INTRODUCTION
Coastal areas contain marine habitats most at risk from human activities (Moore, Reference Moore1999). Consequently, inshore dolphins, which are reliant upon the near-shore environment, are among the most threatened species of cetaceans that are most in need of management intervention to reduce anthropogenic threats (Thompson et al., Reference Thompson, Wilson, Grellier and Hammond2000).
In the Arabian Gulf an economic boom based on exploitation of extensive natural resources including hydrocarbon reserves, has resulted in a rapid development of port and coastal processing facilities (Yagoub & Kolan, Reference Yagoub and Kolan2006). This Gulf is considered to be one of the most heavily impacted and exploited water bodies globally, raising serious concerns about the conservation status of many cetacean species (Braulik et al., Reference Braulik, Ranjbar, Owfi, Aminrad, Dakhteh, Kamrani and Mohsenizadeh2010; IWC, 2012). At least 10 species of cetacean have been identified in the region, but most of these are considered vagrant or seasonal visitors (Preen, Reference Preen2004). There is a lack of information about abundance and distribution of most cetacean species in the region (Robineau & Fiquet, Reference Robineau and Fiquet1994; Baldwin, Reference Baldwin1995a; Baldwin et al., Reference Baldwin, Van Waerebeek, Gallagher, Gallagher, Fisher, Ghazanfar and Spalton1999; Preen, Reference Preen2004; Braulik et al., Reference Braulik, Ranjbar, Owfi, Aminrad, Dakhteh, Kamrani and Mohsenizadeh2010; Owfi et al., Reference Owfi, Braulik and Rabbaniha2016). Therefore, the lack of data on which to base assessment of abundance, distribution and threats, hampers conservation and management efforts and our ability to assess the impact of human activities on cetacean populations in this region (Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015; Owfi et al., Reference Owfi, Braulik and Rabbaniha2016).
Indian Ocean humpback dolphins (Sousa plumbea, hereafter called humpback dolphins) are obligate shallow-water dolphins that occur exclusively in the near-shore waters of the Indian Ocean from South Africa to the Bay of Bengal (Jefferson & Rosenbaum, Reference Jefferson and Rosenbaum2014). Humpback dolphins do occur along the coasts of eastern Iran, western Pakistan and along the Gulf of Oman (Baldwin et al., Reference Baldwin, Collins, Van Waerebeek and Minton2004; Braulik et al., Reference Braulik, Ranjbar, Owfi, Aminrad, Dakhteh, Kamrani and Mohsenizadeh2010; Owfi et al., Reference Owfi, Braulik and Rabbaniha2016). In the Emirate of Abu Dhabi opportunistic sightings of live animals have been mostly recorded in proximity to Marawwah Island, Bu Tinah Island and surrounding islands (Baldwin, Reference Baldwin1995a).
This species typically occurs less than 3 km from shore and/or in water less than 25 m depth and in protected bays and estuaries (Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015). Information from many parts of its range is sparse, and large portions of the range of S. plumbea have not been surveyed (Jefferson & Rosenbaum, Reference Jefferson and Rosenbaum2014; Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015). Thus, in many parts of the Arabian Gulf, these animals are known to be present only from opportunistic sightings or stranding records (Baldwin, Reference Baldwin1995a, Reference Baldwinb; Baldwin et al., Reference Baldwin, Collins, Van Waerebeek and Minton2004; Preen, Reference Preen2004; Owfi et al., Reference Owfi, Braulik and Rabbaniha2016). Humpback dolphins are reliably identified from marks and pigmentation patterns on their dorsal fins (Corkeron et al., Reference Corkeron, Morissette, Porter and Marsh1997). Photographic identification of the naturally identifiable individuals has become a standard method in cetacean research (Würsig & Jefferson, Reference Würsig, Jefferson, Hammond, Mizroch and Donovan1990). This method is widely applied with capture–recapture methods to estimate abundance, demographic parameters and movement patterns of cetaceans (e.g. Wilson et al., Reference Wilson, Hammond and Thompson1999; Reed et al., Reference Reed, O'Grady, Brook, Ballou and Frankham2003; Parra et al., Reference Parra, Corkeron and Marsh2006).
Sousa plumbea has only been recognized as a distinct species in its own right since 2014 (Jefferson & Rosenbaum, Reference Jefferson and Rosenbaum2014). A recent evaluation of the conservation status of this species suggests that the Indian Ocean humpback dolphin should be classified as being Endangered under IUCN criteria A4cd, and due to the continued population declines of this species, in the coming years could satisfy criteria A3cd (Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015). The conservation status of the humpback dolphin in the Arabian Gulf is unknown (Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015).
About 80% of the human population in Abu Dhabi Emirate is living near the coast, and during the past three decades, the Emirate has developed major industrial projects along the coastline consisting of oil and gas facilities, electricity generation plants, desalination plants, industrial and civil ports, commerce and tourism centres (Yagoub & Kolan, Reference Yagoub and Kolan2006). Concerns have been raised with regard to the rate of industrial development along the coast of the Emirate of Abu Dhabi given the lack of appropriate baseline data on inshore dolphins in this rapidly developing region. Thus, in 2014, the Environment Agency – Abu Dhabi (EAD) in collaboration with the Bottlenose Dolphin Research Institute (BDRI) started the implementation of dolphin surveys to assess the abundance and distribution of small cetaceans in Abu Dhabi's coastal waters.
Here, we present for the first time in the Arabian Gulf, data on population size, use of habitat, group dynamics, residence patterns and potential threats to humpback dolphins off the coastline of the Emirate of Abu Dhabi (United Arab Emirates).
MATERIALS AND METHODS
Study area
The Emirate of Abu Dhabi is the largest of the seven emirates that make up the United Arab Emirates (UAE), which has a coastline stretching ~350 km long representing about 76% of the Arabian Gulf coastline of the UAE (Abdessalaam, Reference Abdessalaam2007). The study area is located between 24°08′N 51°40′E and 24°57′N 54°58′E consisting of the coastal waters of the Emirate of Abu Dhabi, in the United Arab Emirates (Figure 1). The coastal zone of Abu Dhabi has an exemplary development of active coastal sabkha surrounded by dune and gravel desert (Yagoub & Kolan, Reference Yagoub and Kolan2006). The sabkha is a supratidal flat area typically lying between the desert and sea, whose surface is characterized by efflorescences of salt, gypsum and calcium carbonate as well as windblown sediments and often tidal deposits (Butler, Reference Butler1969). Mangrove, represented by a single species, Avicennia marina, covers extensive areas although not continuously (Abdessalaam, Reference Abdessalaam2007). The study area is subject to both extreme negative and positive surface water temperature oscillations. Negative anomalies occur in winter (below 20°C), the most extreme caused by the Shamal, a cold north wind which blows from the Iranian highlands into the area of low atmospheric pressure over the Arabian Peninsula. Positive temperature excursions occur from April to September when surface waters attain temperatures up to 36°C (John et al., Reference John, Coles and Abozed1990).
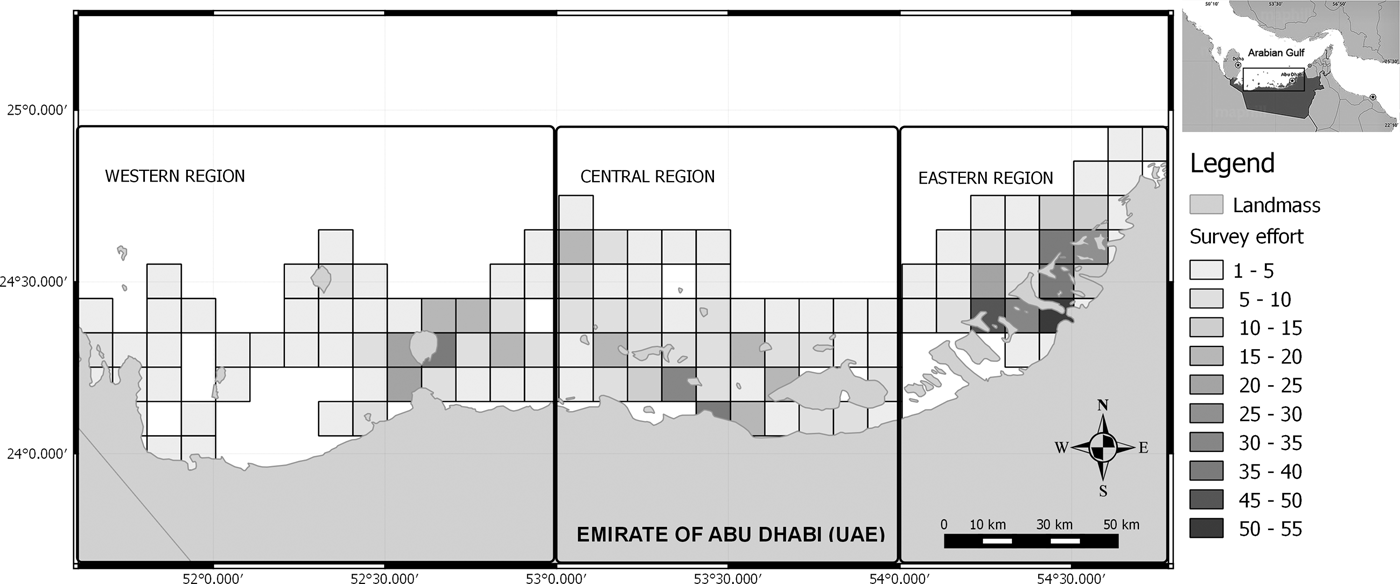
Fig. 1. The study area in Abu Dhabi waters showing the different zones of research. The gradient of colours depicts the survey effort as the number of 20 min sets (N = 1110) recorded within each 10 km2 cell.
The study area was stratified into three zones for logistical reasons, to study different habitats/sub-areas with different degrees of human impact along the Abu Dhabi coastline, and to increase precision in the abundance estimate (Figure 1). (1) The Western Region (between 24°08′N 51°40′E and 24°57′N 53°00′E), including Sir Bani Yas, Dalma, Al Yasat Al Ali and surrounding islands, is the region with lowest human population density. (2) The Central Region (between 24°08′N 53°00′E and 24°57′N 54°00′E) including the Marawwah Marine Biosphere reserve (MMBR), and surrounding islands, comprises several important representatives habitats with national and regional significance (Abdessalaam, Reference Abdessalaam2007). Fishing is only allowed in designated areas of the MMBR and is limited to traditional fishing methods that include fixed net (locally called Hadhra), shore net (locally called Al Sakkar) and seine nets (locally called Daffara). The Eastern Region (between 24°08′N 54°00′E and 24°57′N 54°58′E) including Al Bahrani island and the channels around the city of Abu Dhabi is the area that face the highest anthropogenic pressure.
Data collection
Boat-based observation surveys were carried year round during two consecutive years (2014 and 2015) and used repeated effort in all three zones with systematic transect lines. We established a systematic survey route departing from a different harbour within each zone (Abu Dhabi, Mirfa and Sir Bani Yas harbour to monitor the Eastern, Central and Western zone respectively). Due to time constraints, it was not possible to survey all three zones in a single day. It was not possible to follow a zig-zag pattern because designated channels, islands and shallow water governed the path of the transect lines. Daily surveys encompassed the inshore waters and were attempted to equally cover one monitored zone in a given day in order to locate any persistently isolated group of dolphins. However, the geographic distribution of effort could vary according to weather conditions. In order to analyse the seasonality of humpback dolphins in the study area, two sampling seasons were defined: Winter season (from October to March); Summer season (from April to September). The time duration it took to complete a sampling season (all three zones monitored) was between 15 and 21 consecutive days. Each zone was sampled five times per season and 10 times per year.
Boat-based observations were carried out using a 45 ft customized research vessel with an observation platform powered by two 300 hp outboard engines. Each one of the zones was surveyed during daylight hours at a constant speed, between 8 and 10 knots, with at least three experienced observers, stationed on the observation platform, scanning 360° of the sea surface in search of dolphins (with the naked eye and/or 10 × 50 binoculars). The minimum number of observers and vessel speed remained consistent during the study period, making data suitable for the comparative analysis of encounter rates. Boat-based observations were done when the visibility was not reduced by rain or fog, and sea conditions were <3 on the Douglas sea scale (approximately equivalent to the Beaufort wind force scale).
On each boat survey, the time, latitude, longitude, speed, environmental data (e.g. sea state, wind speed and direction, and visibility), and anthropogenic data (e.g. marine traffic and presence of fisheries) were recorded every 20 min (following Díaz López, Reference Díaz López2012). A hand-held global positioning system (GPS) was used to record the latitude, longitude and speed of travel (knots) and an iPad application was developed to collect and visualize the environmental and anthropogenic data in the field. These 20 min sets were used to summarize field conditions and distribution of the survey effort irrespective of dolphin presence.
Upon sighting a group of humpback dolphins, searching effort ceased and the vessel slowly manoeuvred towards the group in order to minimize disturbance during the approach. We recorded dolphins’ position (while located ~20 m from the animal), depth and time. Group size and composition were also recorded. A group of humpback dolphins was defined as one or more humpback dolphins observed within a 200 m area. Group size was estimated based on the initial count of different individuals observed on the surface. The group size and age categories were assessed visually in situ, and the data were later verified with photographs taken during each sighting by increasing the number of individuals present if more marked individuals were photographed than was estimated by the field data.
Individual humpback dolphins were classified according to the age of individuals within each group at the time of sighting. Age class definitions were: (1) Newborns, dependent humpback dolphins smaller than 1.0 m, with foetal folds or lines. Newborn dolphins were also determined on the basis of uncoordinated surfacing behaviour and swimming in infant position (underneath the mother lightly touching her abdomen); (2) Immature humpback dolphins, with a uniform dark grey colouration across the dorsal surface and two thirds or less the length of an adult, often observed in close association with an adult but never observed in the infant position; (3) Adult humpback dolphins, full-grown individuals primarily grey on the dorsal surface, with variable amounts of white scarring, blotches of white/pink, and dark or light spotting on numerous parts of the body, including the dorsal fin region (Jefferson & Rosenbaum, Reference Jefferson and Rosenbaum2014).
During each encounter, we attempted to photograph all members of the group in order to identify individuals with photographs, using natural marks. Individual dolphins were identified based on the size, location and pattern of notches on the trailing edge of the dorsal fin and along the humpback region (directly ahead and behind the dorsal fin). Digital photographs were taken using DSLR cameras equipped with telephoto zoom lens. An encounter was considered completed once photographs of all individuals in a group were obtained.
Data analysis
As the number of sightings could depend on the survey effort, a daily dolphin encounter ratio (DER) was computed as DER = Ns/Se, where Se (search effort) is the number of hours spent searching and Ns is the total number of humpback dolphin encounters. By calculating DER we eliminated effort-related bias from derived distribution patterns arising from an uneven survey effort, caused by time and weather restrictions. Thus, we examined the total DER for the entire study period, either for each survey season or surveyed coastal zone.
For spatial analysis the study area was divided into 10 km2 cells by creating a polygon grid using the software QGIS (Hugentobler, Reference Hugentobler, Shekhar and Xiong2008). The number of 20 min sets collected within a cell was deemed a fair representation of survey effort. To show the number of encounters in relation to the survey effort, sighting rate for humpback dolphins was calculated as (SPUE) = number of encounters in cell/number of 20 min sets collected in each cell. If a correlation between the survey effort and the sighting rate was observed, partial correlations were calculated between both variables in order to determine a threshold where the correlation was no longer significant.
Photographs selected for inclusion in the Abu Dhabi humpback dolphin photo-identification catalogue were based on the following criteria: (1) they showed a clear, unmasked, lateral view of the dorsal fin and humpback (left or right side); (2) the dorsal fin and humpback were suitably sized in the frame for all notches to be clearly visible; (3) the focus of the image and light intensity was sufficient to allow all notches to be distinguishable; and (4) the dorsal fin and/or humpback had sufficient notches to provide equal probability of recapture (Scott et al., Reference Scott, Wells, Irvine, Leatherwood and Reeves1990). All photographs containing a dorsal fin and humpback region (directly behind the dorsal fin) were graded for quality and degree of distinctiveness so as to minimize misidentification and heterogeneity in capture probabilities (Urian et al., Reference Urian, Gorgone, Read, Balmer, Wells, Berggren, Durban, Eguchi, Rayment and Hammond2015). Accordingly, all photographs were given an absolute value score (1 low, 4 average and 10 high) for: (1) perpendicular angle of the dorsal fin to the camera, focus; (2) the proportion of the frame filled by the fin; and (3) whether the dorsal fin and humpback region was fully visible. The individual scores for each category were summed to obtain an overall quality score (OQS). Overall quality scores from 3 to 9 were considered poor quality, from 12 to 15 average quality, from 18 to 24 good quality, and 30 excellent quality.
Correct identification of individuals is a requirement for unbiased parameter estimates (Yoshizaki et al., Reference Yoshizaki, Pollack, Brownie and Webster2009). To ensure this, only excellent- and good-quality photographs were used for individual identification. Additionally, each adult individual in the catalogue was included in a distinctiveness category, based on the amount of information contained on the dorsal fin and humpback region to ensure that more distinctly marked individuals would not have a higher probability of being captured. (1) A ‘well-marked individual’ was considered one dolphin that is recognized not by a single large feature in the dorsal fin and humpback region, but also by a matrix of evident marks which form a distinctive ‘face’ for the individual. (2) A ‘marked individual’ was considered one dolphin with distinct dorsal fin with an average amount of information (i.e. a single large feature and several secondary characteristics). Features such as body and dorsal fin scars, lesions, decolouration and tooth-rakings were used as secondary characteristics, thereby reducing the possibility of false positives (Wilson et al., Reference Wilson, Hammond and Thompson1999). (3) An ‘unmarked individual’ was considered one identified dolphin with dorsal fin or humpback region with small amount of information (i.e. a small feature and secondary characteristics). Since such characteristics are not necessarily permanent, ‘unmarked individuals’ were not included in the catalogue.
Every photograph was re-examined for false positives (different individuals being assigned the same discrete catalogue number) and false negatives (the same individual being assigned multiple discrete catalogue numbers) and the final data were confirmed by an independent and experienced second observer. Photographs of individuals that matched previously identified animals (i.e. recaptures) were archived. The best photographs of both sides of every individual were kept in an annual identification catalogue. Date, time, location, group size and composition (where known), were recorded in a database. Photographs were regularly replaced in the catalogue as better quality or more current images became available. Photographic re-sightings of identified dolphins were made with reference to this catalogue. Capture histories, corresponding to whether or not an individual was identified within a sampling period (a season), were compiled for each identified individual remaining after the photo-grading process, except calves.
Spatial and temporal distribution of the surveys, time spent at sea, and the choice of the most appropriate data sets and abundance models were made to minimize violation of mark–recapture assumptions. Thus, a number of fundamental assumptions were made: (i) selected (‘well-marked’ and ‘marked’) humpback dolphins will always be recognized; (ii) photo-identified humpback dolphins must be representative of the population being estimated; (iii) every selected humpback dolphin should have the same probability of being photographed within any one sampling occasion. Heterogeneity resulting from mark distinctiveness was minimized by including only captures from excellent- and good-quality photographs and by including only sufficiently marked individuals in analyses. We defined the term ‘population’ as the number of dolphins frequenting the study area and used the terms abundance and population size synonymously (Williams et al., Reference Williams, Nichols and Conroy2002).
Using POPAN in SOCPROG 2.3 (Whitehead, Reference Whitehead2009), abundance estimates were calculated and fitted with three open population models: (1) Mortality, this model assumes a population of constant size, where mortality (which may include permanent emigration) is balanced by birth (which may include immigration). The population size and mortality rate (per sampling period) were estimated by maximum likelihood; (2) Mortality + Trend, this model assumes a population growing or declining at a constant rate. The population size, mortality rate (per sampling period) and growth or decline of the population (instantaneous proportional rate per sampling period) are estimated by maximum likelihood; (3) Reimmigration, this is the model in which members of a population move from (emigration rate) and into (reimmigration rate) a study area (Whitehead, Reference Whitehead2001). The population size in the study area, the total population size, the emigration and reimmigration rates are estimated by maximum likelihood; and (4) Reimmigration and mortality, this is the same as model (3) with the exception that mortality (which may include permanent emigration from the total population) is balanced by birth (which may include immigration). Parameters for these models are detailed in Gowans et al. (Reference Gowans, Whitehead, Arch and Hooker2000). To obtain adequate sample sizes and to ensure an even coverage of the study area, the sampling period was set by season, resulting in four sampling periods (two during 2014 and two during 2015). Model selection was based on the lowest Akaike's Information Criterion (AIC, Akaike, Reference Akaike1973).
Our abundance estimates refer only to the marked individuals in the population. The total abundance was calculated using estimates generated from the most parsimonious model, and corrected by the mark rate for the animals inhabiting this region. The percentage of permanently marked individuals (θ) was estimated by: (1) selecting randomly good- and excellent-quality photographs; (2) counting the number of photographs of recognizable individuals (‘well-marked’ and ‘marked’); and (3) dividing by the total number of photographs randomly selected (Williams et al., Reference Williams, Dawson and Slooten1993). Thus, the total number of images selected at random, including ‘unmarked’ individuals, and the number of images that contain ‘well-marked’ and ‘marked’ individuals were used to make inferences about the proportion of identifiable individuals.
Statistical analysis
All data were checked for normality using a Shapiro–Wilk test. Seasonal fluctuations in group size were tested using a Mann–Whitney paired test for non-parametric data. Spatial fluctuations in group size and age classes were tested using a Kruskal–Wallis test for non-parametric data. If the Kruskal–Wallis test showed significant inequality of the medians (P < 0.05) a ‘post-hoc’ pairwise Mann–Whitney test was used. Correlations were calculated using the Spearman's (non-parametric) rank-order correlation coefficient. Data were presented as mean ± standard error. Statistical significance was tested at α < 0.05 level.
RESULTS
Between June 2014 and December 2015, 55 at-sea surveys were conducted within the coastal waters off the Emirate of Abu Dhabi. In all, 55 days over a period of 5 months were spent in the field. On average, 27.5 ± 1.5 days per season were spent at sea, totalling 368 h (corresponding with 1110 sets recorded every 20 min) and 6703 km (Table 1, Figure 1).
Table 1. Distribution of the survey effort in days (D), hours (H), and kilometres (km), and the number of humpback dolphin encounters (S).
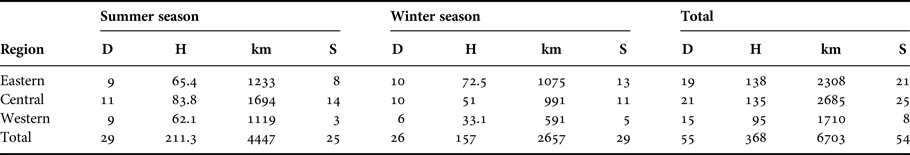
During this time there were 107 small cetacean encounters (54 with Indian Ocean humpback dolphins (Sousa plumbea), 48 with Indo-Pacific bottlenose dolphins (Tursiops aduncus) and five with finless porpoises (Neophocaena phocaenoides). Humpback dolphins were seen on 32 days at sea (58% of total number of surveys) (Table 1).
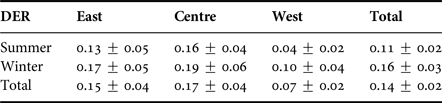
Humpback dolphins were sighted during every season surveyed and their presence did not show any variation among seasons (Mann–Whitney test, P > 0.05), or spatial fluctuations along the three monitored coastal regions (Kruskal–Wallis test, P > 0.05) (Table 2).
Table 2. Average daily sighting rate (DER) for humpback dolphins among seasons and between the three monitored zones.
Group size and group composition
Humpback dolphin group size and composition were examined for 54 independent groups encountered between 2014 and 2015. Group size ranged from 1 to 24 individuals (mean = 6.8 ± 1.7; Table 3). The most observed were aggregations of two dolphins (23% of the encounters), followed by solitary individuals (15%), and with most encountered groups (79%) containing less than 10 animals (Figure 2). Group composition showed that 73% of the observed humpback dolphins were adults; thus the remaining 27% were categorized as dependent calves (here considered together as immature humpback dolphins (23%) and newborn humpback dolphins (4%)).
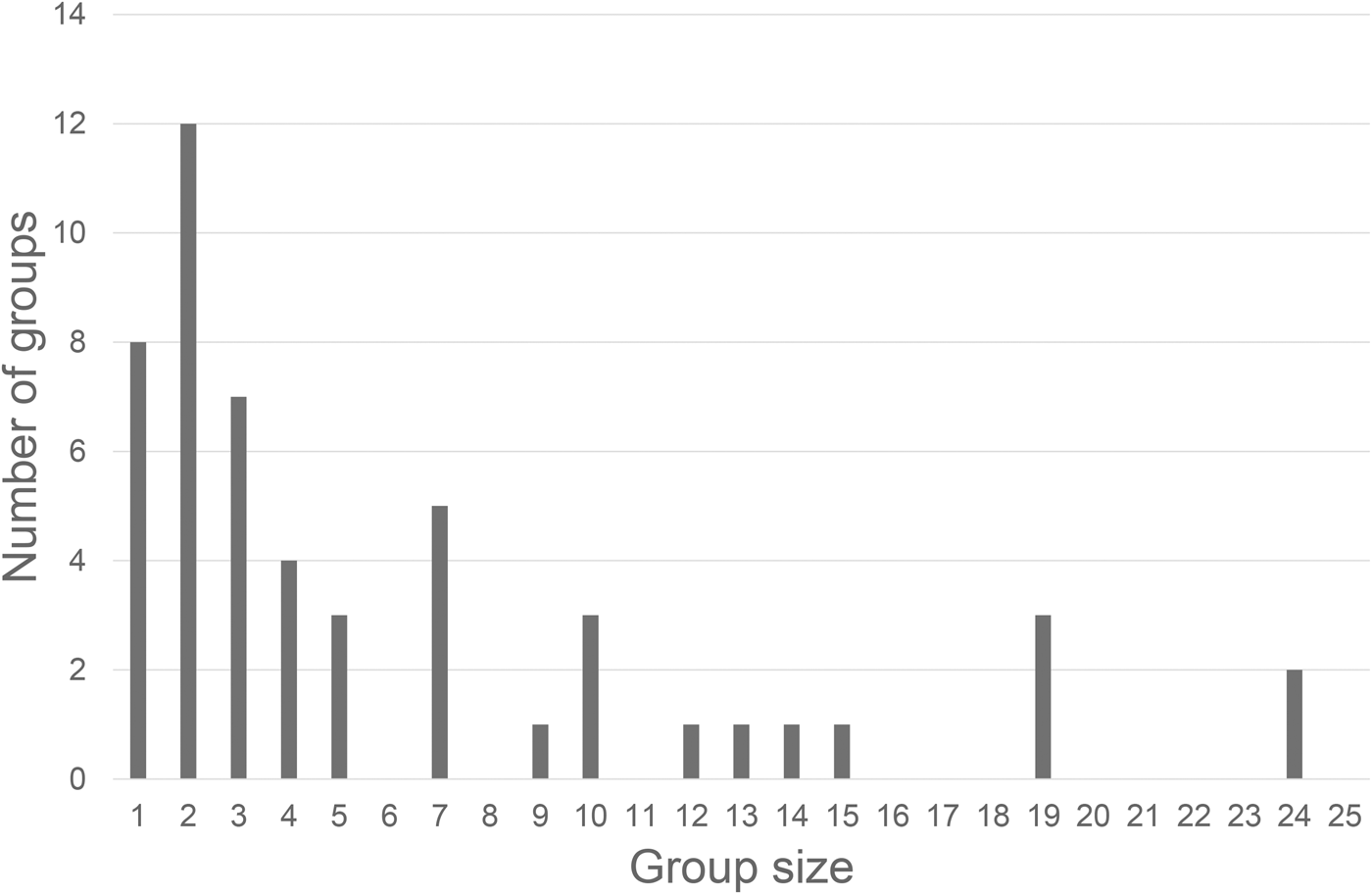
Fig. 2. Frequency distribution for humpback dolphins’ group size.
Table 3. Humpback dolphin group sizes (mean ± SE) observed during the study (G), including all age-classes (A = Adults, Im = Immatures, Nb = Newborns).
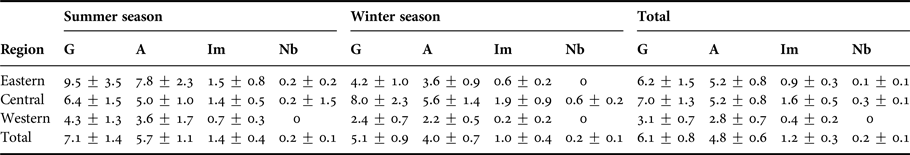
Group size was positively correlated with the presence of dependent calves in the group (Spearman rho = 0.67, P < 0.01). Groups composed entirely of adults (mean = 2.4 ± 0.3) were smaller than groups including dependent calves (mean = 9.8 ± 1.3).
The group size did not differ significantly among seasons (Mann–Whitney test, P > 0.05) or zones (Kruskal–Wallis, P > 0.05). Adult, immature and newborn humpback dolphins were recorded throughout all the survey months and monitored regions.
Photo-identification catalogue and abundance estimates
After the completion of the 55 photographic surveys, a total number of 220 adult humpback dolphins were identified and included in the Abu Dhabi humpback dolphin photo-identification catalogue. The Eastern region is the zone with a higher number of identified humpback dolphins (119 individuals), followed by the Central region (90 individuals), and the Western region (11 individuals).
A discovery curve of photographic captures of new permanently marked individuals (N = 220) showed an influx, and potentially outflux, of humpback dolphins during the four seasons of research, suggesting that the population is open for the duration of the study. Based on the lowest AIC value, the ‘Mortality model’ was selected as the most parsimonious for the studied species (Table 4). Abundance estimates suggested a population size of 589 (95% CI = 397–710) marked individuals. Based on the ratio of marked humpback dolphins, which was 84% of the catalogued individuals, we estimated 701 (95% CI = 473–845) humpback dolphins inhabiting Abu Dhabi waters.
Table 4. Abundance estimates of humpback dolphins in Abu Dhabi waters between 2014 and 2015.

N, estimated population size; CI, 95% confidence interval, bootstrapped (N = 500); Model results; n.a, not available; m, estimated mortality rate; t, estimated trend rate; e, estimated emigration rate; re, estimated reimmigration rate; nT, estimated total population size; Nc, number of humpback dolphins captured; s.p., number of sampling periods; θ, ratio of marked to total animals documented; Nt, estimate of total population size after correcting for proportion of identifiable individuals; AIC, Akaike Information Criterion; LogL, Log Likehood.
Site fidelity
Relative to the seasons surveyed, the average number of photographic recaptures per individual humpback dolphin was 1.37 ± 0.1 (from 1–4 times, N = 220), with 51 individuals (23% of the total) recaptured in more than one season.
Relative to the monitored coastal zones, only five individuals (2% of the total) were recaptured in two different zones. All these five dolphins were observed in both the Central and Eastern regions with individual movements of up to 100 km (mean = 92 ± 12 km).
Use of habitat
Humpback dolphins had a nearshore distribution throughout the whole study area, often found within the 1 m and 18 m isobaths (mean = 8.4 ± 1.2). There were no changes in the depth at which the humpback dolphins were sighted between the three monitored zones (Kruskal–Wallis, P > 0.05), or among seasons (Mann–Whitney, P > 0.05).
A correlation between the survey effort and the sighting rate was observed (Spearman rho = 0.79, P < 0.05). Therefore, partial correlations were calculated between both variables in order to determine a threshold where the correlation was no longer significant. Cells searched on less than two occasions (beneath the observed threshold; Spearman rho = 0.11, P > 0.05) were removed from subsequent analysis, to reduce bias associated with poorly sampled areas. Afterwards, the humpback dolphins’ occurrence along the Abu Dhabi waters was represented using a GIS (Figure 3). The occurrence was measured through the number of encounters in relation to the survey effort (20 min sets) in each 10 km2 cell.
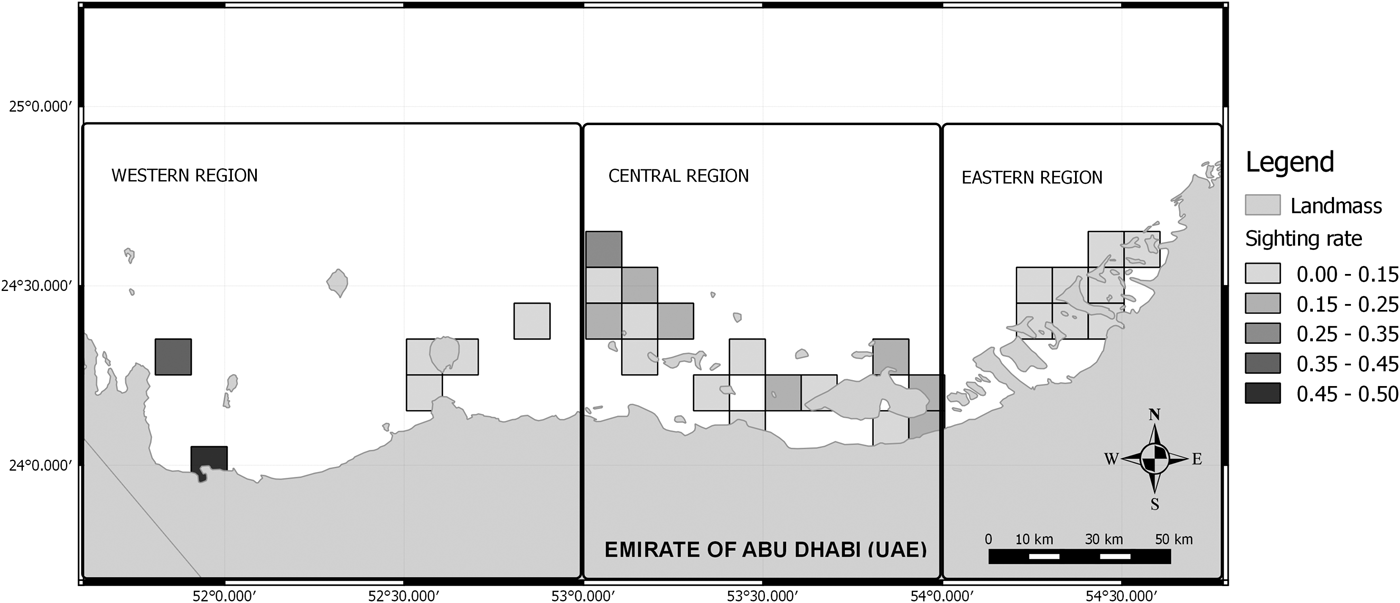
Fig. 3. Generated map showing the distribution of humpback dolphins in Abu Dhabi waters. The change in intensity of colour indicates the change from low occurrence to high occurrence, with black showing the areas of distribution where the sighting rate (SPUE) was the highest.
Potential threats to humpback dolphins in Abu Dhabi
The analysis of the photographs taken during the sightings helped to determine some of the anthropogenic threats occurring in these waters. The type of healing pattern observed in the region of the dorsal fin, humpback, and body cuts, present in 12% of the identified humpback dolphins, suggests injuries caused by anthropogenic activities. The most common type of healing pattern, observed in 8% of the identified humpback dolphins, showed the remains of a propeller strike (Figure 4). A second type of healing pattern, observed in 4% of the identified humpback dolphins (Figure 5), reflected a straight deep injury similar to what we would expect from cuts derived from entanglement in monofilament gill-nets used in fisheries.

Fig. 4. Humpback dolphin peduncle with wounds believed to be caused by collision with a vessel.

Fig. 5. Humpback dolphin with wounds believed to be caused by a gill-net where the square of the open mesh caught over the dorsal fin.
DISCUSSION
For successful conservation strategies, it is important to have an understanding of the population size. This is the first reported study on abundance estimation and use of habitat, from anywhere between north of Kenya and the south-eastern coast of India, in the Sousa plumbea range (Reeves et al., Reference Reeves, Dalebout, Jefferson, Karczmarski, Laidre, O'Corry-Crowe, Rojas-Bracho, Secchi, Slooten, Smith, Wang and Zhou2008; Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015). Estimates of population sizes available for selected areas around the world indicate that most S. plumbea populations that have been quantitatively evaluated have been smaller in size compared with Abu Dhabi waters (always less than 500 individuals and usually fewer than 100). For example, 450 dolphins (95% CI = 447–485) in the Algoa Bay region, Eastern Cape coast of South Africa (Karczmarski et al., Reference Karczmarski, Winter, Cockcroft and McLachlan1999), 105 individuals (95% CI = 30–151) in Maputo Bay, Mozambique (Guissamulo & Cockcroft, Reference Guissamulo and Cockcroft2004), 170–244 in the Richard's Bay region on the KwaZulu–Natal coast, South Africa (Atkins et al., Reference Atkins, Pillay and Peddemors2004), around 60 dolphins in Bazaruto Archipelago, Mozambique (Guissamulo & Cockcroft, Reference Guissamulo and Cockcroft1997) and 65 (95% CI = 56–102) off Zanzibar (Stensland et al., Reference Stensland, Carlen, Sarnblad, Bignert and Berggren2006). Therefore, we can conclude that the abundance estimation for S. plumbea of 701 (95% CI = 473–845) individuals observed during this study, if accurate due to the nature of the assumptions involved in mark–recapture analyses with open population models, would make it the largest population of this species that has been evaluated in the world.
While their occurrence within Abu Dhabi waters is frequent, the study area appears to be only a part of a much larger home range for this population. With no previous estimates of abundance, it is impossible to assess if the population in Abu Dhabi waters has been stable, increasing or decreasing. Quantitative trend data are not available anywhere in the Sousa spp. range, but there are indications that some subpopulations have declined in numbers in recent years (Braulik et al., Reference Braulik, Findlay, Cerchio, Baldwin, Jefferson and Curry2015).
Humpback dolphins occurred mostly close to the Abu Dhabi coastline in the Eastern and Western regions, but also in offshore waters that are relatively sheltered, and near reefs around Delma and Bu Tinah islands in the Central region. The dependence on shallow-water habitats as feeding grounds was evident throughout the year (Corkeron et al., Reference Corkeron, Morissette, Porter and Marsh1997; Karczmarski et al., Reference Karczmarski, Winter, Cockcroft and McLachlan1999; Koper et al., Reference Koper, Karczmarski, Preez and Plön2016), although the preferred habitats may differ between groups and locations (Atkins et al., Reference Atkins, Pillay and Peddemors2004; Stensland et al., Reference Stensland, Carlen, Sarnblad, Bignert and Berggren2006).
The mean group size observed in Abu Dhabi of about six individuals is similar to that reported in other locations for S. plumbea (Durham, Reference Durham1994; Karczmarski et al., Reference Karczmarski, Winter, Cockcroft and McLachlan1999; Koper e t al., Reference Koper, Karczmarski, Preez and Plön2016). Seasonality of occurrence (Koper et al., Reference Koper, Karczmarski, Preez and Plön2016) and group sizes (Durham, Reference Durham1994; Karczmarski et al., Reference Karczmarski, Winter, Cockcroft and McLachlan1999; Guissamulo & Cockcroft, Reference Guissamulo and Cockcroft2004) has not been observed in Abu Dhabi waters. The absence of seasonal fluctuations in group size may indicate a decreased prey abundance, which could result in smaller food patches (Koper et al., Reference Koper, Karczmarski, Preez and Plön2016). When prey is scarce and food patches are small, seasonal fluctuations in prey abundance may be less discernible, which would explain the absence of a seasonal fluctuations in group size. The individual movements along the Abu Dhabi coastline were similar to those movements observed in humpback dolphins along the east coast of Australia (Cagnazzi et al., Reference Cagnazzi, Harrison, Ross and Lynch2011).
During this study, two species of dolphin, the Indian Ocean humpback dolphin (Sousa plumbea) and the Indo-Pacific bottlenose dolphin (Tursiops aduncus), and one species of porpoise, the finless porpoise (Neophocoena phocaenoides), have been observed along the coastline of the Emirate of Abu Dhabi. In Oman and much of the Arabian Gulf, these species were also the most commonly recorded coastal cetaceans (Baldwin et al., Reference Baldwin, Collins, Van Waerebeek and Minton2004; Owfi et al., Reference Owfi, Braulik and Rabbaniha2016). The presence of these three species in the same coastal area is remarkably similar to those observations carried out for Indian Ocean humpback and bottlenose dolphins co-occurring off South Africa (Koper et al., Reference Koper, Karczmarski, Preez and Plön2016), and Indo-Pacific humpback dolphins and finless porpoises co-occurring off Hong-Kong waters (Barros et al., Reference Barros, Jefferson and Parsons2004). In those areas, the species showed substantial dietary overlap, but spatial segregation and behavioural displacement were thought to explain, at least in part, how they are able to share the habitats they occupy (Barros et al., Reference Barros, Jefferson and Parsons2004). The fact that humpback dolphins were the only cetacean species sighted inside the murky waters of the Eastern coast channels around the city of Abu Dhabi could be explained as a spatial segregation and behavioural displacement between species. Thus, humpback dolphins could explore the ‘acoustic visibility’ of their preferred prey, well-known sound producers of estuaries and murky waters (Barros & Cockcroft, Reference Barros and Cockcroft1991; Barros et al., Reference Barros, Jefferson and Parsons2004).
A limited coastal range and its near-shore distribution make humpback dolphins particularly vulnerable to mortality and traumatic injuries from heavy maritime traffic and gill-netting practices (Amir et al., Reference Amir, Berggren and Jiddawi2002; Jefferson & Hung, Reference Jefferson and Hung2004). There is a need to determine the conservation status of this important S. plumbea population where human-caused mortality is expected to be occurring. For example, the narrow channels around the Abu Dhabi city in the Eastern region in which humpback dolphins were observed regularly are used extensively by high-powered vessels, which present a threat to this population. Incidental fishing mortality of this species appears to be high, unsustainable and resulting in rapid local population declines (Stensland et al., Reference Stensland, Carlen, Sarnblad, Bignert and Berggren2006; Cerchio et al., Reference Cerchio, Andrianarivelo and Andrianantenaina2015).
Destruction of inshore habitats is likely to be one of the greatest threats for humpback dolphins, particularly in the Arabian Gulf as well as in many other increasingly developed urban coastal areas (Baldwin et al., Reference Baldwin, Collins, Van Waerebeek and Minton2004). In Abu Dhabi Emirate, dredging, land reclamation, port and harbour construction, boat traffic, oil and gas exploration (including seismic surveying) and other coastal development activities all occur, or are concentrated within, humpback dolphin habitat and threaten their survival.
Further research will improve our capacity to provide effective management actions towards the conservation of this species and also to understand how specific areas are used by the humpback dolphins, and what factors affect their distribution and abundance. Similarly, biopsy samples across this geographic range are required to gain a more detailed understanding of humpback dolphin population genetic structure and connectivity. Greater conservation and research efforts in the Arabian Gulf are considered imperative and could be extended to sympatric species such as the Indo-Pacific bottlenose dolphin, finless porpoise and dugong.
ACKNOWLEDGEMENTS
Thanks to Amin Blooshi and the volunteers who came along to help with the fieldwork: Donna Kwan, Marina Antonopoulou, Oliver Kerr, Yasmin Baker, Winston Cowie, Nessrine Alzahwali and Camilla Argent. Data collection complies with the current laws of the country in which it was performed, Abu Dhabi (UAE).
FINANCIAL SUPPORT
This study was funded by the Environment Agency – Abu Dhabi. We would like to thank HE Razan Al Mubarak and the management of EAD for supporting this work.













