Low socio-economic status (SES) is a determining factor of poor maternal diet( Reference McLeod, Campbell and Hesketh 1 – Reference Baron, Manniën and te Velde 4 ), lifestyle behaviours( Reference Phares, Morrow and Lansky 5 – Reference Larrañaga, Santa-Marina and Begiristain 7 ) and pregnancy outcomes( Reference Agyemang, Vrijkotte and Droomers 8 , Reference Metcalfe, Lail and Ghali 9 ). However, the response of women of low SES to dietary interventions in pregnancy is largely unknown. In terms of health, SES is a measure of an individual’s access to the basic resources required to achieve good health, and can be estimated by income, occupation, education and neighbourhood( Reference Shavers 10 ). The reasons for poorer health and health-related behaviours among disadvantaged groups of the population are manifold, including unemployment, inferior housing conditions, discrimination( Reference Gehlert, Sohmer and Sacks 11 ), greater anxiety and depression( Reference Sooman and Macintyre 12 ), and barriers to healthy lifestyle choices such as limited food budget, lack of cooking skills and poor nutrition knowledge( Reference Wiig and Smith 13 ).
Since poor health and health-related behaviours are more prevalent among disadvantaged groups of the population, it would seem prudent that these individuals are provided with resources and support to allow them equal opportunities to influence their own health. Governments and state bodies aim to address this through public health policies and initiatives such as food subsidy programmes, dietary interventions, additional community funding and family support services( Reference McFadden, Green and Williams 14 , 15 ). The International Weight Management in Pregnancy (i-WIP) collaboration recommends that public health measures are taken to promote lifestyle education and behaviour change before, during and after pregnancy among women of low SES( Reference Dodd and Thangaratinam 16 ). However, if policies that meet the needs of disadvantaged groups are to be successful, research specific to these individuals must be carried out that will inform governments and public health bodies. Currently, research and intervention studies, through their design, analysis and dissemination, are not filling the knowledge gaps which exist in relation to initiatives among individuals of low SES.
Notably, there is a paucity of data on the response of low-SES individuals to interventions, as the majority of clinical trials and observational studies do not analyse the outcomes of interest stratified by SES, and individuals of low SES are not represented in equal numbers( Reference Callander and McDermott 17 – Reference Furler, Magin and Pirotta 19 ). The lack of consideration of the differences in outcomes across socio-economic groups makes it difficult for readers, health-care professionals and policy makers to disentangle the types of interventions that have the greatest impact among those of lower SES( Reference Callander and McDermott 17 ).
For policies to be successful in changing health behaviours, multifaceted approaches with both upstream interventions (those that influence policy) and downstream interventions (those that influence individual-level behaviours) are required in tandem( Reference Brownson, Seiler and Eyler 20 ). However, the majority of research studies carried out in the context of dietary interventions in pregnancy are designed as single downstream interventions( Reference Walsh, McGowan and Mahony 21 – Reference Kernot, Olds and Lewis 24 ). More information is required to understand if these interventions are successful in changing behaviours among low-SES women, and strategies to improve response.
The ROLO study (Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia) consisted of low glycaemic index (GI) dietary education session, delivered in small groups, to pregnant women( Reference Walsh, McGowan and Mahony 21 ). Since nutrition knowledge is a mediator between SES and diet quality( Reference McLeod, Campbell and Hesketh 1 ), a dietary group education session may be appropriate for women of low SES. While the low-GI dietary intervention was successful in reducing dietary GI and gestational weight gain( Reference Walsh, McGowan and Mahony 21 ), differences in these outcomes across socio-economic groups (education level and neighbourhood deprivation) have not been examined to date. We aimed to determine if response to a low-GI dietary intervention, measured by changes in diet and excess gestational weight gain, differed across women of high/low educational attainment and neighbourhood deprivation.
Participants and methods
Study design
The present study was a secondary data analysis of 625 pregnant women originally recruited as part of the ROLO study between 2007 and 2011, at the National Maternity Hospital, Dublin, Ireland. Detailed methodology and results of the ROLO study have been previously published( Reference Walsh, McGowan and Mahony 21 , Reference McGowan, Walsh and Byrne 25 ). In brief, 800 secundigravida women who had previously given birth to a macrosomic infant (>4000 g) were randomised to receive either low-GI dietary advice from the research dietitian or standard usual care (no dietary advice), with the aim to reduce recurrence of macrosomia( Reference Walsh, McGowan and Mahony 21 ). Although the primary outcome of birth weight did not differ significantly between the intervention and control arms, the secondary outcomes, gestational weight gain, dietary GI and glucose intolerance, were reduced significantly in the intervention arm( Reference Walsh, McGowan and Mahony 21 , Reference McGowan, Walsh and Byrne 25 ).
Patient selection
Participants were recruited at the first antenatal hospital visit between 10 and 18 weeks’ gestation. Women were included in the study if they were aged 18 years or above, had previously delivered one infant weighing ≥4000 g and had good understanding of the English language. They were excluded if they were pregnant with twins/triplets, had previous or current gestational diabetes, or had an underlying medical condition requiring medication.
Intervention
Participants were randomised into the intervention or control arm using computer-generated allocations. Two weeks post-randomisation, women in the intervention group attended one two-hour group education session with the research dietitian, in groups of two to six participants. They received low-GI dietary advice and were encouraged to choose low-GI alternatives to high-GI carbohydrate foods. They were also advised on general healthy eating recommendations for pregnancy. Information leaflets were provided to participants that included low-GI recipes, lists of low-GI foods, fact sheets and tips. The research dietitian met with the intervention participants at 28 and 34 weeks’ gestation for brief reinforcement of the low-GI diet and to answer any questions about the diet.
Anthropometry
At the first antenatal consultation (randomisation), participants were weighed in light clothing using a SECA weighing scale (SECA GmbH & Co. KG, Germany) to the nearest 0·1 kg and height was measured without shoes to the nearest 0·1 cm using a wall-mounted stadiometer. BMI (kg/m2) was calculated. Mid-upper arm circumference was measured at the first antenatal consultation using a SECA non-stretch measuring tape.
Gestational weight gain
Maternal weight was recorded and gestational weight gain calculated at multiple time points throughout pregnancy: 24, 28, 34, 38, 40 and 41 weeks’ gestation. A final weight measurement at 38, 40 or 41 weeks’ gestation was available for 343 participants. The measurement taken at the latest gestation was considered the final weight. The last weight measurement was taken at 34 weeks’ gestation for an additional 178 participants. For these women, weight at 38 weeks’ gestation was imputed (see methods for multiple imputation below). A further 104 participants did not have a weight measurement at 34, 38, 40 or 41 weeks’ gestation. These women were excluded from the analysis. The maternal characteristics of the women included and excluded from the gestational weight gain analysis are included in the online supplementary material, Supplemental Table 1.
Total gestational weight gain was calculated by subtracting the measured weight at the first antenatal visit from the final weight. Gestational weight gain per week was calculated by dividing total weight gained by the number of weeks of gestation (measured final weight) or by 38 (imputed final weight). The 2009 Institute of Medicine (IOM) guidelines for total gestational weight gain( 26 ) and BMI at the first antenatal visit were used to categorise gestational weight gain as inadequate, adequate or excessive. For analysis, we dichotomised gestational weight gain as ‘exceeded IOM guidelines’ and ‘did not exceed IOM guidelines’.
Nutrient intakes
Food and beverages consumed over three consecutive days were recorded during the first trimester (prior to intervention education session) and during both the second and third trimesters. Dietary data from food diaries were entered into the dietary analysis software NetWISP version 3.0 (Tinuviel Software, Llanfechell, UK). Change in nutrient intake from pre- and post-intervention was calculated by subtracting the baseline nutrient intake from the mean of the second- and third-trimester intakes.
Lifestyle characteristics
Emotional well-being was examined using the WHO-5 Wellbeing Index( 27 ). The questionnaire contains five statements that describe positive moods. Each statement is rated on 6-point Likert scales, based on mood during the previous two weeks. Responses were standardised to a percentage score, with 100 % indicating maximal well-being( Reference Topp, Østergaard and Søndergaard 28 ). Smoking status, age and gestational age at the first visit were also recorded.
Socio-economic status
Educational attainment was self-reported. Participants selected one of six categories: complete third level (higher-level degree); some third level (certificate/diploma); complete secondary level; some secondary level; primary education only; or no education. The Pobal Haase & Pratschke Deprivation Index (HP Index) was used to assign deprivation indices at street level, or small area( Reference Haase and Pratschke 29 ). The HP Index is based on data from the 2011 Census of Population in Ireland, with information on three measurements of affluence/disadvantage incorporated: demographic profile; social class composition; and labour market situation (Fig. 1). The HP Index is normally distributed, and the mean is set as 0. Therefore, it can be determined if a small area is disadvantaged (negative) or advantaged (positive). HP Index scores can be categorised from ‘extreme affluence’ to ‘extreme deprivation’. The participant’s street address was entered into the online interactive mapping tool (available at http://maps.pobal.ie/#/Map) and the score was recorded. Due to small numbers in the extremely affluent group (n 2), these women were categorised as ‘very affluent’.

Fig. 1 Basic model of the Pobal Haase & Pratschke Deprivation Index (HP Index)( Reference Haase and Pratschke 29 )
We created a deprivation–education variable that categorised participants into one of four groups based on advantaged or disadvantaged neighbourhood deprivation and education level above or below third level (university). Categories were as follows: ‘disadvantaged and <3rd level’; ‘disadvantaged and ≥3rd level’; ‘advantaged and <3rd level’; and ‘advantaged and ≥3rd level’.
Compliance and acceptability
At 34 weeks’ gestation, participants in the intervention group completed a questionnaire to access compliance and acceptability of the low-GI dietary intervention. Participants indicated level of compliance with the intervention on a 5-point Likert scale. The acceptability questionnaire consisted of six questions, also 5-point Likert scales, exploring whether the participant found the dietary intervention easy to follow, enjoyable, economical, provided her with enough energy and variety, and if her family were satisfied with changes.
Multiple imputation
We used multiple imputation to derive missing values for 38-week weight, using the weights at booking, weeks 34, 40 and 41, as well as smoking status and age. Five imputations were performed, convergence verified and the imputed values were reasonably close to observed values. Calculation of the weight gain was then performed on the raw imputed values as described above, and pooled analyses performed over all imputed data sets.
Statistical analysis
Data were assessed for normality using the Kolmogorov–Smirnov test and visual inspection of histograms. One-way ANOVA with post hoc Tukey’s tests were used to explore maternal characteristics across deprivation and education categories. Independent-sample t tests explored differences between maternal characteristics in the intervention and control groups, within deprivation and education categories. Excess gestational weight gain was analysed using binary logistic regression. Changes in nutrient intake from pre-intervention to post-intervention were analysed using independent-sample t tests. Compliance and acceptability of the intervention were analysed using one-way ANOVA with post hoc Tukey’s test and χ 2 tests. Well-being was compared using independent-sample t tests. All statistical analyses were performed using the statistical software package IBM SPSS Statistics for Windows, version 20.0. Results were considered statistically significant if P<0·05.
Results
Maternal characteristics
The demographic details for the total study population (n 625) are displayed in Table 1. Mean early-pregnancy BMI was 26·6 kg/m2. Three (0·5 %) participants were underweight, 270 (43·2 %) were normal weight, 238 (38·1 %) were classified as overweight and 114 (18·2 %) were obese. Half of the population (n 299, 47·8 %) received the low-GI dietary intervention. The comparison of maternal characteristics across deprivation–education categories is displayed in Table 2. Women in the ‘disadvantaged and <3rd level’ category were significantly younger and had the lowest WHO-5 Wellbeing scores compared with women in other deprivation–education categories. This group of women also had higher early-pregnancy BMI and mid-upper arm circumference than those in the ‘advantaged and ≥3rd level’ category. There were few differences in maternal characteristics between the intervention and control groups across each of the deprivation–education categories (see online supplementary material, Supplemental Table 2). WHO-5 Wellbeing score was higher in the intervention participants of the ‘disadvantaged and ≥3rd level’ and ‘advantaged and <3rd level’ categories.
Table 1 Maternal characteristics of women (N 625) recruited to the ROLO randomised control trial, Dublin, Ireland, 2007–2011

ROLO, Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia; MUAC, mid-upper arm circumference; GWG; gestational weight gain; HP Index, Pobal Haase & Pratschke Deprivation Index.
Table 2 Maternal characteristics according to deprivation–education category of women (N 625) recruited to the ROLO randomised control trial, Dublin, Ireland, 2007–2011

ROLO, Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia; MUAC, mid-upper arm circumference; GWG; gestational weight gain.
a,b,c,dMean value was significantly different from that in the deprivation–education category denoted by the letter.
*P value is for one-way ANOVA with post hoc Tukey’s test.
Excess gestational weight gain
Data on deprivation–education category and excess gestational weight gain were available for 521 participants. Within the ‘disadvantaged and ≥3rd level’ and ‘advantaged and ≥3rd level’ categories, those in the intervention group were significantly less likely to gain excess weight compared with those in the control group (Table 3). There was no significant difference in excess gestational weight gain between participants in the intervention and control groups in the ‘disadvantaged and <3rd level’ and ‘advantaged and <3rd level’ categories. Furthermore, total weight gain and weight gain per week were significantly lower among women in the intervention group compared with the control group in the ‘disadvantaged and ≥3rd level’ and ‘advantaged and ≥3rd level’ categories (see online supplementary material, Supplemental Table 2).
Table 3 Intervention and risk of excessive gestational weight gain according to deprivation–education category of women (N 521) recruited to the ROLO randomised control trial, Dublin, Ireland, 2007–2011
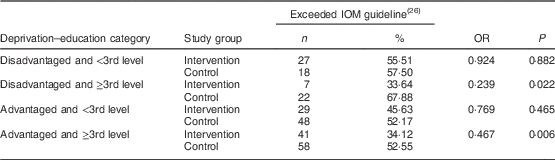
ROLO, Randomised cOntrol trial of a LOw glycaemic index diet in pregnancy to prevent macrosomia; IOM, Institute of Medicine.
OR derived from binary logistic regression.
Women excluded from the gestational weight gain analysis (n 104) had significantly lower weight, BMI and mid-upper arm circumference at the randomisation visit, but their neighbourhood deprivation score was not significantly different from that of the women included (see online supplementary material, Supplemental Table 1).
Nutrient intakes
The mean changes in nutrient intakes from pre-intervention to post-intervention are shown in Table 4. The intervention reduced GI significantly in both the ‘advantaged and ≥3rd level’ and ‘disadvantaged and ≥3rd level’ categories. The reduction in GI in the intervention groups of the other deprivation–education categories did not reach statistical significance. Additionally, the intervention significantly reduced glycaemic load, total carbohydrate and total sugars within the ‘advantaged and ≥3rd level’ category. It also reduced glycaemic load in the ‘advantaged and <3rd level’ category. The intervention did not significantly change any of the nutrient intakes from pre-intervention to post-intervention in the ‘disadvantaged and <3rd level’ category.
Table 4 Change in nutrient intake from pre-intervention to post-intervention according to deprivation–education category of women (N 541) recruited to the ROLO randomised control trial, Dublin, Ireland, 2007–2011

*P value is for independent-sample t test between intervention and control.
WHO-5 Wellbeing score
Compared with participants in the control arm, the WHO-5 Wellbeing score was significantly lower in the intervention arm within the ‘disadvantaged and ≥3rd level’ category (intervention: 56·7 (sd 15·3) v. control: 64·8 (sd 13·3), P=0·028) and ‘advantaged and <3rd level’ category (intervention: 54·8 (sd 16·0) v. control: 60·6 (sd 14·1), P=0·010). There was no difference between the intervention and control arms of the study within the ‘disadvantaged and <3rd level’ (intervention: 50·6 (sd 18·2) v. control: 55·5 (sd 13·9), P=0·148) or ‘advantaged and ≥3rd level’ (intervention: 58·8 (sd 13·3) v. control: 59·5 (sd 16·3), P=0·692) category.
Compliance and acceptability
Of the 183 participants in the intervention arm who completed the compliance and acceptability questionnaire, 80·3 % reported following the prescribed diet always or most of the time. There was no significant difference in compliance between the categories of deprivation and education score; ‘always’ or ‘mostly’ was reported by 79·3 % of ‘disadvantaged and <3rd level’; 89·5 % of ‘disadvantaged and ≥3rd level’; 74·1 % of ‘advantaged and <3rd level’; and 82·7 % of ‘advantaged and ≥3rd level’ (P=0·761). Most participants (68·8 %) found the recommended diet easy to follow, with no differences observed between SES groups (P=0·183). Within the total group, 65·6 % of participants reported that they enjoyed the dietary changes made, which was similar between the categories of deprivation and education score (P=0·666). The majority, 81·8 %, agreed that the changes they made did not increase their weekly grocery bill, with no differences observed between SES groups (P=0·673). Seventy-nine per cent felt they had enough energy while on the diet, which was similar across the groups (P=0·191). Among the total group, 83·1 % agreed that they enjoyed a wide variety of foods in their eating plan, with no differences observed between SES groups (P=0·144). Overall, 71·4 % agreed that their family was happy with the changes made to their diet. This was significantly lower in the ‘disadvantaged and ≥3rd level’ category, within which 40·0 % agreed that their family was happy (P=0·040).
Discussion
The main finding from the present study is that an intervention which consisted of a single dietary group education session, with brief reinforcement, significantly reduced excess gestational weight gain and improved dietary GI only among women with third level education, regardless of neighbourhood deprivation. Neither gestational weight gain nor GI differed between the intervention and control groups among women who had not attended third level education, residing in both advantaged and disadvantaged neighbourhoods. Since the dietary education session was not effective among less educated women, it would seem prudent that tailored approaches are required to increase the effectiveness of interventions among women of lower educational attainment.
Huynh et al. examined both education and neighbourhood disadvantage in relation to excess gestational weight gain. High educational attainment was protective against excess gestational weight gain and residing in a disadvantaged neighbourhood increased the risk( Reference Huynh, Borrell and Chambers 30 ). Unlike our findings, women with a high level of education were at an increased risk of excess weight gain if they resided in a disadvantaged neighbourhood, suggesting that the protective effect of high maternal education was attenuated by the negative social environments( Reference Huynh, Borrell and Chambers 30 ). Many studies have reported that low educational attainment is associated with excess gestational weight gain( Reference Kraschnewski, Chuang and Downs 31 – Reference Al Mamun, Mannan and O’Callaghan 33 ), but this is not conclusive( Reference Sridhar, Darbinian and Ehrlich 34 – Reference Guilloty, Soto and Anzalota 36 ). A large, multicentre cohort study found no association between socio-economic index and excess gestational weight gain( Reference Restall, Taylor and Thompson 37 ). The UPBEAT randomised control trial consisted of a low-GI diet among obese pregnant women residing in areas of high socio-economic deprivation in the UK( Reference Poston, Bell and Croker 38 ). The intervention was successful in reducing total gestational weight gain among these women of low SES( Reference Poston, Bell and Croker 38 ). Although we did not find a reduction in excess gestational weight gain in the most disadvantaged group, the UPBEAT study had a more intense intervention than the ROLO study. Multiple support tools and opportunities to meet health trainers in an individual and group context were provided, which may have facilitated behaviour change. Excess gestational weight gain in pregnancy predicts weight retention at 1 year postpartum, and this predisposes women to a BMI in the overweight category 15 years post-pregnancy( Reference Linné, Dye and Barkeling 39 ). This is particularly pertinent for low-SES women, as we found that women in the most disadvantaged group began pregnancy with the highest BMI and MUAC.
In terms of dietary changes related to the ROLO low-GI intervention, the most disadvantaged women did not change their diet from pre- to post-intervention, but the most advantaged women significantly reduced GI, glycaemic load, total carbohydrate and total sugars. A single education session with brief reinforcement appeared to be insufficient to change dietary behaviour among the most disadvantaged women. The multifaceted approach of the UPBEAT trial significantly reduced GI and glycaemic load among a mostly disadvantaged population( Reference Poston, Bell and Croker 38 ). The LIMIT trial in overweight and obese pregnant women found that a healthy eating intervention improved diet quality, but not GI or glycaemic load, although analyses were not stratified by SES and thus cannot be fully compared with our findings( Reference Dodd, Cramp and Sui 40 ). Identifying successful interventions for dietary change among those of low SES is important, as these women already have poorer dietary habits( Reference McLeod, Campbell and Hesketh 1 – Reference Rifas-Shiman, Rich-Edwards and Kleinman 3 ) and nutritional knowledge compared with their high-SES counterparts( Reference McLeod, Campbell and Hesketh 1 ).
Barriers to effective intervention response
Many women of low SES experience barriers that prevent them from making dietary changes, including lower income, limited food budget, lack of transportation to larger supermarkets, poor cooking skills and family preferences( Reference Wiig and Smith 13 , Reference Siu, Giskes and Turrell 41 ). These women have stronger beliefs in the influence of chance on health( Reference Baron, Manniën and te Velde 4 ), thus they may not prioritise healthy eating. Other barriers include following cravings and difficulty managing time, particularly if the woman has children at home( Reference Heery, McConnon and Kelleher 42) . The ROLO dietary intervention seemed to be equally acceptable among the four deprivation–education categories. All groups similarly reported that the intervention was easy to follow, did not increase their weekly grocery bill and was enjoyable, suggesting that, for the majority, the intervention did not increase barriers relating to finances and food preferences. Participants in the ‘disadvantaged and <3rd level’ category did not report any problems with the intervention compared with their peers; however, since this was the group least likely to make the prescribed changes to their diet, it may be hypothesised that these women did not understand the intervention and did not know what changes were required. Thus, due to lack of changes made in their diet, it is possible that they did not find the intervention difficult. We found that only 40 % of those in the ‘disadvantaged and ≥3rd level’ category reported that their families were happy with the changes made to their diet, compared with the average of 71 % across all groups. This could represent a lack of family support.
Those living in disadvantaged areas are likely to experience greater anxiety and depression( Reference Sooman and Macintyre 12 ), and low-income women with increased stress are more likely to consume a poor diet( Reference Fowles, Stang and Bryant 43 ). In addition, worry about weight gain during pregnancy is positively associated with total gestational weight gain( Reference Heery, Wall and Kelleher 44 ). We found that women in the ‘disadvantaged and <3rd level’ category had the lowest WHO-5 Wellbeing score, which may have been a barrier in making dietary changes. Additionally, the intervention seemed to have a negative impact on well-being score within the ‘disadvantaged and ≥3rd level’ and ‘advantaged and <3rd level’ categories. These women found the dietary changes most stressful to make. Those in the ‘disadvantaged and ≥3rd level’ category may have understood the changes required, but did not have the financial, peer or family support required to easily modify their lifestyle.
Tools to facilitate response to interventions
The principles of health promotion, as set out in the Ottawa Charter for Health Promotion, are building healthy public policy, creating a supportive environment, strengthening community action, developing personal skills and reorienting health services( 45 ). In order for individuals to achieve their greatest health potential, many factors are required, including life skills, information and opportunities to make healthy choices( 45 ). Although engaging with low-SES women may be difficult, as they are less likely to attend antenatal classes( Reference Baron, Manniën and te Velde 4 ), it should be a public health priority to provide these women with the relevant tools and information they require to overcome barriers and make healthy choices in a facilitating environment. As researchers, we need to design interventions better to serve the needs of low-SES women.
This raises the question: are different interventions required to target the same outcomes among populations of varying SES? As demonstrated by our findings, the provision of information through an education session is effective only among those of higher educational attainment. Practical tools to enhance life skills, such as growing, cooking and nutrition courses, may help disadvantaged populations to make the healthy choice the easiest choice. Cooking skill interventions among low-SES groups improve confidence in cooking and fruit and vegetable consumption( Reference Garcia, Reardon and McDonald 46 ), while those involved in community gardening projects also have improved fruit and vegetable intakes( Reference Litt, Soobader and Turbin 47 ). Behaviour change techniques, such as barrier identification, goal setting and self-monitoring, may also have some benefit in improving GI, glycaemic load, carbohydrate (percentage of energy) and fibre in pregnant women of low SES( Reference Poston, Bell and Croker 38 ).
Patient and public involvement in intervention design
To gain greater understanding of the initiatives and tools that are acceptable to low-SES groups, researchers should seek input from these individuals. Patient and public involvement (PPI) is a key component of research and should be utilised particularly in research relating to pregnant women of low-SES groups, who are less likely to engage in health services( Reference Docherty, Bugge and Watterson 48 ). It encourages research to be carried out with the involvement of society members, rather than for them( 49 ), and strengthens the potential of research to effectively influence practice( Reference Moss, Daru and Lanz 50 ). Participants involved in PPI feel empowered, valued, and develop life skills and knowledge about their condition( Reference Brett, Staniszewska and Mockford 51 ). A positive experience with PPI has also been shown to motivate participants to continue involvement in research( Reference Brett, Staniszewska and Mockford 51 ).
Equality in intervention design
Researchers and policy makers have a responsibility to create and monitor interventions that provide equal benefit for all members of society, regardless of socio-economic background. Intervention-generated inequalities occur when an intervention is of greater benefit to advantaged (lower-risk) groups than to disadvantaged (higher-risk) groups( Reference Lorenc, Petticrew and Welch 52 ). The ROLO study improved excess gestational weight gain and dietary GI among highly educated women, but not among those with less than third level education. It has been suggested that downstream interventions (which target individual-level behaviours), such as education and media campaigns, increase intervention-generated inequalities( Reference Brownson, Seiler and Eyler 20 , Reference Lorenc, Petticrew and Welch 52 ), while upstream interventions (policy approaches), through regulation, increased access or economic incentives, reduce intervention-generated inequalities( Reference Brownson, Seiler and Eyler 20 , Reference Lorenc, Petticrew and Welch 52 ). The ROLO study may not have been successful in reaching the most disadvantaged individuals due to its nature as a downstream intervention that provided one education session, at an individual level. Food subsidy programmes are an example of upstream interventions, such as the Healthy Start programme in the UK, the Special Supplemental Nutrition Programme for Women, Infants, and Children in the USA and the OLO (œufs, lait, orange – eggs, milk, orange) food and supplementation programme in Quebec( Reference Hamza, Colin and O’Brien 53 ). Food vouchers can increase fruit, vegetable and whole grains consumption( Reference McFadden, Green and Williams 14 , Reference Schultz, Byker Shanks and Houghtaling 54 ). Home-visiting interventions within disadvantaged communities, such as the Nurse–Family Partnership Program in the USA( Reference Olds 55 ) or Preparing for Life in Ireland( Reference Doyle, McGlanaghy and Palamaro-Munsell 56 ), take a broader view of addressing the health and social needs of low-SES pregnant women. Home visits provide education and support during pregnancy, and have been found to improve pregnancy outcomes( Reference Olds 55 , Reference Doyle, McGlanaghy and Palamaro-Munsell 56 ). They also have a positive effect on diet quality, smoking and seeking support services( Reference Olds 55 ).
Within the ROLO study, pregnant women of lower SES and educational attainment may have benefited from multiple, practical downstream approaches to increase knowledge and skills, in addition to upstream, supportive services and economic incentives that would facilitate behaviour change. Figure 2 provides examples of upstream and downstream interventions that could work in tandem to improve gestational weight gain and diet in pregnancy.

Fig. 2 Potential upstream and downstream interventions to improve excess gestational weight gain and dietary changes in pregnancy (SES, socio-economic status; GWG, gestational weight gain)
Recruitment, analysis and dissemination of interventions
There is a requirement for researchers to include more disadvantaged women in trials and to stratify analyses by socio-economic groups to allow readers, health-care professionals and policy makers to fully understand the interventions that are most likely to benefit those of lower SES( Reference Callander and McDermott 17 ). It is somewhat difficult to compare our findings with the majority of dietary clinical trials and observational studies among pregnant women, as several studies enrol a greater proportion of high-SES participants and fail to stratify their results by SES. This is not unique to pregnancy research; only 5 and 12 % of the literature on CVD and stroke interventions, respectively, report on intervention effectiveness between socio-economic groups( Reference Callander and McDermott 17 , Reference Magin, Victoire and Zhen 18 ). Furthermore, less than 10 % of randomised controlled trials published in international medical journals report demographic characteristics relating to SES of their participants( Reference Furler, Magin and Pirotta 19 ).
Study strengths and limitations
The present study has several strengths worthy of consideration. The study design allowed for the measurement of two SES variables: maternal educational attainment and neighbourhood deprivation (HP Index). Combining the two variables to create a deprivation–education variable allowed for categorisation of the most advantaged and disadvantaged groups of individuals within the study population. Additionally, the HP Index is a novel method of determining SES and is unique and specific to the population of Ireland. The study is not without limitations. Nutrient intake data were collected using self-reported food diaries, thus the possibility of error due to memory or social desirability bias must be considered. For those who did not have a final weight recorded, the 38-week gestation weight was imputed. For analysis, the variable ‘did not exceed IOM guidelines’ included both adequate and inadequate weight gain. Of the 270 participants who did not exceed the IOM guidelines, eighty-six participants gained inadequately. The number of participants in the disadvantaged groups were small, thus the power to detect significant differences between groups may have been reduced. Furthermore, due to multiple testing, some findings may represent false positives and require replication in future studies. If we applied the corrected P value of 0·00135 (due to thirty-seven tests), the only finding to survive correction would be change in glycaemic load from pre-intervention to post-intervention among the participants in the intervention arm within the ‘advantaged and ≥3rd level’ category. Lastly, we acknowledge that data on income or occupation would have provided a more complete set of SES variables, but these were not collected.
Conclusions
Women with third level education, regardless of the neighbourhood in which they lived, were most receptive to the ROLO low-GI dietary intervention. A single, group education session, with brief reinforcement, was not effective in reducing GI and excess gestational weight gain among less educated women. Based on our findings, we recommend the following: (i) researchers should engage in PPI, prior to and during study design, to gain an understanding of participant needs, personal skills required and barriers that prevent lifestyle changes; (ii) create multifaceted, appropriate and practical interventions that combine upstream and downstream approaches, and will engage with and benefit individuals of low SES; (iii) include multiple measures of SES during data collection; and (iv) stratify data analysis by SES to understand the effect of interventions on various population groups. Research is required to drive evidence-based practice, but there remains a paucity of high-quality data on the population of low-SES pregnant women. Thus, there is a requirement for researchers to rethink current strategies and carefully plan meaningful and successful interventions to ultimately reduce the gap in health inequalities.
Acknowledgements
Acknowledgements: The authors would like to thank the mothers who participated in the study. Financial support: This work was supported by the Health Research Board, Health Research Centre for Health and Diet Research, Ireland. The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: F.M.Mc.A. designed the intervention; E.C.O.B. analysed data and wrote the manuscript; all authors read and approved the final manuscript; F.M.Mc.A. had primary responsibility for the final content. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee at The National Maternity Hospital, Dublin, Ireland. Written informed consent was obtained from all subjects.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980017001951











