Antipsychotic drug use in Alzheimer's disease
Alzheimer's disease affects around 35 million people worldwide, 50% of whom will experience psychosis symptoms (delusions and hallucinations). Psychosis symptoms are often distressing, increase the risk of aggression towards caregivers, predict faster cognitive and functional decline, and reduce ability to live independently.Reference Connors, Ames, Woodward and Brodaty1 Although symptoms sometimes respond to psychosocial interventions, for those with severe persistent symptoms, antipsychotic medication is required to reduce distress and associated risks.Reference Ohno, Kunisawa and Shimizu2 The best evidence of efficacy is for second-generation antipsychotic drugs.Reference Tampi, Tampi, Balachandran and Srinivasan3 However, concerns about side-effects (sedation, falls, parkinsonism and stroke) and increased mortality in people with dementia, particularly in those aged over 80 years,Reference Howard, Costafreda, Karcher, Coppola, Berlin and Hough4 has led to a restriction in prescribing. In England, National Institute for Health and Care Excellence guidance emphasises the need to treat with ‘the lowest effective dose for the shortest possible time’ but provides little practical information on the optimal dose range for individual drugs.
We have shown that amisulpride therapeutic plasma concentrations for the treatment of Alzheimer's disease psychosis (40–100 ng/mL), are lower than those recommended for the treatment of schizophrenia (100–320 ng/mL), as a result of a leftwards shift in the dopamine D2/3 receptor concentration–occupancy curve.Reference Reeves, McLachlan, Bertrand, D'Antonio, Brownings and Nair5 These findings raise questions regarding the mechanisms of antipsychotic sensitivity in Alzheimer's disease and suggest that, for amisulpride at least, 50 mg/day (compared with 400–800 mg/day in young adults), may optimally balance the risks and benefits of treatment.Reference Reeves, Bertrand, McLachlan, D'Antonio, Brownings and Nair6 It is, however, not clear how far we can extrapolate this approach to other antipsychotic drugs.
Pharmacokinetics and consensus guidance on risperidone prescribing
Risperidone, an antipsychotic drug with high affinity for dopamine D2/3 and serotonin 5-HT2A receptors, is the only drug licensed for short-term use in the treatment of aggression and psychosis in dementia in the European Union, and is typically prescribed across a 0.5–2 mg/day dose range for this indication.Reference Katz, de Deyn, Mintzer, Greenspan, Zhu and Brodaty7 Oral risperidone has high (70–85%) bioavailability and is extensively metabolised by cytochrome P450 (CYP2D6 and to a lesser extent CYP3A4) to the active metabolite 9-hydroxy(OH)-risperidone.Reference Mauri, Paletta, Di Pace, Reggiori, Cirnigliaro and Valli8 Peak concentrations of risperidone and 9-OH-risperidone are reached after 1 and 3 h, respectively. The elimination half-life (t1/2) of risperidone is dependent on multiple factors: genetic variation in CYP2D6 genotype, which leads to non-functional, decreased and increased enzyme activity in people who are poor, intermediate and extensive metabolisers, respectively, accounts for around 50% of the variability in risperidone concentrations (t1/2 4.7 h in extensive and 22 h in poor metabolisers); with age, hepatobiliary dysfunction and use of CYP (2D6 inhibitors, 3A4 inducers) further contributing to variability.Reference de Leon, Wynn and Sandson9,Reference Feng, Pollock, Coley, Marder, Miller and Kirshner10 The metabolite is predominantly renally excreted (glomerular filtration and tubular secretion by an unknown transporter) with a t1/2 of 20 h; increased to 25 h in the over 65 s and in moderate renal failure. The time taken to achieve steady state concentrations of the active moiety (combined concentrations of risperidone and 9-OH-risperidone) is dependent on t1/2 and estimated as 4–5 days in young adults who are normal metabolisers.
Consensus guidelines, based on therapeutic drug monitoring,Reference Mauri, Paletta, Di Pace, Reggiori, Cirnigliaro and Valli8,Reference Hiemke, Bergemann, Clement, Conca, Deckert and Domschke11 pharmacokinetic modellingReference Vandenberghe, Guidi, Choong, von Gunten, Conus and Csajka12 and imaging of striatal D2/3 receptor occupancyReference Uchida, Takeuchi, Graff-Guerrero, Suzuki, Watanabe and Mamo13 in patients with schizophrenia taking risperidone, recommend active moiety concentrations of 20–40 ng/mL (3–6 mg/day),Reference Vandenberghe, Guidi, Choong, von Gunten, Conus and Csajka12 as higher concentrations increase occupancy beyond 80% and increase the risk of extrapyramidal side-effects (EPS). For those with glomerular filtration rates below 60 mL/min, because of age or other cause of renal impairment, a 50% dose reduction is advised to avoid excessive exposure.Reference Grunder, Augustin, Paulzen and Grunder14 Recent guidance on personalised risperidone prescribing advocates dose reductions for those with concentration-to-dose ratios of the active moiety over 14 ng/mL per mg/day, indicating slower clearance, as a result of the combined effect of CYP2D6, CYP3A4 and renal clearance.Reference de Leon15 There is a lack of empirical data from people with Alzheimer's disease.
Aims
This analysis aimed to combine pharmacokinetic and clinical outcome data from The Clinical Trials of Intervention Effectiveness in Alzheimer's disease (CATIE-AD) study,Reference Schneider, Tariot, Dagerman, Davis, Hsiao and Ismail16 with the following objectives.
(a) To investigate sources of variability in plasma concentration–time profiles of risperidone and 9-OH-risperidone, using an approach that allowed estimation of risperidone clearance (metabolism) in distinct subpopulations.
(b) To estimate pharmacokinetic indices (peak, trough and average concentrations of risperidone, 9-OH-risperidone and active moiety) for each individual, across the prescribed dose range.
(c) To investigate the relationship between the above pharmacokinetic indices with EPS.
Method
Data source
CATIE-AD is a randomised, double-blind, parallel group study comparing olanzapine, quetiapine, risperidone and placebo in the treatment of psychosis and aggression in Alzheimer's disease (Clinicaltrials.gov identifier: NCT00015548). In phase 1, participants were randomised to receive risperidone, olanzapine, quetiapine or placebo (1:1:1:1 ratio), with study physicians having a choice of two capsule strengths (0.5 mg, 1.0 mg). Dose adjustments and treatment discontinuation (possible after 2 weeks, with a further decision point at 12 weeks) were at the discretion of study physicians. Patients with an adequate response continued treatment for up to 36 weeks. Patients whose initial treatment was discontinued during phase 1 could be enrolled in phase 2 and randomly assigned to receive one of the antipsychotic drugs to which they were not initially assigned, or to receive citalopram. In phase 3, treatment was prescribed in an open manner. Within each phase, plasma drug concentration was measured at 2, 4 and 12 weeks, or when a medication switch was made.Reference Schneider, Tariot, Dagerman, Davis, Hsiao and Ismail16,Reference Schneider, Tariot, Lyketsos, Dagerman, Davis and Davis17
Clinical assessment (baseline, every 2–4 weeks during dose titration) included the Simpson Angus Scale (SAS),Reference Simpson and Angus18 and Barnes Akathisia Scale (BAS).Reference Schneider, Tariot, Dagerman, Davis, Hsiao and Ismail16,Reference Barnes19 Plasma concentrations of risperidone and the active metabolite 9-OH-risperidone were determined using a liquid chromatography-tandem mass spectrometry method with a detection limit of 0.1 ng/mL. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from all patients. A comprehensive plan was developed to ensure that all institutional, National Institute for Health and federal regulations concerning informed consent were fulfilled. The plan included careful assessment of risks and benefits, review by the CATIE protocol and ethics committees and review by the National Institute of Mental Health Data Safety and Monitoring Board.
Data extraction
Data available for participants taking risperidone included study identification number, phase, visit, dose (mg), timing of blood draw (hours post dose), number of days of treatment, dosage interval (daily), physiological characteristics (age, gender, height, weight, ethnicity (coded as White/other)) and smoking status (currently smoking or not), Mini-Mental State Examination (MMSE)Reference Folstein, Folstein and Mchugh20 scores, and plasma concentrations of risperidone and 9-OH-risperidone (ng/mL).
Treatment-emergent EPS were coded as present if SAS total scores were ≥6, or BAS global scores were ≥2 at follow-up, in individuals with baseline SAS ratings <6 and BAS scores <2. Only participants without baseline EPS were included in our analysis of outcome data. Data on other adverse events were extracted from the adverse event log and included sedation, postural hypotension and electrocardiogram (ECG) abnormalities. Rating scales and adverse events were checked for consistency with pharmacokinetic data, using phase, number of days of treatment and the timing of blood sampling. The concomitant mediation log was used to confirm that no participant was prescribed CYP2D6 inhibitors (fluoxetine, paroxetine, duloxetine, bupropion) or CYP3A4 inducers (carbamazepine).
Statistical analysis
Demographics
Demographic data were analysed using statistical package for social sciences version 22.0. Mann–Whitney U-tests were used to describe group comparisons. Chi-squared tests were used to compare frequencies between groups.
Pharmacokinetic model development
Plasma concentration–time profiles of risperidone and 9-OH-risperidone were evaluated using a statistical model that linked parent risperidone and metabolite 9-OH-risperidone via a metabolism rate constant (km), with the following parameters: risperidone clearance (CLRISP); risperidone volume of distribution (VRISP); absorption rate constant (ka); 9-OH risperidone volume of distribution (V9-OH-RISP); and 9-OH-risperidone clearance (CL9-OH-RISP). The analysis estimated fixed effects (parameters describing dose–concentration relationships) and random effects, comprised of interindividual variability (difference between individual and predicted model parameter values for the sample) and residual variability (system noise, dosage history errors). Model development was carried out using Monolix software (version 2018r; www.lixoft.eu). Parameters were estimated using an iterative approach that provided maximum likelihood estimates and standard errors. Concentrations below the limit of quantification were coded to specify that their true values (and their contribution to the likelihood) could lie anywhere between 0 and 0.1 ng/mL. Plasma concentration was converted from ng/mL to μg/L for use in model building.
The model allowed estimation of the probability of there being more than one subpopulation in relation to risperidone clearance, by including a latent covariate. No assumption was made that latent categories corresponded solely to CYP2D6 genotype, as multiple factors contribute to hepatic metabolism in older people. Residual variability was estimated separately for risperidone and 9-OH-risperidone. Covariates (height, age, gender, smoking, ethnicity, weight) were incorporated in a stepwise manner, through visual inspection of covariate plots and regression analysis in R for categorical covariates. Models were evaluated using goodness-of-fit criteria, including diagnostic scatter plots, visual predictive checks, degree of shrinkage, change in interindividual variability, model precision and approximate likelihood ratio tests. A change in log likelihood estimate was considered significant if ≥4 (equivalent to P < 0.05, d.f. = 1), and accompanied by no change or a decrease in Bayesian Information Criteria.
Pharmacokinetic biomarkers and clinical outcome
Model-based estimates were used to calculate peak, trough and average concentrations of risperidone, 9-OH-risperidone and active moiety (their combined concentrations) for each individual, across the dosage interval. Concentration-to-dose ratio for the active moiety was calculated from trough estimates, to allow comparison with recommendations regarding personalised dosing of risperidone.Reference de Leon15 Each pharmacokinetic biomarker was individually considered as an independent variable (regressor) in a binary logistic model that described the probability of EPS. The model accounted for random effects and adjusted for potential confounders (age, gender, MMSE, height, weight). Best fit models were used to simulate and predict plasma concentrations and probability of EPS.
Results
Participants characteristics
Of 110 patients taking risperidone, 65 (59.1%) were randomised to risperidone treatment in phase 1; 31 (28.2%) in phase 2; and 14 (12.7%) in phase 3 (188 plasma samples, collected 26.9 h post dose (s.d. = 69.9)). After excluding four samples, taken after 180 h (above 6 half-lives post dose), data from 108 patients remained. Of the participants 57(52.7%) were men, the mean age was 78.4 years (s.d. = 6.7), mean weight 68.9 kg (s.d. = 14.7), mean height 1.6 m (s.d. = 0.1) and with a mean MMSE score of 14.6 (s.d. = 6.2). The mean timing of plasma sampling was 18.1 h post dose (s.d. = 26.8), with a mean days of treatment of 92.4 (s.d. = 76.8) and a mean dose of 1.0 mg/day of risperidone (s.d. = 0.7) (risperidone plasma concentrations 2.4 ng/mL (s.d. = 3.1), 9-OH-risperidone plasma concentrations 10.0 ng/mL (s.d. = 8.4); 20 (18%) risperidone and 2 (1.8%) 9-OH-risperidone samples were below the limit of quantification).
Eight participants with baseline SAS scores of ≥6 (indicating EPS prior to commencing risperidone), were excluded from the analysis of outcome data. Those with baseline EPS had greater global cognitive impairment (MMSE 7.8 (s.d. = 7.0) v. 15.2 (s.d. = 5.8), Mann–Whitney U, P < 0.0001) but there were no differences in other characteristics (Table 1). SAS- and BAS-rated treatment-emergent EPS occurred in 14 (14%), 8 of whom were recorded as having parkinsonism (moderate severity) in the adverse event log. Other adverse events included sedation in 13 (13%) (‘mild’ in 10, ‘moderate’ in 2, and ‘severe’ in 1), falls in 5 (5%), postural hypotension in 2 (2%) and ECG abnormalities in 3 (3%) patients (Table 1).
Table 1 Demographic and clinical characteristics of risperidone-treated The Clinical Trials of Intervention Effectiveness in Alzheimer's disease participants

EPS, extrapyramidal side-effects; MMSE, Mini-Mental State Examination; ECG, electrocardiogram.
a. n = 100, only those without baseline EPS were included.
***P < 0.0001 (all other findings not significant).
Those with treatment-emergent EPS were prescribed a higher risperidone dose (1.7 (s.d. = 0.9) mg v. 0.9 (s.d. = 0.5) mg; Mann–Whitney U-test, P < 0.003), had lower MMSE scores (10.2 (s.d. = 4.2) v. 16.0 (s.d. = 5.6), Mann–Whitney U-test, P < 0.0001) and a greater proportion (5 (37.5%) v. 6 (7.0%) patients) were treated with concomitant antidepressant medication (trazodone) (chi-squared P = 0.007, odds ratio (OR) = 7.4, 95% CI 1.9–29.2).
Pharmacokinetic model
The base model included a latent covariate with two categories (the model failed to converge using a covariate with three categories). Parameters were estimated with good precision, apart from V9-OH-RISP (relative standard error 60.1%). Residual variability was 0.1 μg/L (56.2%) for risperidone and 0.7 μg/L (28.2%) for 9-OH-risperidone. Covariate testing identified a significant contribution of log-transformed age to the variability in risperidone clearance (β = −0.3, P = 9.13e-04). Inclusion of an age effect on CLRISP increased the precision of the model (Supplementary Table 1 available at https://doi.org/10.1192/bjp.2020.225) and reduced the estimated probability of being in latent category 1 from 32% to 22%. Stepwise testing of other covariates on clearance parameters did not improve the precision or model fit.
Model-based predictions, based on the mean age (78.4 years) of the sample, suggested that for patients assigned to latent category one, risperidone clearance was 8.7 L/hr (t1/2 22 h), compared with 34.2 L/hr (t1/2 5 h) for those in latent category two. Patients in latent category one were thus considered to represent ‘functionally poor’ metabolisers. Predictions based on the observed contribution of age to risperidone clearance estimated that, for those aged 88 years, risperidone clearance would be reduced by 22% (t1/2 28.8 h in functionally poor metabolisers and 6.5 h in functionally normal metabolisers. Based on V9-OH-RISP and CL9-OH-RISP, the t1/2 for 9-OH-risperidone was 27 h). Visual predictive checks shown as percentile plots, superimposed on observed data, are shown in Supplementary Fig. 1.
Pharmacokinetic biomarkers and functional metaboliser status
Of the 100 participants included in the analysis of clinical outcome, 18 were categorised as functionally poor metabolisers. There were no differences in clinical or demographic or clinical variables in those who were functionally poor metabolisers and those who were functionally normal metabolisers. Cholinesterase inhibitors were prescribed in a higher proportion of functionally poor metabolisers (14 (77.8%) v. 37 (45.1%), chi-squared P = 0.01, OR = 1.27, 95% CI 1.04–1.53), and a higher proportion (14 (77.8%)) of functionally poor metabolisers were women (chi-squared P = 0.02, OR = 1.24, 95% CI 1.04–1.49). Functionally poor metabolisers were prescribed a lower dose of risperidone (0.7 (s.d. = 0.4) mg/day v. 1.1 (s.d. = 0.7) mg/day, Mann–Whitney U-test, P = 0.007) and had higher concentrations across all pharmacokinetic biomarkers (Supplementary Table 2).
Trough active moiety concentration-to-dose ratio was markedly increased in functionally poor metabolisers (20.2 (s.d. = 7.2) v. 7.6 (s.d. = 4.9) ng/mL per mg/day, Mann–Whitney U-test, P < 0.0001), shown in Fig. 1. There were no differences in the proportion of participants with emergent EPS in those who were functionally poor metabolisers and those who were functionally normal metabolisers.
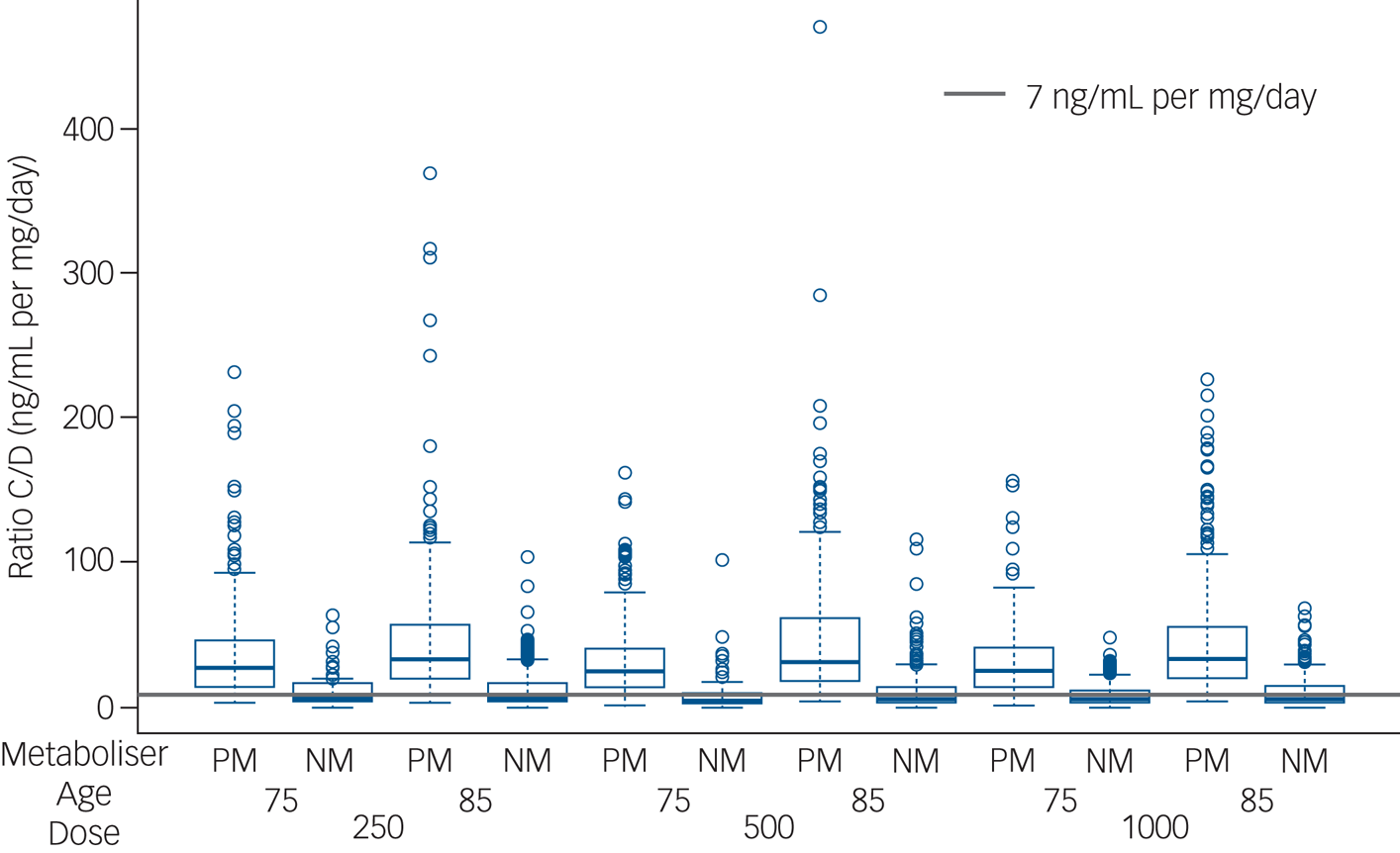
Fig. 1 Active moiety concentration-to-dose ratio. Estimated concentration-to-dose (C/D) ratios for the active moiety (ng/mL per mg/day) are shown at trough in those participants categorised as functionally poor metabolisers (PM) and those participants categorised as functionally normal metabolisers (NM), aged 75 and 85 years, prescribed 250, 500 and 1000 μg risperidone daily.
The grey line (7 ng/mL per mg/day) represents typical estimates for concentration-to-dose ratio in a reference group, based on therapeutic drug monitoring studies of risperidone.
Pharmacokinetic biomarkers and clinical outcome
Associations between individual pharmacokinetic biomarkers and EPS are detailed in Table 2. Pharmacokinetic biomarkers showed a significant association with EPS, achieving greatest significance in relation to trough concentrations of 9-OH-risperidone (adjusted OR = 15.79, 95% CI 4.66–53.51) and the active moiety (adjusted OR = 16.61, 95% CI 5.98–46.0). MMSE also contributed significantly to the best fit regression models (Table 2).
Table 2 Pharmacokinetic biomarkers and emergent extrapyramidal side-effects (n = 100)a
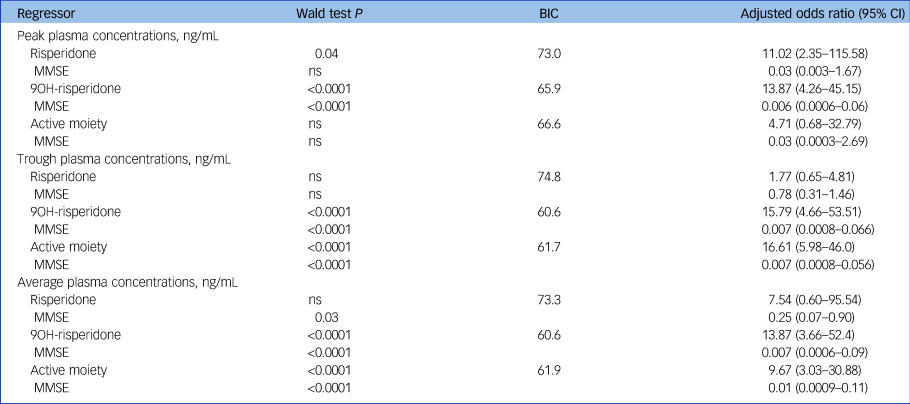
MMSE, Mini-Mental State Examination; ns, not significant; BIC, Bayesian Information Criteria.
a. Binary logistic regression models accounted for random effects, and adjusted for potential confounders including gender and log-transformed age, MMSE, height, weight and gender. A backward method was used, which removed variables that did not contribute to the model (significance threshold P < 0.05). For each best fit model, the β regressor effect coefficient (standard error) value was used to calculate a Wald statistic, its P-value, based on the chi-squared statistic, and adjusted odds ratio (95% confidence interval). Regression models were not significant for sedation and are not shown in the Table.
Model-based simulations (Fig. 2) suggest that for participants who were functionally normal metabolisers aged 75 years with an MMSE of 15, 1 mg/day risperidone would be associated with minimal risk of EPS. For those aged 75 years with an MMSE of 5, a dose reduction to 0.5 mg/day would be required. For those aged 85 years, the dose would need to be reduced by 50% to achieve equivalent plasma concentrations, and patients who aree functionally poor metabolisers would require very low alternate daily dosing (0.25–0.5 mg/48 h in those aged 75 years and 0.125–0.25 mg/48 h in those aged 85 years).
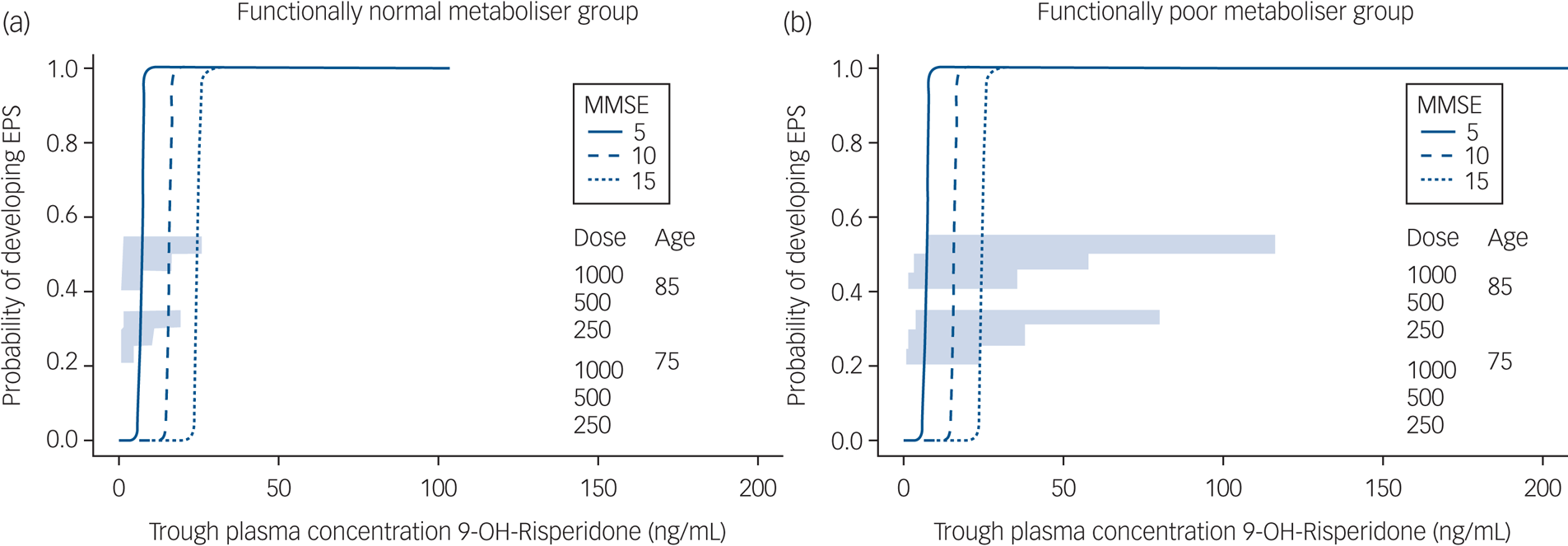
Fig. 2 Simulated trough 9-OH-risperidone concentrations and extrapyramidal side-effects (EPS).
Simulated trough 9-OH-risperidone concentrations and the probability of EPS are shown for a population of 100 people in each of the following categories: 75 or 85 years old; with an Mini-Mental State Examination (MMSE) score of 5, 10 or 15; prescribed 250, 500 or 100 μg risperidone daily in (a) participants who were categorised as functionally normal metabolisers and (b) participants who were categorised as functionally poor metabolisers.
For completeness, the same analysis was carried out in relation to sedation, but there were no significant associations, although the patient rated as having ‘severe’ sedation had active moiety concentrations of 43.39 ng/mL (the highest in the sample), and also had treatment-emergent EPS. The number of participants with other emergent side-effects was too small to investigate through the use of logistic regression.
Discussion
Pharmacokinetic and pharmacodynamic contributions to EPS
The consensus, based on meta-analyses of placebo controlled trials of risperidone in people with psychosis in Alzheimer's disease, is that 1 mg/day risperidone may optimally balance efficacy and adverse effects.Reference Tampi, Tampi, Balachandran and Srinivasan3,Reference Katz, de Deyn, Mintzer, Greenspan, Zhu and Brodaty7,Reference Schneider, Dagerman and Insel21 However, meta-analyses can only inform the ‘average’ dose requirements, but are less able to identify subgroups who are most susceptible to side-effects. In this analysis we have investigated the pharmacokinetic (dose–concentrations) and pharmacodynamic (concentration–outcome) contribution to EPS, to guide safer prescribing.
We estimated that 22% of patients were functionally poor metabolisers, and observed an independent effect of age on risperidone clearance. Pharmacokinetic biomarkers were robust predictors of EPS, with higher trough concentrations of 9-OH-risperidone and active moiety providing the best fit for the data. Lower MMSE, a marker of more severe global cognitive impairment, was an independent predictor of EPS. Model-based simulations suggest that, for functionally normal metabolisers aged 75 years with an MMSE of 15 (moderate Alzheimer's disease severity), 1 mg/day would be associated with minimal risk of EPS, but a dose reduction to 0.5 mg/day would be required for those with an MMSE of 5. For those aged 85 years, a 50% dose reduction would be required and, for those who are functionally poor metabolisers, alternate day dosing would be required to avoid excessive exposure.
Drug metabolism is a major contributor to pharmacokinetic variability and, in older adults, the relative importance of genotype is difficult to disentangle from physiological and clinical factors.Reference de Leon15 In the absence of information on CYP2D6 genotype, the extent of any genetic contribution to functional metaboliser status is unclear, although estimates of risperidone clearance in participants who were functionally poor metabolisers and functionally normal metabolisers were broadly consistent with estimates for CYP2D6-predicted ‘intermediate’ and ‘extensive’ metabolisers,Reference Feng, Pollock, Coley, Marder, Miller and Kirshner10 with a single patient having a risperidone/9-OH-risperidone ratio greater than one (suggestive of a genetically poor metaboliser).Reference de Leon15
Age was independently associated with risperidone clearance and, when incorporated into the model, led to the reassignment of six patients who were initially categorised as being functionally poor metabolisers. Although previous research has not shown an effect of age specifically on risperidone clearance,Reference Feng, Pollock, Coley, Marder, Miller and Kirshner10 a 30% decrease in hepatic metabolism in those aged over 70 years has been observed for other CYP2D6 substratesReference Sotaniemi, Arranto, Pelkonen and Pasanen22 and it is thus likely that our findings are explained by the older age of CATIE-AD participants. The long t1/2 (27 h) of 9-OH-risperidone in the sample as a whole is consistent with age-related impairment in renal clearance in CATIE-AD participants, which resulted in high trough concentrations of 9-OH-risperidone. This is important as the association between trough active moiety concentrations and EPS was largely explained by 9-OH-risperidone.Reference Vandenberghe, Guidi, Choong, von Gunten, Conus and Csajka12
The absence of an association between pharmacokinetic biomarkers and sedation warrants further consideration, as it may reflect the fact that distinct pharmacological mechanisms underpin sedation and EPS. However, methodological limitations need to be taken into account, as sedation was not measured using a standardised scale, but was identified solely from the adverse event log. This is important as only eight participants were rated as having EPS in the adverse event log, compared with the 14 identified using SAS and BAS scores. Prior exposure to antipsychotic drugs before randomisation also needs to be considered, as this may have reduced our ability to detect a relationship between sedation and pharmacokinetic biomarkers. This was not the case for EPS, as patients who scored above the threshold cut-off on SAS or BAS at baseline were excluded from further analysis.
Statistical modelling of the relationship between risperidone active moiety plasma concentrations and D2/3 receptor occupancyReference Uchida, Takeuchi, Graff-Guerrero, Suzuki, Watanabe and Mamo23 in adults with schizophrenia, suggests that trough concentrations of 10.5–38.2 ng/mL are associated with 60–78% occupancy in the striatum, and 6.5 ng/mL (95% CI, 3–10 ng/mL) is associated with 50% occupancy. In CATIE-AD participants, EPS emerged from trough active moiety concentrations of 3.4 ng/mL (of which 3.2 ng/mL was 9-OH- risperidone), and concentrations exceeded 10 ng/mL (60% occupancy) in 8 of 14 patients with EPS. In the absence of occupancy data, it is unclear whether the emergence of EPS at such low concentrations signifies a leftwards shift in the concentration–occupancy curve, similar to that observed during amisulpride treatment.Reference Reeves, McLachlan, Bertrand, D'Antonio, Brownings and Nair5 This is possible, as risperidone and 9-OH-risperidone are substrates for P-glycoprotein,Reference Wang, Ruan, Taylor, Donovan, Markowitz and DeVane24 a blood–brain barrier efflux transporter that is marked reduced in older people with Alzheimer's disease.Reference van Assema, Lubberink, Bauer, van der Flier, Schuit and Windhorst25 However, age- or disease-specific changes in brain–drug distribution, clearance and competition with endogenous dopamine at receptor sites need to be considered.Reference Reeves, McLachlan, Bertrand, D'Antonio, Brownings and Nair5
Pharmacodynamic changes (reduced D2/3 receptor reserve, altered signal transduction) that lead to a greater functional outcome for a given occupancy are also important. We have previously observed EPS at low striatal D2/3 receptor occupancies (60% compared with 80% in young people), in older people with schizophrenia taking risperidone,Reference Uchida, Suzuki, Graff-Guerrero, Mulsant, Pollock and Arenovich26,Reference Graff-Guerrero, Rajji, Mulsant, Nakajima, Caravaggio and Suzuki27 and older people with psychosis in Alzheimer's disease taking amisulpride.Reference Reeves, McLachlan, Bertrand, D'Antonio, Brownings and Nair5 Given the error margin of occupancy predictions, we cannot rule out the possibility that occupancy was underestimated in a proportion of those with active moiety concentrations less than 10 ng/mL.Reference Uchida, Takeuchi, Graff-Guerrero, Suzuki, Watanabe and Mamo23
The observed association between lower MMSE score, a marker of greater neuropathological change, and emergent EPS is consistent with previous clinical observations in patients taking risperidone.Reference de Leon15 Furthermore, an association between agitation, antipsychotic use and death in those with more severe dementia has been reported in a recently published cohort study.Reference Aworinde, Werbeloff, Lewis, Livingston and Sommerlad28 The mechanisms of antipsychotic-induced EPS are not fully understood, but D2/3 receptor antagonism of inhibitory dopaminergic inputs to striatal medium spiny neurons and cholinergic interneurons may play a key role.Reference Ohno, Kunisawa and Shimizu2 It is unclear whether the risk of EPS in those with lower MMSE scores reflects greater impairment in networks that modulate motor control, or is associated with as yet unidentified factors that potentiate EPS.
Limitations
Limitations to the analysis include sparse sampling, which meant it was not possible to estimate within-participant variability in clearance of risperidone or 9-OH-risperidone, or to investigate the contribution of concomitant medications to pharmacokinetic variability. Neither was it possible to investigate the contribution of comorbid medical conditions to variability in pharmacokinetics or emergent side-effects. The lack of information on renal function is a major limitation, given the significant association between plasma concentrations of the renally eliminated metabolite and emergent EPS. This needs to be addressed in future studies.
We cannot account for the fact that those with emergent EPS were more likely to have been prescribed concomitant trazodone, given the small sample size and uncertain exposure (dose, continuity of the prescribed drug) of individual participants to trazodone. However, we cannot rule out the possibility of potential drug–drug interactions, including an interaction with P-glycoprotein, for which trazodone is a substrate. Preclinical studies have shown that selective serotonin reuptake inhibitors potentiate antipsychotic-induced EPS and this may be relevant as trazodone acts as a weak reuptake inhibitor.Reference Ohno, Kunisawa and Shimizu2 The issue of polypharmacy is an important one, as antipsychotic drugs are often initiated alongside other drug treatments, including sedating antidepressants and the downstream effects of central drug–drug interactions are poorly understood. Other limitations relate to the CATIE-AD study design, which may have reduced our ability to detect associations between pharmacokinetic indices and clinical outcome. This includes flexibility in starting dose (low or high), and the option of making adjustments or of discontinuing a phase, based on clinician judgement.
Dose adjustments needed to avoid treatment-emergent EPS
This analysis represents a step towards safer risperidone prescribing and argues strongly for age- and MMSE- related dose reductions. From a pragmatic perspective, clinicians should ‘start low go slow’ (0.5–1 mg/day) in those aged 75 years with moderate stage Alzheimer's disease, and ‘start low, stay low’ (maximum 0.5 mg/day) in those aged 75 years with severe Alzheimer's disease. For those aged 85 years, the dose should be halved, and alternate daily dosing should be considered if side-effects emerge, as it is likely that the person has slower active moiety clearance.
Personalised prescribing should ideally incorporate knowledge of genetic, environmental and personal variables to determine dosing. This is not currently happening in clinical practice and there has been a lack of empirical data in older people to justify the use of routine therapeutic drug monitoring. A recently proposed personalised prescribing algorithm for risperidone suggests that high trough concentration-to-dose ratios are indicative of slower active moiety clearance, as a result of the combined effect of CYP2D6, 3A4 and renal clearance.Reference de Leon15 Our analysis, which used model-based estimates of trough concentrations, were consistent with this recommendation and suggested that alternate daily dosing may be required in those with higher concentration-to-dose ratios to avoid emergent EPS. Therapeutic drug screening offers the opportunity to guide dose adjustments with more precision, as the concentration-to-dose ratio of the active moiety could be derived from a single steady state trough plasma sample, to identify those at higher risk of excessive exposure. It will, however, be important to replicate these findings in a larger data-set that includes information on renal function, and allows further investigation of the impact of antidepressant use on the observed associations. Alongside this, future studies should evaluate the feasibility and clinical utility of therapeutic drug screening in older people with Alzheimer's disease.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjp.2020.225.
Data availability
Data used in the preparation of this article were obtained from the limited access data-sets distributed from the NIH supported ‘Clinical Antipsychotic Trials of Intervention Effectiveness in Alzheimer's disease’ (CATIE-AD) (MH64173, MH90001).
Acknowledgements
Data used in the preparation of this article were obtained from the limited access data-sets distributed from the NIH supported ‘Clinical Antipsychotic Trials of Intervention Effectiveness in Alzheimer's disease’ (CATIE-AD) (MH64173, MH90001). This is a multisite, clinical trial of people with psychosis and/or agitation in the content of Alzheimer's disease, which compared the effectiveness of randomly assigned medication treatment. This manuscript reflects the views of the authors and may not reflect the opinions or views of the CATIE Study Investigators or the NIH. No funding was provided for this analysis. Suzanne Reeves had full access to the data included in this analysis, and had final responsibility for the decision to submit for publication.
Author contributions
S.R. led the study design, carried out data extraction, carried out data analysis and led on the writing of the paper; J.B., H.U., R.B., B.P. and R.H. gave input into the study design and analysis plan; K.Y., Y.O. and K.Y.L. extracted data on clinical outcome measures; M.O. and S.R. extracted and checked adverse event data. J.B. supervised S.R. in the analysis of the data, carried out simulations and led on data presentation; E.B. and R.H. gave input into the interpretation and presentation of pharmacokinetic data; all authors contributed to the interpretation of the clinical findings and the writing of the paper, and approved the submitted manuscript.
Funding
R.H. is supported by the NIHR University College London Hospital Biomedical Research Centre (UCLH BRC). K.Y.L. is supported by the Medical Research Council (MR/S021418/1)
Declaration of interest
There are no competing interests.










eLetters
No eLetters have been published for this article.