Introduction
Insect species have high epidemiological importance as vectors of hundreds of diseases worldwide and, consequently, are responsible for hundreds of thousands of deaths every year (Vasconcelos Reference Vasconcelos2015). Mosquitoes of the family Culicidae are among the most important vectors, responsible for the transmission of diseases such as malaria, yellow fever, dengue, chikungunya, and Zika, especially in tropical and subtropical areas (Vasconcelos Reference Vasconcelos2015). In the Americas, for instance, US$ 2.1 billion is spent on dengue-related illness each year (Shepard et al. Reference Shepard, Coudevelli, Halasa, Zambrano and Dayan2011). In Brazil, it has been estimated that Aedes aegypti and arboviruses that they transmit caused an economic impact of R$ 2.3 billion in 2016 (approximately US$ 460 million) (Teich et al. Reference Teich, Arinelli and Fahham2017).
One of the first common methods of reducing Aedes albopictus, for example, which breed in artificial environments, is removing or turning over temporary water accumulated in containers or other water tanks, thus preventing oviposition and proliferation, with temporary suppression of immature mosquitos (Baldacchino et al. Reference Baldacchino, Caputo, Chandre, Drago, Della Torre, Montarsi and Rizzoli2015; Benelli Reference Benelli2015). This method also impacts the distribution of native mosquitos, limiting the places for oviposition (Baldacchino et al. Reference Baldacchino, Caputo, Chandre, Drago, Della Torre, Montarsi and Rizzoli2015). Several alternative strategies have been studied and tested, each with its own strengths and drawbacks. Chemical insecticides are effective, but mosquitoes commonly evolve resistance (Benelli Reference Benelli2015). Predatory fish reduce mosquito densities, but cannot survive in small environments and have the potential to cause damage if exotic species are introduced and become invasive, impacting the natural communities (Azevedo-Santos et al. Reference Azevedo-Santos, Vitule, Pelicice, García-Berthou and Simberloff2017; Kumar & Hwang Reference Kumar and Hwang2006).
An alternative to predatory fish is the use of crustacean copepods, which have a high ability to prey on mosquitoes, low chances of invading and affecting natural communities when using native species, and low costs (Baldacchino et al. Reference Baldacchino, Bruno, Visentin, Blondel, Arnoldi, Hauffe and Rizzoli2017; Tran et al. Reference Tran, Olsen, Viennet and Sleigh2015; Udayanga et al. Reference Udayanga, Ranathunge, Iqbal, Abeyewickreme and Hapugoda2019).
Cyclopoid copepods have been found to be effective predators of mosquito larvae under experimental conditions and in the field in several regions of the world (e.g. Estrada & Vázquez-Martínez Reference Estrada and Vázquez-Martínez2015; Marten et al. Reference Marten, Borjas, Cush, Fernandez and Reid1994; Marti et al. Reference Marti, Micieli, Scorsetti and Liljesthröm2004; Nam et al. Reference Nam, Yen, Phong, Ninh, Mai, Lo, Nghia, Bektas, Briscombe, Aaskov, Ryan and Kay2005, Reference Nam, Yen, Duc, Con Tu, Thang, Hoang, San, Loan, Huong, Khanh, Trang, Lam, Kutcher, Aakov, Jeffery, Ryan and Kay2012; Tranchida et al. Reference Tranchida, Miceli, Maciá and García2009; Udayanga et al. Reference Udayanga, Ranathunge, Iqbal, Abeyewickreme and Hapugoda2019). One of the fully successful examples is given by Marten et al. (Reference Marten, Cabllero, Larios and Bendaña2022), with the use of copepods and two other predators (baby turtle and tilapia fry) for mosquito control in Honduras, highlighting that this country has used copepods since 1985, approximately. Another successful experience came from Vietnam (Nam et al. Reference Nam, Yen, Phong, Ninh, Mai, Lo, Nghia, Bektas, Briscombe, Aaskov, Ryan and Kay2005, Reference Nam, Yen, Duc, Con Tu, Thang, Hoang, San, Loan, Huong, Khanh, Trang, Lam, Kutcher, Aakov, Jeffery, Ryan and Kay2012; Tran et al., Reference Tran, Olsen, Viennet and Sleigh2015). Additionally, for some species of copepods (Acanthocyclops robustus, Macrocyclops albidus, Diacyclops uruguayensis, and M. longisetus), close to 80% of the population can survive 15 days of desiccation, and 60% can survive 30 days, making this group an excellent tool for the biological control of culicids (Tranchida et al. Reference Tranchida, Miceli, Maciá and García2009).
Among the copepod species most used for mosquito control, especially the Aedes genus, Mesocyclops longisetus (Thiébaud, 1912) is a large predator cyclopoid copepod (1.26 to 2.8 mm length) that commonly feeds on Culicidae larvae (Reid, 1985; Marten et al. Reference Marten, Borjas, Cush, Fernandez and Reid1994; Soumare & Cilek Reference Soumare and Cilek2011). This species has a Neotropical distribution and is commonly found in Brazilian ecosystems as well as in other countries in the region (Reid, 1985). Due to these features, this species has the potential to be used on a large scale for mosquito control also in Brazil. However, even though mosquito-borne diseases are a central problem in public health in this country, only two preliminary studies have been performed (Santos et al. Reference Santos, Andrade and Carvalho1996; Santos & Andrade Reference Santos and Andrade1997).
Here, we carried out two experiments in two types of habitats: plastic containers – plates and pot (first experiment) and concrete slabs (second experiment), simulating small environments commonly related to mosquito proliferation. The experiments were set up on the border of a forest fragment, and the containers and the concrete slab were naturally colonized by Chironomidae and Culicidae insects. Inventory studies of both these families can be found in Trivinho-Strixino (Reference Trivinho-Strixino2011a) and Paula et al. (2015). After the colonization, M. longisetus was introduced at different densities and its effectiveness in decreasing mosquito populations without impacting the natural community was assessed. The study attempted to answer the following questions: (i) Will the introduction of copepods effectively control mosquito larvae? (ii) Is the density of copepods important for ensuring biological control?
Materials and methods
Study area and culture of copepods
The study was conducted between February and July 2019 in a natural subtropical (Köppen) environment located in an Atlantic Forest fragment (Buri County, São Paulo State, Brazil, 23°, 35',45" S, and 48°, 31',53" W, altitude 590 m.a.s.l.). There was a small town (Campina do Monte Alegre, c.a. 6,000 habitants) about 4 km from the site.
Copepods were produced under indoor controlled conditions (12:12 h; 25°C) from ovigerous females captured in a nearby lake, separated and placed in polypropylene tanks of 150 liters. A mix of algae from the genera Scenedesmus and Chlorella and cladocerans of the species Moina minuta Hansen, 1899, were added daily ad libitum as food for the copepods; chemical fertilizer NPK 10:10:10 was added to stimulate the growth of phytoplankton.
First experiment – Plastic containers
The first experiment considered three copepod densities and two container shapes (plates and pots, see below). In this experiment, we tested the following hypotheses: (i) predation by copepods decreases the abundances of both Culicidae and other insects (e.g. other Diptera), (ii) better control occurs at the highest density of copepods, and (iii) the type of the container (or aquatic environment) affects the natural colonization and copepod control of dipteran larvae.
Two types of plastic containers, each with a volume of 2 L, were used: shallow circular black plates commonly used in flower vases, measuring 41 cm in diameter and 6 cm in depth, and rectangular white and blue ice cream pots, measuring 15 cm long by 10 cm wide by 15 cm high. Filtered mineral water was used to fill these containers. The two types of containers were chosen to study the effect of shape on the rate of colonization by mosquitoes. The containers were randomly arranged on a large table positioned on the border of a forest fragment, with the aim of studying natural colonization by dipterans from the forest.
As treatments, we used two densities of copepods (5 and 15 ind. L−1), plus a control group without copepods (0 ind. L−1). We used four replicates for each density level (control, 5, and 15 copepods. L−1), totalling 12 plastic containers of each type.
Chironomidae and Culicidae larvae were sampled every 5 days, totalling 90 individual samplings per month for 4 months (360 experimental units in the beginning), but some units were lost due to external factors and, in the end, we had 13 useful cycles for Chironomidae and 12 for Culicidae ad 336 experimental units). After the insects were captured, all containers were washed and filled with mineral water and new copepods for the next run. During each sampling, the air temperature was measured using a mercury thermometer.
Second experiment – Concrete slab
The second experiment was performed on a 1 m × 1 m concrete slab with a capacity of 10 L. It was performed from January to July, 2019, totalling 171 days of experiment divided into five sequential runs. Due to the logistic situation, the duration of the experiments varied from 24 to 33 days. The monitoring and counting of organisms (Culicidae and Chironomidae larvae and copepods after addition) were performed at intervals of 3–5 days. For each of these, the slab was fully marked with sections of 10 by 10 cm, aiming at the best quantification of the organisms. The slab was filled with mineral water.
At the beginning of each round of the experiment, copepods were introduced at the following densities: 2 ind. L−1 (20 individuals, run number 4), 5 ind. L−1 (50 individuals, runs 1, 3 and 5) and 10 ind. L−1 (100 individuals, run 2). In this last run, copepods were added twice, once at the beginning of the experiment, as in the other rounds, and then on the 21st day of the experiment, at the same density as the initial addition, since the copepods had died. At the end of each experimental run, the slab was dried and washed to remove all organisms and start a new colonization and a new introduction of copepods.
When counting in both experiments, dipteran larvae were identified at the family level – Culicidae and Chironomidae – using Rueda (Reference Rueda2004) and Trivinho-Strixino (Reference Trivinho-Strixino2011b), respectively. The insect larvae were fixed in 70% ethanol.
Data analysis – First experiment
A descriptive analysis was conducted first. Means and standard errors were used to summarize the abundance of Chironomidae and Culicidae at different times and copepod densities. The association between quantitative variables was assessed using Pearson’s coefficient of linear correlation (r statistics) and tested by the Student’s t-distribution. Furthermore, the natural abundance of Culicidae and Chironomidae was compared among receptacles (pots and plates) using Wilcoxon’s signed-rank test for paired data.
The count data were explored through Generalized Linear Mixed Models to accommodate random effects for experimental runs/cycles. Poisson and Zero-Inflated Poisson (ZIP) models were fitted to the data using the R packages ‘glmer’ and ‘glmmTMB’, respectively. The model’s adequacy was examined with the DHARMa package (Hartig, 2020). Data from pots and plates were quite different, and no model described well the two types of habitats together. Counts for Culicidae larvae were better described by the Zero-inflated Poisson model; no suitable model was found to describe Chironomidae counts over time.
Chironomidae data were analyzed within the collection times (runs). Due to the over-dispersed counts, the quasi-Poisson model was considered (Demétrio et al. Reference Demétrio, Hinde and Moral2014). The copepod densities were compared within each type of receptacle (pots or plates), due to interactions between type of receptacle and copepod density. Effects of runs, type of receptacle and treatments were assessed through the likelihood ratio test (LRT), considering the F-statistics for scaled deviances.
All tests of hypotheses were carried out at the 5% significance level (P < 0.05) and all data analyses were performed in R Core Team (2020).
Results
First experiment – Plastic containers
The habitats were naturally colonized by larvae of the dipteran families Chironomidae and Culicidae. Through the 75 days of experimentation, the environmental temperature declined linearly (Figure 1(a)), decreasing about 10ºC from February to May (32ºC to 22ºC). The abundance of Chironomidae larvae in the water was positively associated with the environmental temperature (r = 0.93, t = 9.02, df = 12, P < 0.001) (Figure 1(b)).

Figure 1. (a) Relationship between the environmental temperature (ºC) and time (days after the start of the experiment). (b) Relationship between the natural abundance of Chironomidae larvae (at the control treatment) and environmental temperature (ºC). Linear fittings by the least square method are represented by dashed straight lines.
The natural (control) abundances of larvae of Culicidae and Chironomidae were not significantly associated with each other (r = 0.41, t = 1.50, df = 11, P = 0.16). Additionally, the mean natural abundance of Culicidae did not respond to changes in environmental temperature (r = 0.37, t = 1.34, df = 11, P = 0.20). These patterns remained the same when the abundance data were analyzed within the types of habitats.
The natural abundance of Chironomidae was similar when comparing plates and pots (Figure 2(a)). On the other hand, the Culicidae counts were not correlated between types of receptacles (r = 0.30, t = 1.06, df = 11, P = 0.31) and had different distributions for them (Wilcoxon signed-rank test, paired data, P < 0.001). Culicidae counts exhibited more variation between plates and pots than Chironomidae counts (Figure 2(a) and (b)).
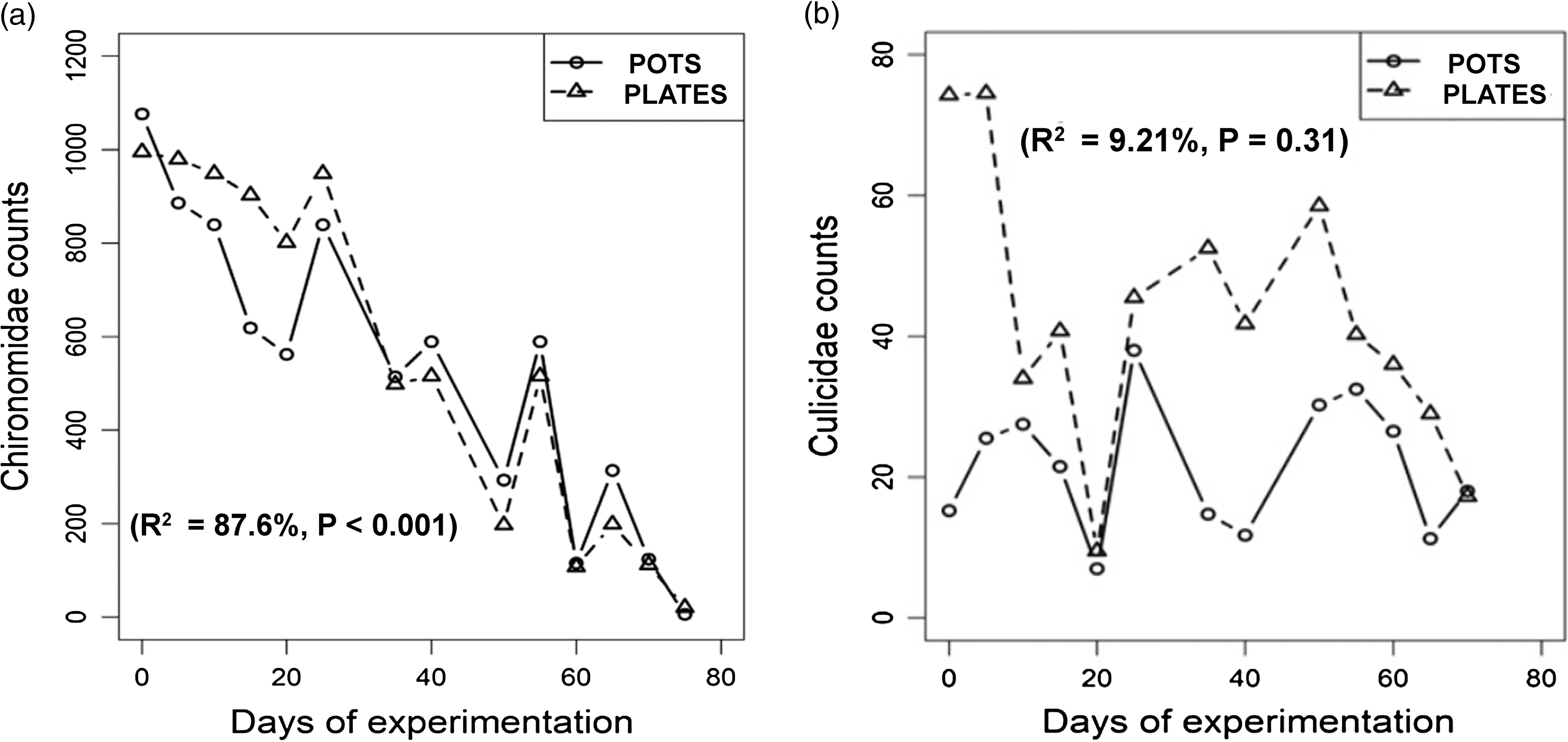
Figure 2. (a) Natural abundance of Chironomidae larvae in the control over time in plates and pots of 2 L. Dots are weekly averages. (b) Natural abundance of Culicidae larvae in the control over time in plates and pots of 2 L. Dots are weekly averages.
A ZIP model with random time effects (intercepts) reasonably described the Culicid counts. For both types of habitats (pots and plates), the counts were clearly affected by copepod density (Wald’s
![]() ${\chi ^2}$
, P < 0.001; Figure 3). The overall mean reduction in the 5 ind. L−1 treatment was about 32% for pots and 47% for plates, while the 15 ind. L−1 treatment resulted in a 61% of reduction for pots and 64% of reduction for plates. In both the habitats, the 15 ind. L−1 treatment differed statistically from the control, providing lower counts. Furthermore, the 5 ind. L−1 treatment differed statistically from the control for plates; for pots, there was a tendency toward lower counts, but it was not significant at the 95% confidence level (P
${\chi ^2}$
, P < 0.001; Figure 3). The overall mean reduction in the 5 ind. L−1 treatment was about 32% for pots and 47% for plates, while the 15 ind. L−1 treatment resulted in a 61% of reduction for pots and 64% of reduction for plates. In both the habitats, the 15 ind. L−1 treatment differed statistically from the control, providing lower counts. Furthermore, the 5 ind. L−1 treatment differed statistically from the control for plates; for pots, there was a tendency toward lower counts, but it was not significant at the 95% confidence level (P
![]() $ \approx $
0.05). The copepods were still effective at high levels of Culicidae abundance, since the cycles with the highest levels of colonization showed reduction rates varying from 60% to 80%.
$ \approx $
0.05). The copepods were still effective at high levels of Culicidae abundance, since the cycles with the highest levels of colonization showed reduction rates varying from 60% to 80%.

Figure 3. (a) and (b) Conditional means (dots) and 95% confidence intervals for overall counts of Culicidae for three densities of copepods and two habitats (pots and plates). Means are the overall results from 13 consecutive 5-day cycles of experimentation. Estimates were obtained from the fitting of the ZIP model with random intercepts for ‘time’. Treatments separated through the dotted line differ from each other at the significance level of 5%.
No suitable model was found to describe Chironomidae counts over time. Therefore, Chironomidae data were analyzed within the collection times (runs). The densities of copepods and types of receptacles influenced Chironomidae counts most of the time (Deviance, P < 0.05), and interactions between these factors were eventually found (Deviance, P < 0.05). Comparisons of the treatments (densities of copepods) were made within types of habitats.
As observed for Culicidae, the use of copepods led to statistically significant reductions in Chironomidae counts most of the time (LRT, P < 0.05; Figure 4). The median reduction in Chironomidae counts was approximately 30% for pots and 23% for plates for the 15 ind. L−1 treatment, but only 7% for 5 ind. L−1 copepods, both for the pots and plates.

Figure 4. (a) and (b) Comparison of treatments (copepod densities) to the number of Chironomidae (mean
![]() $ \pm $
S.E.) in plates and pots after 5 days of exposure at different collection times. Effects of treatments assessed by quasi-Poisson regression models and tested through likelihood ratios. *: differences for treatments at 5% significance.
$ \pm $
S.E.) in plates and pots after 5 days of exposure at different collection times. Effects of treatments assessed by quasi-Poisson regression models and tested through likelihood ratios. *: differences for treatments at 5% significance.
Second experiment – Concrete slab
In the second experiment, increases in Culicidae and Chironomidae were associated with declines in copepod densities (Figure 5). Culicidae larvae were completely absent in the third and fourth runs, and their presence was only occasionally noted during the other monitoring times, increasing in abundance after copepods decreased during runs one and five. During run two, the highest density of copepods used kept the numbers of Chironomidae and Culicidae low at the beginning of the run, but after the twelfth day, following a reduction in the number of copepods, an increase in Chironomidae occurred. Even after the introduction of more copepods on day 21, the densities of Culicidae and Chironomidae no longer decreased. Culicidae larvae persisted to the end of the experiment, even in the presence of copepods and high concentrations of Chironomidae.

Figure 5. Time progression of populations of Chironomidae (black bars), Copepods (white bars), and Culicidae (gray bars) across the monitoring days of five experimental runs. Copepod treatments: 2 ind. L−1 in the fourth run; 5 ind. L−1 at the first, third, and fifth runs; 10 ind. L−1 in the second run.
Discussion
The copepod species M. longisetus used in our studies was efficient to control culicid mosquito larvae in the environments studied, especially in high densities. The results from the first experiment confirmed the effectiveness of copepods in controlling Culicidae larvae, but not the Chironomidae, which are not of medical interest. A density of five copepods per litre eliminated almost 50% of Culicidae larvae; moreover, with 15 copepods per litre the effectiveness of the copepods can increase to almost 70%. In the second experiment, the replacement of copepods over time was important to maintain decreases in larvae densities.
Copepods preyed upon Culicidae more intensively, even when chironomids were abundant. Russel et al. (Reference Russel, Qureshi, Wilson and Cator2019) highlighted the importance of size relationships in determining predation efficiency among copepods in laboratory experiments. Früh et al. (Reference Früh, Kampen, Günter and Werner2019) also found results showing that copepods preferred to prey on the smallest culicid larvae. Biological relationships in small environments with few components indicate that predation occurs almost exclusively on the same organism unless other small prey is available (Naeem 1988). We verified that if Chironomidae are abundant, the pressure on Culicidae is intense (Figure 2). Thus, in small systems close to a border fragment forest like the one in our study, high levels of Chironomidae colonization suppress Culicidae larvae controlled by cyclopoid copepods.
The efficiency of cyclopoid copepods (M. longisetus) in the control of Culicidae has been well documented in other countries, with a reduction of 98% of larvae in one study in Vietnam (Tran et al. Reference Tran, Olsen, Viennet and Sleigh2015), and Tranchida et al. (Reference Tranchida, Miceli, Maciá and García2009) pinpointed this species as one of the best for mosquito control. Marten (Reference Marten1990) found that copepods preyed on approximately 30 larvae per day and were more efficient when copepods were renewed every 15 days, interfering in the mosquito oviposition period. All of this agrees with our results, indicating that larvae increased when copepods decreased, especially in the second experiment and highlighting the effectiveness of M. longisetus in the first. Another study using M. longisetus in tires (Schreiber et al. Reference Schreiber, Hallmon, Eskridge and Marten1996) showed that there was a remarkable decrease in the number of mosquito larvae of different species compared to the control experiment without copepods. Among the main species preyed on in that study were A. albopictus (reduction of 88%), Anopheles crucians, Culex quinquefasciatus, and Culex salinarius. Gorrochotegui-Escalante et al. (Reference Gorrochotegui-Escalante, Fernandez-Salas and Gomez-Dantes1998) conducted an experiment in Mexico using M. longisetus for the control of A. aegypti, which was carried out in three different environments over 4 months with monitoring performed every 15 days: 200 L metal drums (200 copepods with reduction of 37.5% of the larvae), flower pots (50 copepods with a reduction of 67.5% of the larvae) and tires (50 copepods with a reduction of 40.9% of the larvae). Sarwar (2015) points out that the predation of copepods is approximately 98% to 100% of mosquito larvae and they may prey on 30 or more larvae per day. Thus, our results agree with other authors, although the control rates of Culicidae by copepods in our study are lower than desired.
Our results showed an influence of environmental temperature on the development of Chironomidae during the period, since the experiment was conducted during summer and autumn; in autumn, the organisms naturally decrease their metabolic activities. The insects studied here are tropical colonizers and thus sensitive to decreases in temperature. Strixino & Strixino (Reference Strixino and Strixino1985) point to optimum temperatures between 22ºC and 26ºC for tropical Chironomidae larvae in a region located 300 km from our study site. Our study started in summer, when there are more food resources, and the temperatures were close to the optimum. When runs were made in autumn and winter, the minimum temperature values negatively affected the growth and survival of larvae in the small environments. However, other studies found contrary results. Beserra et al. (Reference Beserra, Ribeiro and Oliveira2014) found that the population fluctuations of A. aegypti larvae did not show a correlation with climatic variables in the South of Brazil, since there are no optimal temperature conditions for establishing this species annually. Russel et al. (Reference Russel, Qureshi, Wilson and Cator2019), working in the United Kingdom, discarded the temperature effect and pointed to prey size as the dominant factor.
The results of the second experiment showed a trend of increasing densities of dipterans accompanying the decrease of copepods during the experimental run. When copepods were present, few or no dipterans were found. However, the copepods died after a time in almost all runs, After the reintroduction of copepods in run 2, the densities of dipterans were not reduced, especially when stages up to and including the first instar were found, which copepods eat less than the first life stage.
The results show that high densities of copepods are better than low densities for the control of dipterans. Biological control is only efficient if the density is restored every 15 days, which, in practice, may be difficult to implement on a large scale. Furthermore, the succession cycles on the slab showed an irregular variation, indicating that the factors evaluated should not be considered in isolation, since no correlations were established between the variables studied and the vector’s presence. So, for a better analysis of the predation of the Culicidae, multiple factors permitting their infestation and continuing presence must be considered, reinforcing the necessity for further studies.
Conclusion
We found effective biological control of culicid mosquito larvae by 15 copepods per litre in pots and plates at the edge of a forest fragment. Even 5 copepods per litre caused important reductions in larvae. This occurred even at high abundances of chironomids and seasonal temperature variations. However, copepods are only useful if added when the water starts to fill the receptacle, coinciding with the egg-laying phase of mosquitoes. This issue is the next obstacle to be overcome for the use of copepods at safe and efficient levels, in the attempt to eliminate mosquito vectors of severe diseases in humans.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467423000020
Acknowledgements
To CNPq Universal process 425799/2016-6, Leticia Gonçalves Ribeiro and Amanda Maria Roque to help in the experiment counting and laboratory analysis, Valter Azevedo-Santos to his valuable commentaries and Dr. James A. Nienow of Valdosta State University to English revision and valuable commentaries.









