To promote a sustainable aquaculture production, alternatives to fishmeal (FM) and fish oil are needed, as these commodities are still major protein and lipid sources in commercial aquafeeds for carnivorous species( Reference Tacon and Hasan 1 ). Plant feedstuffs (PF) are nowadays the more available alternatives to FM, and overcome problems associated with fish by-products such as organic and inorganic contaminants, shortage of supply and net effect of demand-and-supply economics. However, PF usually contain anti-nutritional factors that may limit their use in aquafeeds( Reference Tacon and Hasan 1 , Reference Gatlin, Barrows and Brown 2 ).
Prebiotics are defined as ingredients that are not digested by the host but that are fermented by bacteria present in the gut, therefore promoting bacteria growth and/or activity( Reference Gibson and Roberfroid 3 ). Some of the beneficial effects of prebiotics in fish are improved growth rate, feed efficiency (FE), feed digestibility, fish survival, immunological status and resistance to bacterial and viral diseases( Reference Merrifield, Dimitroglou and Foey 4 – Reference Ringø, Dimitroglou and Hoseinifar 7 ). Fructo-oligosaccharides (FOS) have already been evaluated as prebiotic in fish, but xylo-oligosaccharides (XOS) remain barely studied( Reference Merrifield, Dimitroglou and Foey 4 – Reference Ringø, Dimitroglou and Hoseinifar 7 ). FOS are composed by short and medium chains of β-d-fructans in which fructosyl units are bound by β-(2-1) glycosidic linkages attached to a terminal glucose unit( Reference Ringø, Olsen and Gifstad 6 ). Short-chain fructo-oligosaccharides (scFOS) have a chemical composition similar to that of FOS but a smaller degree of polymerisation (between 1 and 5( Reference Bornet, Brouns and Tashiro 8 )). XOS are composed of xylose-based oligomers varying in the degree of polymerisation. Most of these oligomers consist of ester-linked phenolic acids such as ferulic, coumaric and caffeic acids( Reference Broekaert, Courtin and Verbeke 9 , Reference Veenashri and Muralikrishna 10 ).
In fish fed diets containing plant ingredients, supplementation with prebiotics, namely mannan-oligosaccharides (MOS), trans-galacto-oligosaccharides (GOS) and FOS, improved intestinal morphology, by increasing gut absorptive area, microvilli density and height, and villi structure complexity( Reference Dimitroglou, Merrifield and Moate 11 – Reference Zhou, Buentello and Gatlin 14 ). In rats fed XOS-supplemented diets, total intestine weight increased relative to body weight, indicating augmented epithelial cell proliferation( Reference Hsu, Liao and Chung 15 ). Up to now, there are, however, no studies accessing XOS effects on fish intestinal morphology.
PF and prebiotics were both reported to affect reactive oxygen species (ROS) generation in fish, decreasing oxidative damage and/or increasing antioxidant potential( Reference López-Bote, Diez and Corraze 16 – Reference Zhang, Li and Xu 21 ). Prebiotics such as inulin were even reported as antioxidants, with ROS scavenging ability( Reference Stoyanova, Geuns and Hideg 22 , Reference Van den Ende, Peshev and De Gara 23 ). FOS was already reported as having antioxidant activity in fish, although mechanisms beyond that effect were not yet clarified( Reference Zhang, Li and Xu 21 , Reference Pejin, Savic and Petkovic 24 ). XOS has ferulic acid in its composition, which was reported as having a very strong antioxidant activity( Reference Graf 25 ), and studies in mammals showed that XOS decreased lipid peroxidation (LPO) and modulated antioxidant enzymatic activity( Reference Sheu, Lee and Chen 26 – Reference Wang, Cao and Wang 28 ). However, XOS antioxidant potential has not yet been assessed in fish.
Thus, the present work aimed to evaluate the effect of dietary scFOS and XOS supplementation in gut morphology and hepatic oxidative status of European sea bass (Dicentrarchus labrax) fed FM- or PF-based diets. This will contribute to elucidate the potential of prebiotics to mitigate some of the negative effects on fish health of using PF-based diets.
Methods
Diets
Two control diets were formulated to be isoproteic (46 % crude protein) and isolipidic (15 % crude lipid). One diet included FM as the main protein source (FM control diet: FMC diet) and the other diet included FM and PF (soyabean meal (SBM), wheat meal, wheat gluten and maize gluten) to provide a protein source ratio of 30 FM:70 PF (PF control diet: PFC diet). In both diets, cod liver oil was the main lipid source. Four other diets were formulated identical to the controls but including 1 % commercial prebiotics: scFOS (PROFEED Maxflow; Jefo) or XOS (Qingdao FTZ United International Inc.), replacing α-cellulose (diets PFFOS, PFXOS, FMFOS and FMXOS). All dietary ingredients were finely ground, well mixed and dry extruded in a laboratory pellet mill (California Pellet Mill) through a 3-mm die. The pellets were then dried in an oven (40°C) for 24 h and stored in plastic containers until use. Ingredients and proximate composition of the experimental diets are presented in Table 1.
Table 1 Ingredient composition and proximate analysis of the experimental diets

PFC, plant feedstuff control diet; PFFOS, plant feedstuff fructo-oligosaccharides; PFXOS, plant feedstuff xylo-oligosaccharides; FMC, fishmeal control diet; FMFOS, fishmeal fructo-oligosaccharides; FMXOS, fishmeal xylo-oligosaccharides; CP, crude protein; GL, gross lipid.
* Inproquise (CP: 70·1 % DM; GL: 8·8 % DM).
† Sopropèche G (CP: 79·4 % DM; GL: 19·7 % DM).
‡ Sorgal, S.A. (CP: 50·5 % DM; GL: 1·7 % DM).
§ Sorgal, S.A. (CP: 11·8 % DM; GL: 1·9 % DM).
|| Sorgal, S.A. (CP: 82·8 % DM; GL: 1·9 % DM).
¶ Sorgal, S.A. (CP: 65·7 % DM; GL: 3·5 % DM).
** C-Gel Instant – 12016; Cerestar.
†† PROFEED Maxflow; Jefo.
‡‡ Qingdao FTZ United International Inc.
§§ Vitamins (mg/kg diet): retinol, 6.19; cholecalciferol, 0.04; α-tocopherol, 35; menadion sodium bisulphate, 10; thiamine, 15; riboflavin, 25; Ca pantothenate, 50; nicotinic acid, 200; pyridoxine, 5; folic acid, 10; cyanocobalamin, 0·02; biotin, 1·5; ascorbyl monophosphate, 50; inositol, 400.
|||| Minerals (mg/kg diet): cobalt sulphate, 1·91; copper sulphate, 19·6; iron sulphate, 200; sodium fluoride, 2·21; potassium iodide, 0·78; magnesium oxide, 830; manganese oxide, 26; sodium selenite, 0·66; zinc oxide, 37·5; dibasic calcium phosphate, 5·93 (g/kg diet); potassium chloride, 1·15 (g/kg diet); sodium chloride, 0·44 (g/kg diet).
¶¶ Aquacube (guar gum, polymethyl carbamide, manioc starch blend, hydrated calcium phosphate) (Agil).
Animals and experimental conditions
The experiment was directed by FELASA category C certified scientists, and all procedures were conducted according to the European Union Directive 2010/63/EU on the protection of animals for scientific purposes. The growth trial was performed at the Marine Zoology Station, Porto University, Portugal, in a thermoregulated recirculating water system equipped with fibreglass tanks (100 litre capacity) supplied with continuous flow of filtered seawater (6·0 l/min), temperature regulated to 25·0 (sem 1·0)°C, salinity of 36·0 (sem 1·0 g/l and dissolved oxygen kept near saturation (7·0 mg/l).
European sea bass juveniles (D. labrax) were obtained from a commercial fish farm (Maresa S.A.) and after transportation to the experimental facilities fish were submitted to a quarantine period of 15 d. During that period fish were fed with a commercial diet (48 % protein, 11 % lipids, 5 % starch; A. Coelho & Castro Lda). Thereafter, eighteen groups of twenty fish with an initial mean body weight of 60 g were established and each diet was randomly assigned to triplicate tanks. The trial lasted 7 weeks, and during that period fish were fed by hand, 6 d/week, until visual satiation. No mortality occurred during the trial.
Sampling
After 7 and 15 d of the start of the feeding trial and at the end of the trial, three fish from each tank were randomly selected 2 h after the morning meal and euthanised with a sharp blow to the head. Fish were dissected on chilled trays and the digestive tract was freed from the adjacent adipose and connective tissues. Two pyloric caeca (PC) and a section of the distal intestine (DI, distinguished from the mid intestine by an enlarged diameter and darker mucosa) were sampled for histological evaluation. The samples were rinsed in PBS, carefully blotted dry with a paper towel, immediately fixed in phosphate-buffered formalin (4 %, pH 7·4) for 24 h and subsequently transferred to ethanol (70 %) until further processing. At the end of the trial, livers were also sampled from the same fish, immediately frozen in liquid N2 and stored at −80°C until measurement of enzymes activities and LPO levels.
Proximate analysis of the diets
Chemical analyses of the diets were performed following the Association of Official Analytical Chemists methods( 29 ). Energy content was determined by direct combustion in an adiabatic bomb calorimeter (PARR model 1261; PARR Instruments) and starch content was analysed according to Beutler( Reference Beutler 30 ).
Histological processing and morphological evaluation
PC and DI samples were processed and sectioned using standard histological techniques, and sections were stained with haematoxylin–eosin. Blind evaluation of histological preparations was performed with particular attention given to any inflammatory changes( Reference Baeverfjord and Krogdahl 31 , Reference Krogdahl, Bakke-McKellep and Baeverfjord 32 ), namely shortening, widening and fusion of intestinal folds (FH), changes in enterocytes nucleous (ENT) and supranuclear absorptive vacuolisation (SNV), connective tissue hyperplasia in the lamina propria (LP) and submucosa (SM), and infiltration of inflammatory cells (intraepithelial leucocyte (IEL)). A continuous scale scoring system was used as described by Penn et al. ( Reference Penn, Bendiksen and Campbell 33 ), with the range of tissue scores set at 0–5. The overall value of histomorphological alterations was calculated by averaging scores of the separate parameters described above. Images were acquired with the Zen software (Blue edition; Zeiss).
Enzyme activity
Liver samples were homogenised on ice in five volumes of ice-cold 100 mm-Tris-HCl buffer containing 0·1 mm-EDTA and 0·1 % (v/v) Triton X-100, pH 7·8. Homogenates were centrifuged at 30 000 g for 30 min at 4°C, and the resultant supernatants were separated in aliquots and stored at −80°C until use. All enzyme assays were carried out at 25°C in a microplate reader (ELx808; BioTek Instruments). The optimal substrate and protein concentrations for measurement of maximal activity for each enzyme were established by preliminary assays. The molar extinction coefficients used for H2O2 and NADPH were 0·039 and 6·22 mm –1×cm–1, respectively. Superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), glutathione peroxidase (GPX, EC 1.11.1.9), glutathione reductase (GR; EC 1.6.4.2) and glucose 6-phosphate dehydrogenase (G6PD, EC 1.1.1.49) activities were determined as described by Enes et al.( Reference Enes, Pérez-Jiménez and Peres 34 ). Protein concentration in the homogenates was determined by the Bradford method( Reference Bradford 35 ) using the Sigma protein assay kit (ref. B6916) (Sigma-Aldrich Química, S.L.) with bovine serum albumin as a standard.
Enzyme activity was expressed as units (SOD, CAT) or milliunits (GPX, GR and G6PD) per mg of hepatic soluble protein. Except for SOD, one unit of enzyme activity was defined as the amount of enzyme required to transform 1 μmol of substrate/min under the assay conditions. One unit of SOD activity was defined as the amount of enzyme necessary to produce 50 % inhibition of the ferricytochrome c reduction rate.
Lipid peroxidation
Malondialdehyde (MDA) concentration was used as a marker of LPO level in the liver. In the presence of thiobarbituric acid, MDA reacts producing coloured thiobarbituric acid-reacting substances that were measured as described by Enes et al.( Reference Enes, Pérez-Jiménez and Peres 34 ). Results were expressed as nmol MDA per g of wet tissue, calculated from a calibration curve.
Statistical analysis
Data analysis was done by two-way ANOVA using SPSS 21 software package (SPSS® Inc.). Previous to ANOVA, data were tested for normality and homogeneity (Shapiro–Wilk and Levene’s tests, respectively) and when necessary transformed to achieve ANOVA assumptions. When significant interaction between factors were found, one-way ANOVA was performed within each protein source (PF and FM). Significant differences among means were determined with the Tukey’s multiple range test. Histological data were neither normal nor homogeneous and could not be normalised, and they were analysed by the Kruskal–Wallis non-parametric test followed by pairwise comparisons. For all data, the probability level for rejection of the null hypotheses was 0·05.
Results
Growth performance, feed efficiency and feed intake
Growth performance was not the aim of this study, and it was presented in detail elsewhere( Reference Guerreiro, Oliva-Teles and Enes 36 ). Briefly, protein source had no effect on fish growth, but feed intake (FI) was higher and FE was lower in fish fed PF diets (Table 2). Prebiotic supplementation had no effect on growth of fish fed FM but XOS improved growth performance in fish fed PF diets. Prebiotic supplementation had no effect on FI and FE, independently of dietary protein source.
Table 2 Growth performance and feed utilisation efficiency of European sea bass fed the experimental diets (Mean values with their pooled standard errors; n 3)( Reference Guerreiro, Oliva-Teles and Enes 36 )

a,b Mean values within the same line with unlike superscript letters were significantly different (P<0·05).
* Two-way ANOVA: if interaction was significant, one-way ANOVA was performed for prebiotics within each protein source.
† Average body weight (ABW): initial body weight+final body weight/2.
‡ Feed efficiency (FE): wet weight gain/dry feed intake
Gut morphology
Mean scores of the average of the separate parameters (FH, LP, SM, IEL, ENT and SNV) evaluated in PC and DI during the course of the trial are presented in Table 3.
Table 3 Intestinal histology (pyloric caeca (PC) and distal intestine (DI)) of European sea bass fed the experimental diets for 7 and 15 d and by the end of the trial (final)Footnote * (Mean values with their pooled standard errors; n 9)

PFC, plant feedstuff control diet; PFFOS, plant feedstuff fructo-oligosaccharides; PFXOS, plant feedstuff xylo-oligosaccharides; FMC, fishmeal control diet; FMFOS, fishmeal fructo-oligosaccharides; FMXOS, fishmeal xylo-oligosaccharides.
A,B,a,b,c,d Mean values with unlike superscript lowercase letters stand for statistical differences across dietary groups within each sampling day; mean values with unlike superscript uppercase letters stand for statistical differences for each parameter with time, as determined by the Kruskal–Wallis all pairwise comparisons (P<0·05).
* Mean scores were calculated by averaging the scores of the separate parameters evaluated (shortening, widening and fusion of intestinal folds, changes in enterocytes nucleous and supranuclear absorptive vacuolization, connective tissue hyperplasia in the lamina propria and submucosa, and infiltration of inflammatory cells). Score from 0 to 5, with 5 indicating major alterations.
No histomorphological alterations were observed with time in fish fed the FM-based diets. In fish fed the PF-based diets, histomorphological alterations with time were significant in the PC, but not in the DI.
In the PC, replacement of FM by PF resulted in an increase of mean scores of the evaluated parameters, and this increase was significantly higher after 15 d of feeding the experimental diets. Addition of FOS and XOS to the diets had no effect on PC morphology relatively to the respective control diets. Further, at 15 d of feeding, the mean scores of the evaluated parameters in fish fed the PFXOS diet was not different from that of the FMC diet. Histomorphological alterations observed in PF groups were transient, as by the end of the trial mean scores returned to values similar to the FMC diet. Overall, the most relevant observation in PC was the presence of enterocyte swelling (Fig. 1) in fish fed diets containing PF, which resulted in higher ENT and SNV scores in the mentioned groups (for both characteristics: P<0·001, P<0·001 and P=0·001 at time points 7, 15 and final, respectively). To allow a better visualisation of the data, a graph with the several histological parameters evaluated in the PC was made (Fig. 2(a)). As no major differences were observed regarding the effects of prebiotics, and to simplify visualisation, data were pooled and presented only as FM and PF groups.

Fig. 1 Detail of enterocyte nucleousswelling (ENT) in the pyloric caeca of fish fed fishmeal xylo-oligosaccharides (a) and plant feedstuff xylo-oligosaccharides (b) at time point 7 d. Scale bar: 50 µm; haematoxylin–eosin staining.

Fig. 2 Mean scores, with their standard errors for pyloric caeca (a) and distal intestine (b) of FH (shortening, widening and fusion of intestinal folds), LP (connective tissue hyperplasia in the lamina propria), SM (connective tissue hyperplasia in the submucosa), IEL (infiltration of inflammatory cells), ENT (changes in enterocytes nucleous) and SNV (changes in supranuclear absorptive vacuolisation). As no major differences were observed regarding the effects of prebiotics, and to simplify visualisation, data were pooled and presented only as FM (![]() ) and PF (
) and PF (![]() ) groups. Data are separated for sea bass fed the experimental diets for 7 and 15 d and by the end of the trial (final). Score from 0 to 5, with 5 indicating major alterations.
) groups. Data are separated for sea bass fed the experimental diets for 7 and 15 d and by the end of the trial (final). Score from 0 to 5, with 5 indicating major alterations.
PF diets induced more pronounced effects in DI histomorphology, as denoted by the higher mean scores. Replacement of FM by PF resulted in an increase of mean scores of the evaluated parameters in the DI at all sampling days. The addition of prebiotics to PF diets did not ameliorate the negative effects in DI histomorphology.
The most pronounced histological alterations observed in the DI of fish fed PF diets (Fig. 3) were as follows: decreased height of the mucosal folds (P<0·001, P<0·001 and P=0·004 in time points 7, 15 and final, respectively), increased number of IEL (P<0·001, P<0·001 and P<0·001 in time points 7, 15 and final, respectively) and abnormal size variation of SNV (P=0·013, P=0·002 and P=0·001 in time points 7, 15 and final, respectively). As explained above, a graph was also made for the DI portion (Fig. 2(b)). Opposite to what was observed in PC, histological alterations did not recede and were similar at days 7, 15 and by the end of the trial.

Fig. 3 Distal intestine alterations in fish fed FM control diet (a, c) and PF control diet (PFC) (b, d), showing decreased fold height, increased width of the lamina propria and intraepithelial leucocytes (IEL), as well as abnormal enterocyte vacuolisation and nucleous position in PFC group at time point 15 d. MF, mucosal fold; SM, submucosa; GC, goblet cell; SNV, supranuclear vacuole. Scale bars: 100 µm (a, b); 20 µm (c, d); haematoxylin–eosin staining.
Antioxidant enzymatic activity and lipid peroxidation
Antioxidant enzymatic activity and LPO levels at the end of the trial are present in Table 4. Protein source affected all parameters analysed. LPO levels, SOD and CAT activities were higher, whereas GPX, GR and G6PD activities were lower in fish fed PF diets than the FM diets. LPO level and GPX activity were not affected by prebiotic incorporation, whereas CAT activity was decreased by XOS supplementation.
Table 4 Specific activities of hepatic antioxidant enzymes and lipid peroxidation (LPO) levels of European sea bass fed the experimental dietsFootnote * (Mean values with their pooled standard errors; n 9)
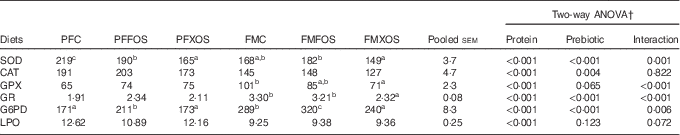
PFC, plant feedstuff control diet; PFFOS, plant feedstuff fructo-oligosaccharides; PFXOS, plant feedstuff xylo-oligosaccharides; FMC, fishmeal control diet; FMFOS, fishmeal fructo-oligosaccharides; FMXOS, fishmeal xylo-oligosaccharides; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; G6PD, glucose 6-phosphate dehydrogenase.
a,b,c Mean values within the same line with unlike superscript letters are significantly different (P<0·05).
* SOD and CAT are expressed as U/mg protein. GPX, GR and G6PD are expressed as mU/mg protein. LPO is expressed as nmol malondialdehyde/g tissue.
† Two-way ANOVA: for CAT, two-way ANOVA results showed significant differences among control, fructo-oligosaccharides and xylo-oligosaccharides, regardless of protein source, being Tukey’s test results as b-b-a, respectively. For other parameters, if interaction was significant, one-way ANOVA was performed for prebiotics within each protein source.
In fish fed PF diets, both scFOS and XOS decreased SOD activity, whereas in fish fed FM diets XOS led to a reduction of SOD, GPX, GR and G6PD enzymatic activities. Compared with the control groups, scFOS increased G6PD activity in both PF and FM diets.
Discussion
A replacement of 95 % of FM by PF in diets for European sea bass was already accomplished without affecting fish growth, diets digestibility or voluntary FI( Reference Kaushik, Covès and Dutto 37 ). However, in that study authors did not perform intestinal histomorphology observations. However, it is well known that the anti-nutritional factors presented in PF, namely those present in SBM, can cause moderate and severe enteritis in fish( Reference Baeverfjord and Krogdahl 31 , Reference van den Ingh, Krogdahl and Olli 38 – Reference Opstvedt, Aksnes and Hope 41 ). In the present study, although no overt inflammation was observed, fish fed PF diets presented alterations in the DI histomorphology when compared with fish fed FMC diets. Such an effect may be related to the inclusion of 25 % of SBM in the PF diets, as it was previously observed in Atlantic salmon (Salmo salar)( Reference Krogdahl, Bakke-Mckellep and Røed 39 , Reference Refstie, Storebakken and Baeverfjord 40 ). However, it was previously reported in European sea bass that inclusion of 30 % SBM had no significant consequences in the DI histomorphology( Reference Bonaldo, Roem and Fagioli 42 ). Thus, the DI modifications observed in the present study were more likely a consequence of the combination of different PF, and thus different anti-nutrients, in the PF diets. Accordingly, Couto et al.( Reference Couto, Kortner and Penn 43 ) fed European sea bass with two purified soyabean anti-nutrients, saponins and phytosterols, and observed no severe effects on fish gut histology, supporting the fact that the inflammation observed in the present study could be because of interaction/cumulative effects of anti-nutrients and PF sources.
In this study, the majority of permanent gut histological alterations were observed on the DI portion. A similar observation was reported by van den Ingh et al.( Reference van den Ingh, Krogdahl and Olli 38 ) in salmon fed diets in which FM was partially replaced by 30 % full-fat SBM. The presence of inflammation only in the DI was related to the higher sensibility of this intestinal portion to PF anti-nutrients. In fact, the same was already reported in another study with salmon( Reference Hendriks, VandenIngh and Krogdahl 44 ) and in European sea bass( Reference Couto, Kortner and Penn 43 ).
With regard to the PC section, although at 15 d fish fed PFC diets presented higher mean scores for the evaluated parameters than fish fed the FMC diet, at the end of the trial no histomorphological differences between the two groups were found. These results indicate that fish were able to adapt to the high dietary PF levels, reversing the initial histomorphological alterations. Such recovery from previous histological alterations because of diet changes has already been reported for European sea bass( Reference Couto, Kortner and Penn 43 ) and common carp (Cyprinus carpio)( Reference Urán, Gonçalves and Taverne-Thiele 45 ). Urán et al.( Reference Urán, Gonçalves and Taverne-Thiele 45 ) hypothesised that this recovery was related to the omnivorous feeding habits of carp, making it prone to adapt to diets with high PF levels. This is not the case for sea bass, as it is a carnivorous species and thus does not feed on PF in nature. However, the present results indicate that European sea bass has higher tolerance for PF than salmon, which is also a carnivorous species. Indeed, salmon seems to be particularly intolerant to high levels of dietary PF. Actually, even rainbow trout (Oncorhynchus mykiss), which is also a carnivorous species that belongs to the same family as salmon, exhibits higher tolerance for SBM anti-nutritional factors( Reference Refstie, Korsøen and Storebakken 46 ).
Although prebiotics such as MOS, GOS and FOS have been reported as increasing gut absorptive surface area and microvilli density and height in fish fed plant-based diets( Reference Dimitroglou, Merrifield and Moate 11 – Reference Zhou, Buentello and Gatlin 14 ), in the present study no histomorphological alterations were noticed in fish fed the PF diets. Other studies similar to the present one also reported no differences at optical microscopy level in the intestinal morphology of fish fed diets containing PF and prebiotics( Reference Dimitroglou, Merrifield and Moate 11 , Reference Torrecillas, Makol and Caballero 47 , Reference Guerreiro, Enes and Rodiles 48 ). Dimitroglou et al.( Reference Dimitroglou, Merrifield and Spring 12 ) fed gilthead sea bream (Sparus aurata) FM- and SBM-based diets supplemented with MOS, and also observed that MOS had no effect on the mucosal folds morphology of the anterior intestine. However, MOS appeared to improve the absorptive surface area in the posterior intestine of fish fed the FM diet, as denoted by higher perimeter of the intestinal lumen. However, using electron microscopy techniques, it became evident that MOS affected both anterior and posterior intestine at the ultrastructural level in fish fed FM- and SBM-based diets. As we did not assess the intestinal ultrastructure of fish intestine, it cannot be ruled out that histological alterations may have occurred at this level.
Differences between studies on the effects of prebiotics in gut histomorphology may also be related to prebiotic type, dosage, species assessed and age, rearing conditions, differences in gut microbiota or methodological approaches. For instance, Dimitroglou et al.( Reference Dimitroglou, Merrifield and Moate 11 ) showed that gut histology of fish fed the same prebiotic (MOS) and diet may change in the same species depending on fish age. Authors observed improvements in gut morphology, such as increased absorptive surface, microvilli density and length, of subadult rainbow trout fed MOS, whereas no effects were observed in juveniles.
It was previously reported in rainbow trout, Atlantic salmon, gilthead sea bream and European sea bass that PF can reduce oxidative damage in fish( Reference López-Bote, Diez and Corraze 16 , Reference Sitjà-Bobadilla, Penña-Llopis and Gómez-Requeni 17 , Reference Olsvik, Torstensen and Hemre 20 ). This may be linked to PF components with strong antioxidant activity, namely phenolic compounds, flavonoids, α-tocopherol or astaxanthin( Reference Sitjà-Bobadilla, Penña-Llopis and Gómez-Requeni 17 , Reference Olsvik, Torstensen and Hemre 20 ).
Although the PF used in the present study are rich sources of flavonoids (SBM) and phenolic compounds, mostly in the form of ferulic acid (maize and wheat meal)( Reference Adom and Liu 49 , Reference Guo, Rimbach and Moini 50 ), no improved antioxidant effect was observed in the PF diets. Indeed, fish fed PF diets presented higher hepatic LPO level than fish fed FM diets. Increased LPO occurs when ROS production is higher than ROS removal. SOD, which catalyses the dismutation of the superoxide anion (O2 –) to molecular oxygen and H2O2, is the first enzyme responding to the presence of oxygen radicals, preventing the radical chain reaction initiated by O2 –. In the present study, hepatic SOD activity was higher in fish fed PF diets, indicating that there was a rise of O2 – generation in fish fed PF diets. Hepatic CAT activity was also increased, whereas hepatic GPX activity was lower in fish fed PF diets, indicating that CAT was the major route for reducing H2O2 to molecular oxygen and water. The different behaviour of the two peroxidation reduction routes may be related to the overall concentration of H2O2 generated, as it is known that CAT is more active when H2O2 production is high, whereas GPX is induced by low H2O2 levels( Reference Halliwell and Gutteridge 51 ). Such low H2O2 levels are probably present in fish fed FM-based diets, as in this group significantly lower SOD activities were noticed. GR has a role in the modulation of GPX activity, as it catalyses the NADPH-dependent regeneration of GSH from GSSG generated by GPX( Reference Halliwell and Gutteridge 51 ). Thus, and in order to maintain the GSSG:GSH ratio, higher hepatic GR activity was observed in fish fed FM diets because of the high GPX activity observed in fish fed those diets. Hepatic G6PD was also increased in fish fed FM diets, as it is involved in NADPH regeneration, which is a coenzyme required for GR activity( Reference Scott, Zuo and Lubin 52 , Reference Pandolfi, Sonatí and Rivi 53 ).
Although LPO levels were unaffected by dietary prebiotic incorporation, XOS supplementation seemed to contribute to a reduction of overall ROS production, as CAT activity was lower in fish fed both PFXOS and FMXOS, and activities of GPX, GR and G6PD were also reduced in fish fed the FMXOS diet. Comparison of the present results with other studies performed in fish was not possible, as to the authors’ knowledge there are no studies available regarding the effect of XOS on antioxidant status. In type 2 diabetes mellitus rats, XOS also contributed to a reduction of CAT activity in erythrocyte samples, but not of SOD and GPX activities( Reference Sheu, Lee and Chen 26 ). The lower effects of XOS on the antioxidant enzymatic activity observed in fish fed the PF diets might be related to soyabean oligosaccharides that may have acted as prebiotics and masked the XOS effects( Reference Delzenne 54 – Reference Swennen, Courtin and Delcour 56 ).
In mammals, prebiotics fermentation by gut microbiota leads to the production of SCFA, which have a role in oxidative stress modulation. In addition, butyrate, more than other SCFA, was reported as being related with a significant reduction in H2O2-induced DNA damage in rats and humans( Reference Abrahamse, Pool-Zobel and Rechkemmer 57 – Reference Toden, Bird and Topping 59 ). Although fish have a less abundant gut microbiota than mammals and lack a large bowel, it is known that fermentation by fish gut microbiota also produces SCFA( Reference Burr, Hume and Ricke 60 ). Nonetheless, the reported antioxidant ability of XOS is most likely related to its composition, as most of the oligomers present in XOS consist of ester-linked phenolic acids such as ferulic, coumaric and caffeic acids( Reference Broekaert, Courtin and Verbeke 9 , Reference Veenashri and Muralikrishna 10 , Reference Graf 25 ). Ferulic acid has potent antioxidant potential mainly because of its phenolic nucleus and extended side-chain conjugation that readily forms stabilised phenoxy radicals and terminate chain reactions( Reference Graf 25 ). In fact, ferulic acid scavenges superoxide anion radicals in a similar way to SOD( Reference Toda, Kumura and Ohnishi 61 ).
FOS or a combination of FOS and probiotics were already reported as having antioxidant capabilities in several fish species( Reference Li, Wang and Wang 18 , Reference Ai, Xu and Mai 19 , Reference Zhang, Li and Xu 21 , Reference Zhang, Tian and Li 62 , Reference Guerreiro, Pérez-Jiménez and Costas 63 ). Zhang et al.( Reference Zhang, Tian and Li 62 ) explained this antioxidant potential of FOS by its bifidogenic effect and suggested that the antioxidant properties of FOS might help in the gut microbial defence mechanism, as FOS may help surpassing both exogenous and endogenous oxidative stress. Another possibility is that FOS might have a role in the translation and post-translational process of the antioxidant enzymes( Reference Zhang, Li and Xu 21 ). However, further investigation is needed to understand the mechanisms behind FOS antioxidant capabilities. In the present study, except for a decrease of SOD activity in fish fed the PFFOS diet, scFOS had little effect in European sea bass oxidative status.
In conclusion, PF-based diets increased LPO levels and had negative impacts in the histomorphology of DI compared with fish fed FM-based diets. Prebiotic incorporation in PF diets was not effective in counterbalancing the negative effects of PF diets in gut morphology. However, XOS incorporation in both PF and FM diets reduced antioxidant enzymatic activity, suggesting a role in the reduction in ROS production.
Acknowledgements
The authors would like to express their thanks to Pedro Correia for the assistance during fish rearing. The authors would also like to thank Jefo Species-specific additives France, for providing the short-chain fructo-oligosaccharides prebiotic.
This work was partially funded by Projects AQUAIMPROV (reference NORTE-07-0124-FEDER-000038) and PEst-C/MAR/LA0015/2011, co-financed by the North Portugal Regional Operational Programme (ON.2–O Novo Norte), under the National Strategic Reference Framework, through the European Regional Development Fund, and through the COMPETE – Operational Competitiveness Programme and national funds through FCT – Foundation for Science and Technology, respectively. I. G., A. P.-J., A. C. and P. E. were supported by grants (SFRH/BD/76139/2011, SFRH/BPD/64684/2009, BD/47495/2008 and BPD/39688/2007, respectively) from FCT, Portugal. The funder had no role in the design, analysis or writing of this article.
A. O.-T. and P. E. designed and coordinated the study. I. G, A. C., A. P.-J. and P. E. were responsible for the data collection, analysis and statistical analysis. All authors contributed to the interpretation of the data. I. G. wrote the first draft of the manuscript and all authors critically reviewed and revised the manuscript.
The authors have no conflicts of interest.











