Introduction
The Asian tiger mosquito, Aedes albopictus (Skuse, 1894) (Diptera: Culicidae), is one of the 100 most invasive species on Earth (Lowe et al., Reference Lowe, Browne, Boudjelas and De Poorter2000). Indigenous to Southeast Asia, it has undergone an impressive expansion of its native range in the last few decades. In fact, it is currently present in large areas of every inhabited continent except Antarctica, in both tropical and temperate environments (Kraemer et al., Reference Kraemer, Sinka, Duda, Mylne, Shearer, Barker, Moore, Carvalho, Coelho, Van Bortel, Hendrickx, Schaffner, Elyazar, Teng, Brady, Messina, Pigott, Scott, Smith, Wint, Golding and Hay2015).
The first record of this mosquito outside its place of origin was registered in Europe, specifically in Albania in 1979 (Adhami and Reiter, Reference Adhami and Reiter1998), although there were no reports in any other European country until 1990, when it was identified in Italy (Sabatini et al., Reference Sabatini, Raineri, Trovato and Coluzzi1990). This species is mainly spread passively by ground, aircraft and maritime traffic (Ibáñez-Justicia, Reference Ibáñez-Justicia2020), highlighting pathways such as the trade of used tires or lucky bamboo plant cuttings in Europe (Scholte et al., Reference Scholte, Den Hartog, Dik, Schoelitsz, Brooks, Schaffner, Foussadier, Braks and Beeuwkes2010; Demeulemeester et al., Reference Demeulemeester, Deblauwe, De Witte, Jansen, Hendy and Madder2014), where the species is currently established in at least 26 countries (ECDC, 2022).
Aedes albopictus causes an important nuisance given its biting behaviour, provoking discomfort in people when found in large numbers (Curcó et al., Reference Curcó, Giménez, Serra, Ripoll, García and Vives2008), limiting outdoor activities. Bites can cause serious allergic reactions in sensitive individuals, especially in newly infested areas, which could also be considered an early warning of the introduction of this dipteran (Abramides et al., Reference Abramides, Roiz, Guitart, Quintana, Guerrero and Giménez2011). However, the major concern regarding this species is its capacity to transmit mosquito-borne diseases of public health importance (Näslund et al., Reference Näslund, Ahlm, Islam, Evander, Bucht and Lwande2021). In fact, the establishment of this vector in Euro-Mediterranean countries has led to autochthonous transmission events of several arboviruses; chikungunya cases were first reported in 2007 in Italy (Rezza et al., Reference Rezza, Nicoletti, Angelini, Romi, Finarelli, Panning, Cordioli, Fortuna, Boros, Magurano, Silvi, Angelini, Dottori, Ciufolini, Majori and Cassone2007), dengue in 2010 in France (La Ruche et al., Reference La Ruche, Souarès, Armengaud, Peloux-Petiot, Delaunay, Desprès, Lenglet, Jourdain, Leparc-Goffart, Charlet, Ollier, Mantey, Mollet, Fournier, Torrents, Leitmeyer, Hilairet, Zeller, Van Bortel, Dejour-Salamanca, Grandadam and Gastellu-Etchegorry2010) and Zika in 2019, also in France (Giron et al., Reference Giron, Franke, Decoppet, Cadiou, Travaglini, Thirion, Durand, Jeannin, L'Ambert, Grard, Noël, Fournet, Auzet-Caillaud, Zandotti, Aboukaïs, Chaud, Guedj, Hamouda, Naudot, Ovize, Lazarus, de Valk, Paty and Leparc-Goffart2019). Further cases of arboviruses linked to this species were also reported from other European countries such as Croatia or Spain (Gjenero-Margan et al., Reference Gjenero-Margan, Aleraj, Krajcar, Lesnikar, Klobučar, Pem-Novosel, Kurečić-Filipović, Komparak, Martić, Duričić, Betica-Radić, Okmadžić, Vilibić-Čavlek, Babić-Erceg, Turković, Avsić-Županc, Radić, Ljubić, Sarac, Benić and Mlinarić-Galinović2011; MSCBS, Reference MSCBS2018). Recently, two autochthonous confirmed dengue cases were reported in the Balearic Islands, Spain (Campelli et al., Reference Campelli, Expósito, Reyes, Cano, Moros and Simón2023) and multiple transmission events in Lazio Region, Italy (De Carli et al., Reference De Carli, Carletti, Spaziante, Gruber, Rueca, Spezia, Vantaggio, Barca, De Liberato, Romiti, Scicluna, Vaglio, Feccia, Di Rosa, Gianzi, Giambi, Scognamiglio, Nicastri, Girardi, Maggi and Vairo2023).
The skip-oviposition pattern of synanthropic Aedes implies egg dispersion in multiple and cryptic sites (Reinbold-Wasson and Reiskind, Reference Reinbold-Wasson and Reiskind2021), often small human-made reservoirs largely present in urban private areas (Stefopoulou et al., Reference Stefopoulou, Balatsos, Petraki, LaDeau, Papachristos and Michaelakis2018). In this sense, there is very limited information concerning larval habitat identification of Ae. albopictus in Europe (Baldacchino et al., Reference Baldacchino, Bussola, Arnoldi, Marcantonio, Montarsi, Capelli, Rosà and Rizzoli2016). Better knowledge of the specific domestic breeding habitats occupied by this species in each region would provide important epidemiological and anthropological information, with direct implications for health education, environmental awareness and vector control (Alarcón-Elbal et al., Reference Alarcón-Elbal, Rodríguez-Sosa, Ruiz-Matuk, Tapia, Arredondo Abreu, Fernández González, Rodríguez Lauzurique and Paulino-Ramírez2021, Reference Alarcón-Elbal, López-de-Felipe, Gil, García, Mateo and Bueno2024). The use of entomological indicators such as the house index (HI), container index (CI), pupal index (PI) or Breteau index (BI) could be very useful for the proper monitoring of breeding sites of synanthropic aedine species (Reiter and Gubler, Reference Reiter, Gubler, Gubler and Kuno1997; Focks, Reference Focks2003), being Aedes aegypti (Linnaeus, 1762) particularly noteworthy among them. However, these tools have been scarcely used in Europe, although they are widely utilised in many countries of Central and South America, along with the Caribbean, where their employment is considered essential for the development of vector control programmes (Ministério da Sáude, 2009; César et al., Reference César, Fiestas, García-Mendóza, Palomino, Mamani and Donaires2015; Bardach et al., Reference Bardach, García-Perdomo, Alcaraz, Tapia, Ruano, Ruvinsky and Ciapponi2019).
In this sense, the project ‘New strategies for the control of the tiger mosquito in residential areas’, also known as NESCOTIGER, was conducted in 2022 in Valencia, Spain, and aimed to explore various mosquito control strategies in both public and private settings. Within the scope of this research project, the aim of the current study was to identify and characterise the domestic breeding sites of Ae. albopictus in a recently invaded region of the Spanish Mediterranean. Ultimately, enhancing our understanding of this invasive mosquito species is crucial for the development of effective prevention and control measures.
Material and methods
Study area
The study was conducted in El Vedat (39°25′25″N 0°29′35″W), a residential area situated on the small mountain of the same name in Torrent, in the province of Valencia (Valencian Community, Eastern Spain). It is considered the municipality with the second highest population index of the province, with around 85,000 registered inhabitants in 2022, and it is located 9 km from the city of Valencia, the capital of the autonomous community and the third largest city in Spain. The area is characterised by a high economic level, with a mean annual homestead income ranging from 43,000€ to 61,000€ (x̄ = 54,058€), Spain's average being 27,000€ to 32,000€ for the year 2020. Over 16–26% of the population is 65 years or older (INE, 2023). This large residential area sits 142 masl, on the edge of the Sierra Perenxisa, and it covers a surface area of 5.86 km2, over 40.5% of which comprise urban green spaces, woodlands, pine trees and agriculture fields (mainly fruit trees, especially olives and orange trees). This high-income area had public water services and was characterised by big-size single-family homes (parcel size: x̄ = 642 m2; x~ = 591 m2; Mo = 591 m2; min = 124 m2; max = 2133 m2; SD = 390 m2), each usually having a private swimming pool and garden areas.
Geographic sectorisation and control interventions
In the NESCOTIGER project, a prior analysis of the study area was conducted, leading to the selection of six study sectors (0–5), each of which had simultaneously implemented a different Aedes control strategy (table 1). Such analysis was performed with the spatial analysis software QGIS, and through the employment of socioenvironmental variables such as type of housing, vegetation and water-catching systems in the area (unpublished data). Residents in the area were invited to participate in the project, and were provided with the control tools for its deployment in their private gardens.
Table 1. Description of implemented interventions in El Vedat de Torrent during the NESCOTIGER project classified by study sectors (0–5)

Grey markings represent the sector in which each intervention was implemented.
Collection, processing and identification
A house-to-house cross-sectional entomological survey was carried out to detect larval breeding sites in outdoor areas (gardens, yards, terraces, etc.) of households in El Vedat. Houses were randomly selected among the voluntary participants in the NESCOTIGER project. For sampling, a team composed of the same two researchers visited each evaluated household, informed the residents of the purpose and procedures of the study and obtained informed consent from the head of the household.
The field survey was conducted in the period from July to August 2022. These weeks are the hottest and driest of the year, allowing for a more accurate identification of larval habitats strictly related to human activities. A mean temperature of 28.1°C (min. 19.6°C; max. 40.7°C) and an accumulated precipitation of 26.4 mm between the two studied months was recorded in the closest meteorological station during the study period (meteorological station 8414A ‘Valencia, Aeropuerto’, 6.2 km away from the study area) (AEMET, 2024). However, September is typically a rainy month in the Spanish Levante, leading to the formation of breeding sites also associated with water accumulation from rainfall.
In each house, every real and potential breeding site was recorded and evaluated. For each site, the container type, material (plastic, metal, ceramic, etc.), presence/absence of water, water-holding potential maximum capacity (ml) and presence of either mosquito's larvae or pupae were registered. For each mosquito-positive breeding site, all individuals were retrieved (when possible) with a plastic Pasteur pipette into plastic containers. For those reservoirs with high amounts of water, a fraction of the total was retrieved, and the total larvae and pupae density were calculated based on the estimated content of the container. Each household inspection lasted between 15 and 45 min, based on the number and size of reservoirs inspected.
In the laboratory, preimaginal stages were introduced alive into mosquito breeding containers (Bioquip Products, Rancho Domínguez, CA, USA) filled with the original breeding water. Larvae were reared under laboratory conditions until the 3rd/4th instar, afterwhich, individuals were euthanised by submersion in hot water (60°C) for 1 min. Subsequently, they were preserved in labelled vials filled with 70% ethanol until identification. The collected pupae were allowed to emerge into adults for taxonomic identification after being killed by placing them in a freezer (−20°C) for 30 min. Both immature and adult mosquitoes were identified using the e-taxonomy key of MoskeyTool (MediLabSecure, 2023).
Data analysis
Data analysis was performed with the statistics programs RStudio (R version 4.1.2) and Microsoft Excel. Based on the gathered data, the HI (n houses infested with larvae and pupae/n houses inspected × 100), CI (n containers infested with larvae and pupae/n containers inspected × 100), BI (n containers infested with larvae and pupae/n houses inspected × 100) and PI (n pupae/n inspected houses × 100) were calculated for El Vedat as well as for each independent studied sector. A χ2 test comparing the BI among sectors was performed, employing sector 5 as the control. A Kruskal–Wallis test was developed to evaluate the effect of material type over the larval abundance, employing the ‘kruskal.test()’ function from base R. Finally, a linear model was carried out, employing the ‘lm()’ function from base R to evaluate the effect of total water in containers over Ae. albopictus larvae abundance. Statistical differences were accepted for p < 0.05.
Results
A total of 60 households (10 houses per sector) were surveyed for the presence of immature mosquitoes in El Vedat (table 2). A total of 1444 potential breeding sites were examined, of which 35 (2.4%) harboured immature mosquitoes. A total of 13,125 larvae and 385 pupae of three mosquito species were captured: Ae. albopictus (10,476 larvae and 385 pupae in 33 foci); Culex pipiens (Linnaeus, 1758) (2648 larvae in 2 foci); and Culex modestus (Ficalbi, 1889) (2 larvae in 1 focus). The following results only reflect Ae. albopictus breeding sites for both larvae and pupae (tables 2 and 3).
Table 2. Aedes entomological indices stratified by sector in El Vedat, Valencia, Spain, July–August 2022.
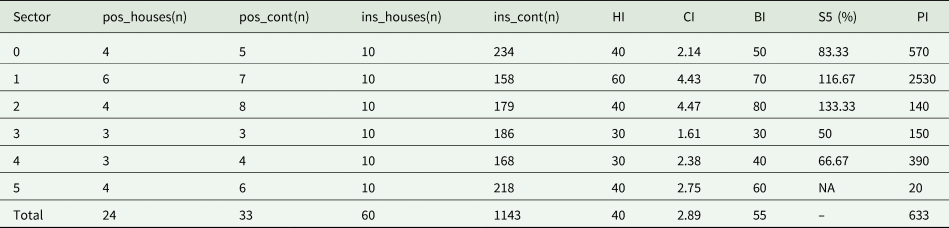
pos_houses (n), no of positive houses; pos_cont (n), no of positive containers; ins_houses (n), no of inspected houses; ins_cont (n), no of inspected containers; HI, house index; CI, container index; BI, Breteau's index; S5(%), percentual difference between BI for each sector and Sector 5; PI, pupal index; NA, not applicable.
Table 3. Aedes albopictus larvae breeding sites identification in El Vedat de Torrent, Valencia, July–August 2022

BS: breeding site, BSw: breeding site with water, PBS: positive breeding sites, H: inspected houses (60), TOT: total preimaginal stages (larvae and pupae), L: total number of larvae, P: total number of pupae.
Aedic indices
The mean HI showed that 40% of the houses in the studied area had presence of Ae. albopictus larvae and that 2.9% of the evaluated containers (CI) were infested by Ae. albopictus. Statistical differences for the BI were recorded among the different sectors (χ2 = 31.81, df = 5, p < 0.001), with a mean value of 55%. The highest BI was found in sectors 1 and 2 (BI = 70 and 80%, respectively) (table 2), both being sites where no larvicide treatment was implemented, in contrast to sectors 3 and 4, where a reduction of the BI compared to sector 5 was seen (50.0 and 66.7%, respectively) (fig. 1). Sectors 1 and 2 recorded higher CI values than that of the control zone (sector 5), while sector 3 accounted for the lowest indices. A total of 380 pupae were identified in 14 breeding sites (average of 27.14 pupae/positive breeding site). A mean PI value of 633 was observed (table 2).

Figure 1. Aedes albopictus and its breeding sites in Torrent, Valencia, July–August 2022. (a) Ae. albopictus larva posterior end; (b) Ae. albopictus imago (dorsal view); (c) water drain; (d) plant pot; (e) water drainage system; (f) construction materials; (g) gutter; (h) ornamentation items (fountain); (i) water depuration system (major mosquito foci, Ae. albopictus larvae n = 7715; Culex pipiens larvae, n = 2600); (j) structural deficiency; (k) bucket; (l) plant pot plate.
Container analysis
Plant pot plates were the most common potential breeding site in the study area, with a mean of 6.8 units/household, followed by flowerpots (n = 2 units/household), construction materials (n = 1.3 units/household), drains (n = 1.2 units/household) and buckets (n = 1 unit/household). While plant pot plates were the most abundant breeding site, only 12.8% contained water, of which only 17% had larvae presence. On the contrary, other less abundant containers were more commonly filled with water, being real potential breeding sites during the study period, such as bromeliad plants (80%), animal drinkers (53.6%) and even empty plant pots (50%) (table 3).
In general, flowerpots and plant pot plates constituted more than half of the real water-holding containers positive for Ae. albopictus larvae. From the potentially infested breeding sites (PBS), among the total evaluated (BS), those with the highest intrinsic larvae positivity rates (PBS/BS) were water irrigation tanks (33.3%), exotic tank bromeliads (20%) and water depuration systems (16.7%). However, when considering only the breeding sites containing water (BSw), the highest rates of larval presence (PBS/BSw) were observed in water irrigation tanks (66.7%), construction tools (carrycot) (66.7%), bromeliads (50%), flowerpots (45.5%) and structural deficiencies (40%). Nevertheless, several identified breeding sites showed larvae presence, even though no pupae were observed. Such containers were bromeliad plants (n larvae = 21; n pupae = 0), buckets (n larvae = 8; n pupae = 0) and structural deficiencies (n larvae = 46; n pupae = 0). Also, only one pupa was found in ornamental items, even though a total of 77 larvae were observed during the study in those container types.
In addition, a single disused swimming pool's water depuration system filled with water presented a great infestation level, being the most productive habitat in the study area (n larvae = 7,715, 73.6%; n pupae = 205, 53.3%). Other highly productive reservoirs were water irrigation tanks (n larvae = 911, 8.8%; n pupae = 39, 10.1%), flowerpots (n larvae= 895, 8.5%; n pupae= 73, 19.0%) and animal drinkers (n larvae= 386, 3.7%; n pupae = 43, 11.2%) (table 3, fig. 1).
Material type and total water effect
No association was found between the material type and the abundance of Ae. albopictus larvae (Kruskal Wallis test; n = 35, df = 6, p-value = 0.72). Based on a descriptive analysis, PVC plastic showed the highest median abundance value, although a single recipient was found positive in this case, with pottery apparently being the most productive breeding site.
Based on data from the positive containers, a linear correlation was observed between total water volume and larvae abundance, with an increase of 1 larvae/11.76 ml (lm; 0.085 ml larvae−1; adjusted R 2 = 0.78, p < 0.0001).
Discussion
To our knowledge, this is the first study concerning aedic indices carried out in Europe, although some researchers have delved into the diversity of breeding sites of the Asian tiger mosquito in countries such as Italy (Baldacchino et al., Reference Baldacchino, Bussola, Arnoldi, Marcantonio, Montarsi, Capelli, Rosà and Rizzoli2016). Nevertheless, this methodology is commonly employed in vector surveillance programmes in endemic areas in Asia, Africa and the Americas (Correia et al., Reference Correia, Isaias, Hailton, Alves and Duarte2015; MSCBS, Reference MSCBS2018; Aryaprema and Xue, Reference Aryaprema and Xue2019; Diéguez Fernández et al., Reference Diéguez Fernández, Borge de Prada, Rodríguez Sosa, Vásquez Bautista and Alarcón-Elbal2021).
Aedes albopictus was first detected in Spain in 2004 (Aranda et al., Reference Aranda, Eritja and Roiz2006), and since then, it has quickly dispersed countrywide, having been reported for the first time in the Valencia province in September 2013 (Alarcón-Elbal et al., Reference Alarcón-Elbal, Delacour-Estrella, Collantes, Delgado, Ruiz-Arrondo, Pinal-Prieto, Melero-Alcíbar, Molina, Sierra, Almela and Lucientes2013; Bueno et al., Reference Bueno, Bernués, Muñoz and Jiménez2013). In July 2015, the species was first detected in the city of Valencia (Bueno and Quero de Lera, Reference Bueno and Quero De Lera2015). In September of that same year, the increase in insect bites and the consequent public alarm led to suspicion that this species might had arrived in the municipality of Torrent, although this was not officially confirmed until 2016 (GVA, 2016). This invasive exotic species causes significant discomfort, a fact confirmed by the vast majority of residents during informal conversations while households were inspected. Nonetheless, the main concern resides in its capacity for disease transmission, as exemplified by its role as a vector in several autochthonous dengue outbreaks in Spain over recent years (ASP, 2013; ECDC, 2017; MSCBS, Reference MSCBS2018; Monge et al., Reference Monge, García-Ortúzar, López Hernández, Lopaz Pérez, Delacour-Estrella, Sánchez-Seco, Fernández Martinez, García San Miguel, García-Fulgueiras and Sierra Moros2020; Campelli et al., Reference Campelli, Expósito, Reyes, Cano, Moros and Simón2023).
Aedic indices
Among the different evaluated indicators, the BI is considered the most noteworthy since it has been employed as an early indicator of adult mosquito density (Parra et al., Reference Parra, Lorenz, Dibo, Gonçalves de Aguiar-Milhim, Monteiro-Guirado, Lacerda-Nogueira and Chiaravalloti-Neto2022) and for dengue transmission risk (Aryaprema and Xue, Reference Aryaprema and Xue2019; Liyanage et al., Reference Liyanage, Tozan, Tissera, Overgaard and Rocklöv2022). However, some authors question the validity of those relations (Bowman et al., Reference Bowman, Runge-Ranzinger and McCall2014; Parra-Amaya et al., Reference Parra-Amaya, Puerta-Yepes, Bejarrano and Arboleda2016), and the World Health Organization (WHO) does not recommend the use of aedic indices as a primary entomological indicator (WHO, 2016). While these indices do not quantify population size or density, this method, proposed a century ago (Connor and Monroe, Reference Connor and Monroe1923), is the only entomological surveillance tool used in most programmes.
According to the Pan American Health Organization's (PAHO) recommendation (PAHO, Reference PAHO1994), an area with endemic disease potential is at a high risk of outbreaks when BI > 5% and CI > 3%. In the case of El Vedat de Torrent, the aedic indices are approaching these criteria (HI = 40%; CI = 2.89%; BI = 55%), even though Eastern Spain is not considered for the time being a high-risk area for the transmission of arboviruses. Nevertheless, it should be taken into consideration that such risk thresholds are established for Ae. aegypti populations, while the present study delves into aedic indices gathered for Ae. albopictus. Similar values for the HI and BI were obtained in surveys conducted in the Congo (HI = 33.3%; CI = 49.6%; BI = 26.6%) (Wilson-Bahun et al., Reference Wilson-Bahun, Kamgang, Lenga and Wondji2020), in Thailand (HI = 39%; CI = 2.5%; BI = 47%) (Preechaporn et al., Reference Preechaporn, Jaroensutasinee and Jaroensutasinee2006) and an extremely high BI in Mexico (>200) (Winch et al., Reference Winch, Barrientos-Sanchez, Puigserver-Castro, Manzano-Cabrera, Lloyd and Mendez-Galván1992), just to mention three examples of tropical countries.
The identification of 33 positive reservoirs for Ae. albopictus (0.55 foci per household) and a mean PI value of 633 pupae per 100 houses (table 3) underscore the significance of residential settings in the proliferation of this mosquito species within urban areas. It is worth noting that this study took place during a major drought period, characterised as the hottest summer recorded in Spain (AEMET, 2022). Consequently, a substantial rise in active foci per household can be anticipated following a rainy period.
Container analysis
Numerous studies have pointed out the preferred breeding sites of synanthropic Aedes, although cultural practices concerning water management have a strong influence over the results. Water gathering and/or collection systems play a key role as mosquito breeding sites, with water tanks, barrels or drums consistently identified as the primary Aedes sources in numerous prior studies (Baldacchino et al., Reference Baldacchino, Bussola, Arnoldi, Marcantonio, Montarsi, Capelli, Rosà and Rizzoli2016; Diéguez et al., Reference Diéguez, Pino, Andrés, Hernández, Alarcón-Elbal and San Martín2016; Morales-Perez et al., Reference Morales-Pérez, Nava-Aguilera, Balanzar-Martínez, Cortés-Guzmán, Gasga-Salinas, Rodríguez-Ramos, Meneses-Rentería, Paredes-Solís, Legorreta-Soberanis, Armendariz-Valle, Ledogar, Cockcroft and Andersson2017; Vannavong et al., Reference Vannavong, Seidu, Stenström, Dada and Overgaard2017; Abilio et al., Reference Abílio, Abudasse, Kampango, Candrinho, Sitoi, Luciano, Tembisse, Sibindy, de Almeida, Garcia, David, Maciel-de-Freitas and Gudo2018; Rodríguez-Sosa et al., Reference Rodríguez Sosa, Rueda, Vásquez Bautista, Fimia-Duarte, Borge de Prada, Guerrero and Alarcón-Elbal2019; Leal et al., Reference Leal, Fernandes Varela, Lopes Gonçalves, Sousa Monteiro, Ramos de Sousa, Lima Mendonça, De Pina, Alves and Osório2020; Alarcón-Elbal et al., Reference Alarcón-Elbal, Rodríguez-Sosa, Ruiz-Matuk, Tapia, Arredondo Abreu, Fernández González, Rodríguez Lauzurique and Paulino-Ramírez2021). The studied area counts with constant public water supply, making water gathering and collection unnecessary and eliminating the risk that breeding sites such as water drums may imply in other epidemiological scenarios. Nevertheless, other big water-holding capacity sites such as water depuration tanks seem to be major Ae. albopictus focus in the study area. In this sense, bigger water-holding capacity containers have been positively correlated with Ae. aegypti egg density (Harrington et al., Reference Harrington, Ponlawat, Edwan, Scott and Vermeylen2008), as was observed for Ae. albopictus larvae in our survey. Other major Aedes breeding site identified in the literature are discarded tires (Abilio et al., Reference Abílio, Abudasse, Kampango, Candrinho, Sitoi, Luciano, Tembisse, Sibindy, de Almeida, Garcia, David, Maciel-de-Freitas and Gudo2018; González et al., Reference González, Rodríguez-Sosa, Vásquez-Bautista, Rosario, Durán-Tiburcio and Alarcón-Elbal2020). Nevertheless, in the researched area, only three dry tires were identified, and they were not perceived as relevant mosquito sources during the studied period. Finally, other key breeding sites in residential areas identified in the literature are flowerpots and several plastic-type containers (Diéguez-Fernández et al., Reference Diéguez Fernández, Borge de Prada, Rodríguez Sosa, Vásquez Bautista and Alarcón-Elbal2019; Leal et al., Reference Leal, Fernandes Varela, Lopes Gonçalves, Sousa Monteiro, Ramos de Sousa, Lima Mendonça, De Pina, Alves and Osório2020; Alarcón-Elbal et al., Reference Alarcón-Elbal, Rodríguez-Sosa, Ruiz-Matuk, Tapia, Arredondo Abreu, Fernández González, Rodríguez Lauzurique and Paulino-Ramírez2021; Yuliani et al., Reference Yuliani, Hadi, Soviana and Retnani2021). Even though no statistical preference was observed for either plastic or any other specific material type, the most frequently infested breeding sites found were pot plates (38.10%) and flowerpots (28.60%). The high positivity of these larval habitats is explained by the regular watering of the plants by the inhabitants, in addition to the lack of cultural control measures.
Aedes albopictus control in residential areas
While public areas are subject to control by municipal services, private areas are inaccessible to public vector control practitioners, presenting a constraint in public control programmes (Stefopoulou et al., Reference Stefopoulou, Balatsos, Petraki, LaDeau, Papachristos and Michaelakis2018). In our research, public water-catch basins were previously treated in the scope of the NESCOTIGER project with insecticide paint (Inesfly 5A IGR NG; Alphacypermethrin 0.7%; D-Alletrin 1.0%; Pyriproxyfen 0.063%) (table 1) and were shown to be non-significant foci for Ae. albopictus mosquitoes (unpublished data). As such, domestic larval habitats were the main ones during the field inspection.
In this context, the role of citizens in the control of the Asian tiger mosquito is considered key, given the importance of the elimination of breeding sites in private areas (Gratz, Reference Gratz1994; ECDC, 2017). In this sense, prioritising the identification of potential breeding sites by residents and understanding the mosquito's biology becomes essential (Caputo et al., Reference Caputo, Manica, Gianluca and Solimini2020). Nevertheless, it has been shown that both children and adults in the study area have limited knowledge on this topic, with a general tendency to misidentify swimming pools as breeding sites (Alarcón-Elbal et al., Reference Alarcón-Elbal, López-de-Felipe, Gil, García, Mateo and Bueno2024). In fact, swimming pools without proper maintenance are not suitable places for the development of this species, as they contain a large amount of water, although unmaintained children's swimming pools do constitute an ideal habitat in urban environments (Rust, Reference Rust, Resh and Cardé2009). For this reason, pools (both maintained and unmaintained) were not considered as potential breeding sites during the development of this study, although they were also prospected, always with negative results. Nevertheless, certain elements tightly associated with pools were found infested by Ae. albopictus, as was the case of the most productive breeding site found in the study, a disused pool's water depuration system (fig. 1i), or structural deficiencies filled with splashed water (fig. 1j). As such, the identification and management of the main larval habitats becomes a priority in the implementation of any Aedes spp. control programme.
It should be noted that sectors 3 and 4, where the larvicide Inesfly Larva IGR was deployed independently or in combination with other control tools respectively (table 1), presented the lowest BI values (table 2). This spraying larvicide was employed by citizens in sectors 3 and 4 to coat potential Aedes mosquitoes breeding sites in their homesteads. Even though adults in Torrent showed during previous research a limited capacity to identify potential Ae. albopictus' breeding sites (Alarcón-Elbal et al., Reference Alarcón-Elbal, López-de-Felipe, Gil, García, Mateo and Bueno2024), it could be argued that the employment of this tool could have contributed in some way to the observed reduction of mosquitoes' preimaginal population densities in comparison with other studied areas. Nevertheless, due to the limited household sample size (n = 60), further research would be needed to assess the effect of the Inesfly Larvae IGR over the BI in any residential area.
In any case, it must be considered that a single highly infested house may affect a whole neighbourhood (Unlu et al., Reference Unlu, Farajollahi, Healy, Crepeau, Bartlett-Healy, Williges, Strickman, Clark, Gaugler and Fonseca2011). In our study, a single heavily infested disused water depuration system contributed to over 73% of the total identified Ae. albopictus larvae and 53% of pupae within the study area while harbouring an estimate of 2600 Cx. pipiens larvae. This depuration system had an estimate of 60 l of water, with a potential total capacity of 300 l. While this focus cannot be regarded as a typical breeding site of Ae. albopictus based on prior literature, it underscores the challenges associated with source reduction in expansive residential areas. Nevertheless, recognising the significance of such highly productive breeding sites is crucial in the planning of future control campaigns in the Mediterranean area of either Ae. albopictus or Cx. pipiens.
In general, excluding flowerpots, the most abundant breeding sites exhibited low larval production. For instance, out of the 388 plant pot plates identified, only eight were positive, accounting for a total of 244 larvae (2.33% of total larvae) and five pupae (1.30% of total pupae). A general density reduction (individuals/breeding site) from larvae to pupae was noted, with only 385 pupae identified out of 10,476 counted larvae, showing a calculated ratio of 0.037 pupae per larva. Based on this data, pupae density per breeding site type should serve as the primary indicator, as pupae offer a more reliable gauge of adult mosquito populations compared to larvae (Focks, Reference Focks2003). Among the various breeding sites identified in our study, tank bromeliads, structural deficiencies and buckets exhibited the presence of larvae but lacked pupae. Consequently, they did not seem to play a significant role as sources of adult Ae. albopictus in El Vedat de Torrent during the study period despite the species being known to breed in natural phytotelmata environments (Paupy et al., Reference Paupy, Delatte, Bagny, Corbel and Fontenille2009), a behaviour previously observed in the city of Valencia (Bueno et al., Reference Bueno, Güemes, Carbonell and Acosta Aleixandre2016). Similar findings were previously reported by Mocellin et al. (Reference Mocellin, Simões, Nascimento, Teixeira, Lounibos and Oliveira2009) in an urban area of Rio de Janeiro, Brazil. However, it is important to exercise caution as different outcomes may arise in various research areas or under distinct climatic conditions.
In this sense, among the study's limitations, we adopted a descriptive cross-sectional design for this research, i.e. each container was sampled just once. Furthermore, the field research took place during a time of the year when populations of this species were expected to be not very abundant because of high temperatures and low rainfall, typical in the mainland Spanish Levante (Collantes et al., Reference Collantes, Delacour, Alarcón-Elbal, Ruiz-Arrondo, Delgado, Torrell-Sorio, Bengoa, Eritja, Miranda, Molina and Lucientes2015). Undoubtedly, conducting such studies in different climatic periods provides a more comprehensive perspective on the entomological situation (Sánchez et al., Reference Sanchez, Vanlerberghe, Alfonso, Marquetti Mdel, Guzman, Bisset and van der Stuyft2006; González et al., Reference González, Rodríguez-Sosa, Vásquez-Bautista, Diéguez-Fernández, de Prada Miguel, Kelvin and Alarcón-Elbal2019; Monzón et al., Reference Monzón, Rodríguez, Diéguez, Alarcón-Elbal and San Martín2019). Therefore, it would be advisable in the future to collect these types of observations over a more extended period, while gathering data from different municipalities with variable climatic conditions. Another significant limitation is that aedic indices rely on visually locating containers, which may not accurately reflect the true prevalence of synanthropic mosquitoes given the presence of cryptic and/or inaccessible containers such as roof gutters, catch basins and septic tanks (Arana-Guardia et al., Reference Arana-Guardia, Baak-Baak, Lorono-Pino, Machain-Williams, Beaty, Eisen and García-Rejón2014), although these sites were considered (when possible) during the development of the field research. Lastly, it is also important to note the limited number of households inspected (n = 60). A larger data sample would be of interest for a better understanding Ae. albopictus' breeding sites in residential areas of similar or greater size.
To conclude, gaining knowledge about the breeding sites of Ae. albopictus in residential areas of the Mediterranean Basin, and effectively communicating this information to the citizens, is of utmost importance. This is especially the case in areas where both adults and students are proven to lack profuse knowledge concerning the biology and ecology of the vector species, as was the case of El Vedat de Torrent (Alarcón-Elbal et al., Reference Alarcón-Elbal, López-de-Felipe, Gil, García, Mateo and Bueno2024). This information enhances our comprehension of the biology of this vector and, more importantly, contributes to the formulation of targeted control programmes, for which community-based strategies are deemed indispensable.
Conclusions
To our knowledge, no aedic index research had previously been published in Spain or even Europe. The identification of the most productive breeding sites is considered key for the development of environmental education and awareness campaigns. In this study, we found that an isolated breeding site accounted for more than half of the total identified Ae. albopictus larvae in this residential area, and should be taken into consideration during the design phase of any control programme in the area. Irrigation tanks, flowerpots and animal drinkers were found as additional important productive sites. Consequently, the implementation of routine aedic index studies that comprise the identification of domestic breeding sites is highly recommended in infested areas of Southern Europe. Twenty years after its first detection in the country, the control of this species is still extremely challenging and requires strategies that necessarily involve the community.
Author contributions
Pedro María Alarcón-Elbal: conceptualisation, data curation, investigation, methodology, supervision, project administration and writing – original draft preparation, review and editing. Marcos López-de-Felipe: conceptualisation, data curation, investigation, methodology, formal analysis and writing – original draft preparation, review and editing. Ignacio Gil-Torró: conceptualisation, funding acquisition, methodology, project administration, supervision and writing – original draft preparation, review and editing. Isaac García-Masiá: funding acquisition, project administration and writing – review and editing. Pilar Mateo-Herrero: funding acquisition, project administration and writing – review and editing. Rubén Bueno-Marí: funding acquisition, project administration and writing – review and editing.
Financial support
This research was partially funded by the Instituto Valenciano de Competitividad Empresarial from the Generalitat Valenciana and the European Union through the FEDER program. The granted project entitled ‘New strategies for tiger mosquito control in residential areas’ included this study.
Competing interests
As authors of this research, we declare to not have any competing interest that could influence the results here shown.







