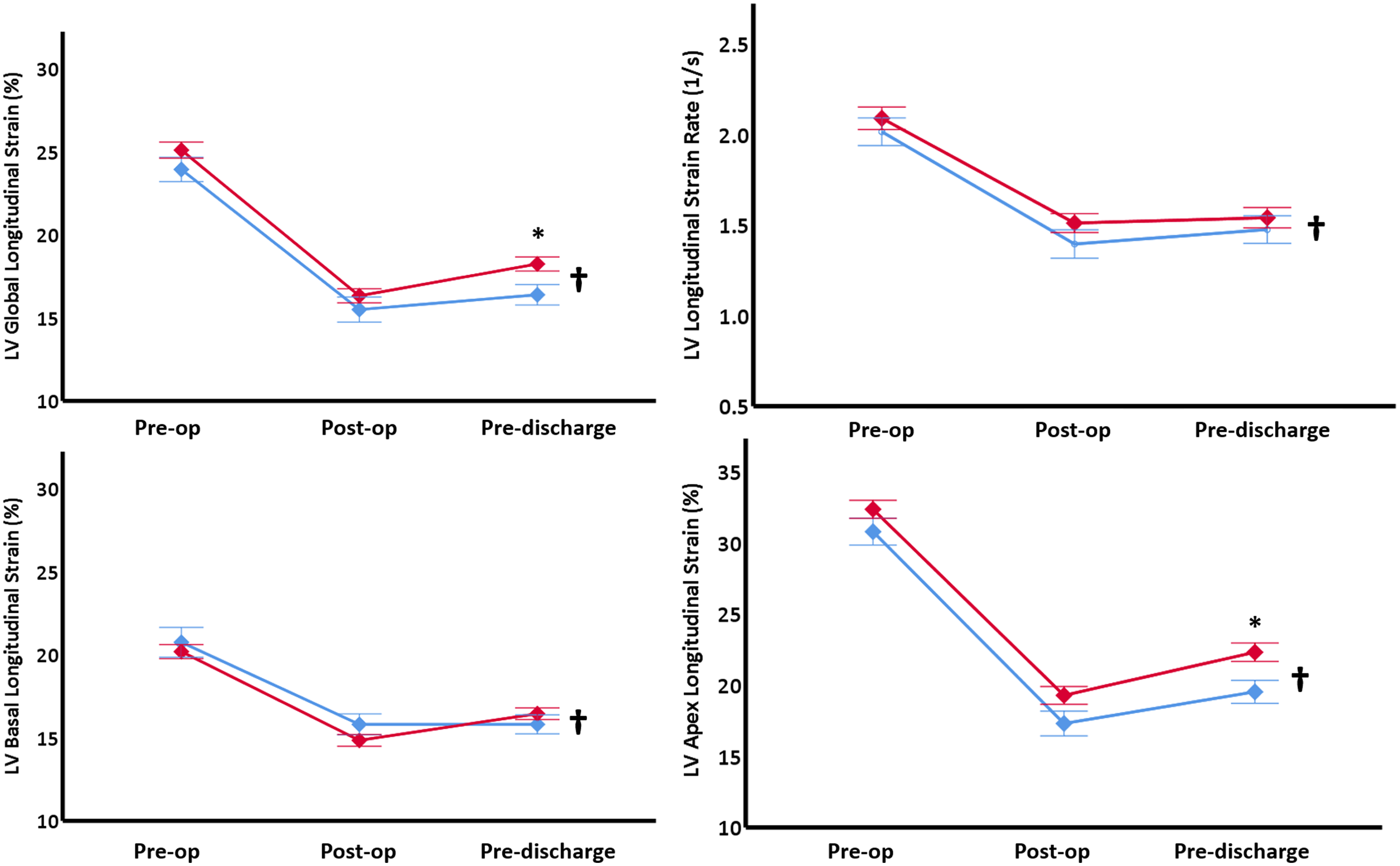
Figure 2. LV deformation measurements. Values are presented as means (diamonds) and one standard error (Whiskers). * p < 0.05 comparing Down syndrome group (red) to Control group (blue), † p < 0.05 change over time in the entire cohort. LV = left ventricle, Pre-op = pre-operatively, Post-op = post-operatively.
In infants with Down syndrome, left ventricular twist, torsion, twist rate, and untwist rate remained significantly higher than in the Control group post-operatively and at discharge (p < 0.05) (Fig 3). There was a significant reduction in the twist and torsion values in both groups post-operatively and neither of the groups returned to baseline values pre-discharge (Fig 3). In the Down syndrome group, apical rotation values were higher throughout the study period with highest rotation values measured pre-discharge (7.3 ± 5.0 versus 5.0 ± 4.6 degrees, p = 0.04) (Fig 3). There was a significant reduction in the values for basal rotation over time in both study groups (Fig 3). Left ventricular twist rate (176 ± 71 versus 118 ± 73 degrees/second, p < 0.01) was lower in the Down syndrome group at discharge (Fig 3).

Figure 3. LV rotational mechanics. Values are presented as means (diamonds) and one standard error (Whiskers). * p < 0.05 comparing Down syndrome group (red) to Control group (blue), † p < 0.05 change over time in the entire cohort. LV = left ventricle, Pre-op = pre-operatively, Post-op = post-operatively.
Infants with Down syndrome had the lowest right ventricular strain on all scans with significantly lower values pre-discharge (14 ± 4 versus 18 ± 6%, p < 0.01; Fig 4). Right ventricular free wall strain rate in the study groups did not significantly differ in values through the study period (Fig 4).

Figure 4. RV function measurements. Values are presented as means (diamonds) and one standard error (Whiskers). * p < 0.05 comparing Down syndrome group (red) to Control Group (Blue), † p < 0.05 change over time in the entire cohort. RV = right ventricle, Pre-op = pre-operatively, Post-op = post-operatively.
On univariate analysis, there were no differences in left and right strain values or twist/torsion measurements between infants with an atrioventricular septal defect and those with a ventricular septal defect throughout the study period (all p > 0.05, data not shown). In addition, there were no correlations between the duration of cross-clamp and any of the post-operative functional measurements (all p > 0.05, data not shown). There were no differences in either end-systolic wall stress or right ventricular systolic pressure according to the type of lesion within each group (Fig 5). On multilinear linear regression, a diagnosis of Down syndrome was an independent predictor of left ventricular torsion and right ventricular free wall strain at discharge (echo 3) when controlling for lesion type, and cross-clamp time. There was no association between Down syndrome and left ventricular global longitudinal strain (Table 3). In addition, a diagnosis of Down syndrome was an independent predictor of duration of invasive ventilation, intensive care stay, and post-operative vasoactive score when controlling for lesion type and pre-operative myocardial performance. Pre-operative right ventricular free wall strain was also an independent predictor of intensive care stay (Table 4).

Figure 5. ESWS and RVSP according to lesion type within each group. Box plot represents medians, interquartile range and 5th and 95th percentile (whiskers). ESWS = end-systolic wall stress, RVSP = right ventricle systolic pressure.
Table 3. Association between clinical characteristic and myocardial function at discharge

Values presented are standardised β and p values. AVSD = atrioventricular septal defect, LV = left ventricle, RV Fw LS = right ventricular free wall longitudinal strain, VSD = ventricular septal defect.
Table 4. Association between clinical characteristic/pre-operative myocardial function and clinical outcomes
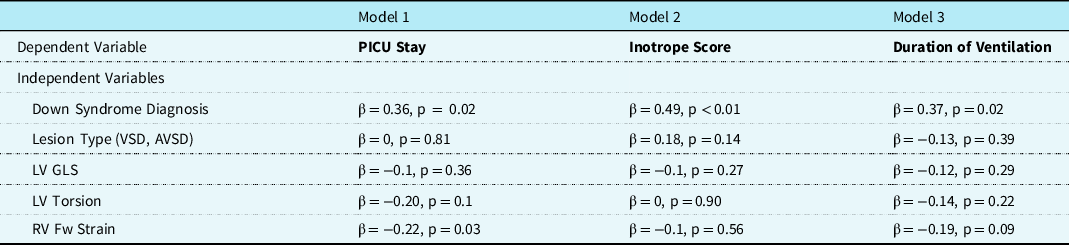
Values presented are standardised β and p values. AVSD = atrioventricular septal defect, LV = left ventricle, LV GLS = left ventricular global longitudinal strain, RV Fw LS = right ventricular free wall longitudinal strain, PICU = Pediatric Intensive Care Unit, VSD = ventricular septal defect.
Discussion
In this study, we provide a comprehensive appraisal of myocardial performance during the peri-operative period, and a description of the post-operative clinical outcomes in children with Down syndrome and those with a normal chromosomal complement undergoing CHD repair. There was a reduction in left and right function measured using deformation analysis in the entire cohort following CHD repair, with infants with Down syndrome demonstrating partial recovery in left ventricular strain, while Control infants demonstrated partial recovery in right ventricular strain pre-discharge. A diagnosis of Down syndrome was an independent predictor of pre-discharge myocardial performance and important clinical outcomes. Interestingly, the type of lesion and cross-clamp time did not appear to influence left or right ventricular function or loading conditions suggesting that a diagnosis of Down syndrome is the main determinant of post-operative myocardial performance. There was also a higher rate of heart block and chylothoraces in the Down syndrome group. The rate of mortality was low in both cohorts, and it was therefore difficult to examine important relationships with this outcome.
The relationship between down syndrome and myocardial function
Infants with Down syndrome undergoing surgery had a distinct morphological and loading condition profile when compared with their counterparts with a normal chromosomal complement. This was characterised by smaller left and right ventricles demonstrated by smaller lengths and annular measurements. The smaller left ventricular lengths and cavity size are likely a consequence of reduced pulmonary venous return secondary to increased pulmonary vascular resistance. In addition, left ventricular end-systolic wall stress was significantly lower and right ventricular systolic pressure was significantly higher in infants with Down syndrome throughout the study period. Wall stress is determined by the afterload of the systemic blood pressure. Infants with Down syndrome presented with significantly lower systolic blood pressure in three time points with lower diastolic blood pressure in the post-operative period in comparison with infants with CHD, but their heart rate did not significantly differ. This may reflect the autonomic dysfunction described in infants with Down syndrome who have lower systemic but higher pulmonary vascular resistance. Reference Bloemers, van Bleek, Kimpen and Bont13,Reference Brun, Holmstrom and Thaulow14
Those morphological and load differences explain the differential change in left and right function prior to hospital discharge: the higher left ventricular function and lower right ventricular function seen in infants with Down syndrome prior to hospital discharge. The lack of difference in left and right strain rate values between the two groups throughout the study period and particularly prior to discharge further suggest the differences seen in function between the group is secondary to differences in loading conditions rather than impairment in contractility. Strain is influenced by pre-load and afterload and is relatively uninfluenced by chamber size during diastole, while strain rate reflects contractility and less pre-load-dependant. Reference Breatnach, Levy, Franklin and El-Khuffash15-Reference Alvarez, Fortin-Pellerin and Alhabdan17 It must be acknowledged, however, that the pre-discharge scan in the Down syndrome group was conducted at a later time point as discharge was delayed by an excess of post-operative co-morbidities. This may have allowed more time for recovery of left ventricular global longitudinal strain measurements toward normal values. Other studies have demonstrated that the recovery of strain rate values to normal can take up to 1 month post-operatively. Reference Rafieyian, Roodpeyma, Vahidshahi and Moghadasi18 This exceeds the time frame for measurements for our study. The better performance on assessment of apical strain as opposed to suboptimal basal strain values was likely explained by the significantly greater surgical manipulation, including patch placement in this area in the Down syndrome group who had a significant excess of inlet ventricular septal defects with additional atrio-ventricular valve pathology.
Interestingly, we did not find an association between the type of lesion and any of the functional measurements either on univariate analysis or on multivariate linear regression. In addition, we did not demonstrate an association between duration of cross-clamp time on either univariate or multivariate analysis. This further suggests that the differences observed in function at discharge are secondary to the unique morphological and loading profile exhibited by infants with a diagnosis of Down syndrome rather than the accompanying CHD or intra-operative factors.
During this study, we have demonstrated that infants with Down syndrome had enhanced left ventricular twist, torsion, and twist rate at discharge when compared to controls. Increased twist and untwist rates are reported in older adults and pre-term infant populations, as a compensatory response to reduced longitudinal function. Reference Burns, La, Prior and Macisaac19 In our study population, the twist and untwist rates were higher in Down syndrome which was more likely to represent a maladaptive response mechanism in a population with increased left ventricular fibrosis and increased dysfunction. Both groups exhibited a significant reduction in twist and torsion post-operatively. This is likely due to impaired rotational mechanisms (particularly in the basal segment) secondary to the surgical stress on the myocardium and higher pulmonary pressures post-operatively, which remains higher in Down syndrome population and persists beyond discharge.
The relationship between Down syndrome and clinical outcomes
Infants with Down syndrome exhibited a higher rate of post-operative morbidities including higher incidence of heart block, chylothoraces, use of inotropes and nitric oxide, and a longer duration of intensive care and over all hospital stay. The higher rate of atrioventricular septal defects was the most likely contributing factor to the observed increase in the cross-clamp times in the DS/CHD group. Operative complexity was also likely a factor in the increased risk of complete heart block requiring pacing in the post-operative Down syndrome group, a finding described in the previous studies. Reference Fudge, Li and Jaggers20,Reference Tucker, Pyles, Bass and Moller21 In addition, DS-related lymphangiectasis is a well-recognised complication of this chromosomal abnormality. Chylothorax was seen exclusively in the DS/CHD group in the post-operative period. Reference Bloemers, van Bleek, Kimpen and Bont13,Reference Esther and Barker22-Reference Mueller, Gegenhuber, Poelz and Haltmayer24 This was a further contributor to the increased duration of total hospital stay seen in the Down syndrome group.
Limitations
There are some limitations to this study and the results should be interpreted with caution. There is a relatively smaller number of infants in the control group. Due to the nature of the patient population, we could not match the rates of atrioventricular septal defect between the two groups and the post-operative differences between the groups may still be influenced by the type of lesion. The difference in the timing of the pre-discharge scans between the groups may also have influenced the difference in left and right ventricular function observed during that time point.
Conclusion
This study demonstrates the difference between the two groups in relation to left and right ventricular function, particularly prior to discharge, and outlines the additional impact a diagnosis of Down syndrome has on myocardial performance during the peri-operative period. Indices of left ventricular function in both groups significantly decline following surgical intervention. Further long-term follow-up of these children to assess their myocardial performance including the time point of recovery to normal is warranted.
Financial support
This work was supported by the National Children’s Research Centre, Dublin, Ireland [07-2018].
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Irish Health Service Executive) and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (Children’s Hospital Ireland at Crumlin).














