To the Editor–Breast cancer is now the most common cancer in Indian women, with surgery being an essential treatment for all patients treated with curative intent. Although breast oncosurgery is considered a clean procedure, reported surgical site infection (SSI) rates are significant worldwide, ranging from 3% to 26%.Reference Jones, Bunn and Bell-Syer 1 – Reference Degnim, Throckmorton and Boostrom 3 SSI increases morbidity, causes psychological trauma, and increases hospital-associated costs.Reference Olsen, Chu-Ongsakul, Brandt, Dietz, Mayfield and Fraser 4 , Reference Bertin, Crowe and Gordon 5 For a patient with a malignancy, infections may compromise oncological outcomes by causing delays in adjuvant chemotherapy or radiotherapy. The aims of this study were (1) to quantify SSI rates after breast oncosurgery in a cancer hospital in eastern India, (2) to identify significant preoperative variables associated with SSI, and (3) to explore the feasibility of early discharge following breast oncosurgery.
A single center, retrospective study was conducted from September 2011 to August 2013 at a tertiary-care oncology center. A consecutive series of data from the medical records of 542 surgical and postoperative patients was analyzed. Breast surgery procedures included lumpectomy, modified radical mastectomy, breast conservation surgery, reconstructive surgery and resurgery following initial surgery in other hospitals. All patients received a single preoperative dose of intravenous coamoxiclav 1.2 g (or cefuroxime 1.5 g if allergic to penicillin), given within 30–60 minutes before incision. Povidone iodine (10%) was used for skin disinfection. Occlusive dressings were applied after surgery and were only changed if soiled.
Treatment protocols included a plan for early discharge from hospital. Patients were discharged the day after the surgery in most cases or were discharged as soon as they were comfortable, with closed suction drains in place. Nursing staff demonstrated drain care, including daily emptying, to the patient and family on the first postoperative day. Patients were reviewed between 3 and 5 days after discharge, when dressings were removed, the wound was left open, and daily baths with soap and water were advised. Drains were removed when the output was below 50 mL. The follow-up period for identification of SSI was 30 days.
Centers for Disease Control and Prevention criteria were used to identify SSI, which meant cellulitis (redness/warmth/swelling by itself was not a criterion for superficial SSI. 6 Additional infection prevention interventions included (1) soap and water baths for patients before surgery, (2) handwashing and hand sanitizer training for patient care staff, (3) nurse-led discussion with patient and family on wound and drain care at home, (4) encouragement to bathe daily at home with soap and water, and (5) written instructions in the local language (ie, Bengali/ Hindi).
Of the 542 total patients, female patients comprised 99.3% of the cohort. Body mass index (BMI) was median, 24.21kg/m2; range, 12.8–50.7kg/m,2 and 125 patients had diabetes mellitus (23%). Neoadjuvant chemotherapy (NACT) before chemotherapy was administered to 148 patients (27.3%). Also, 22 patients had immediate breast reconstruction (4.1%); 30 patients (5.7%) had a recent history of diagnostic breast surgery in another institution; and 2 or more risk factors were present in 90 of 542 patients (16.6%).
Furthermore, 69 of 542 patients had SSI (overall incidence, 12.7% with cellulitis and 4.8% without cellulitis). Organisms were isolated from infected surgical site wounds in only 26 patients (27.7% of the 69 patients with clinical diagnosis of SSI). The most common organism isolated was Staphylococcus spp (64%): 2 patients had methicillin-resistant Staphylococcus aureus (MRSA) and 2 had extended-spectrum β-lactamase (ESBL)–producing gram-negative bacilli. In our center, the MRSA bacteremia rate among inpatients is <1 per 1,000 inpatients, whereas the corresponding figures for third-generation cephalosporin–resistant bacteremia (20 per 1,000 inpatients) and carbapenem-resistant Enterobacteriaceae bacteremia (10 per 1,000 inpatients) are much higher. The median hospital stay following surgery for the whole study period was 2.1 days (range, 0–12 days), which was reduced to 1 day during the latter part of the study when the additional infection control interventions began to have greater effect. Common predictors of infection (eg, BMI, diabetes, previous diagnostic surgery, neoadjuvant chemotherapy) did not influence SSI, but patients who had reconstructive surgery had higher infection rates (Table 1).
TABLE 1 Univariate and Multivariate Analysis of Risk Factors of Surgical Site Infections
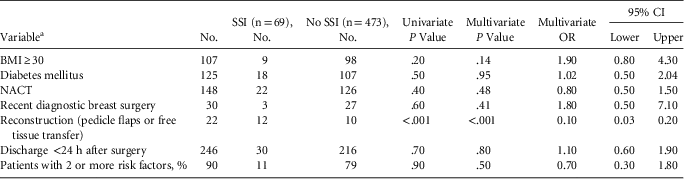
NOTE. CI, confidence interval; NACT, neoadjuvant chemotherapy.
a Patients reporting regular smoking and alcohol intake were negligible so were not analyzed.
We divided factors that contribute to SSI into 2 types. First, modifiable factors include surgical technique, glycemic control, patient hygiene, nutrition, smoking cessation, and standard infection control precautions, and are easier to control. Second, other risk factors include issues such as obesity and patient beliefs and habits, correction over a realistic period before surgery is difficult, if not impossible. Because many factors are beyond the control of surgeons, a “magic bullet” strategy is often followed to compensate for the difficulty in controlling risk factors. This strategy frequently involves prescription of antibiotics, which are called “prophylactic” but are continued for several days after surgery. The World Health Organization (WHO) Surgical Safety Checklist recommends the use of appropriate prophylactic antibiotics before surgery. 7 However, the choice of antibiotics and its duration is often based on the perceptions of individual surgeons, which can be influenced by the local prevalence of drug-resistant bacteria and incidence of SSI in the region.Reference Khan, Rodrigues, Kumar and Rao 8 – Reference van Kasteren, Kullberg, de Boer, Mintjes-de Groot and Gyssens 10
A recent Cochrane review supports the use of preoperative antibiotic prophylaxis for breast cancer, without significant adverse reactions compared with placebo or no treatment.Reference Jones, Bunn and Bell-Syer 1 However, standard infection prevention protocols with focused implementation are more effective in controlling SSIs. Our study demonstrates that postoperative antibiotics can be avoided in most patients having breast oncosurgery, despite the high prevalence of resistant organisms in the hospital. Early discharge following surgery, with the involvement of a multidisciplinary team, is feasible in these circumstances, with relatively low surgical site infection rates.
ACKNOWLEDGMENTS
We would like to thank the nursing team and the infection control team of Tata Medical Center, Kolkata, India, for their support.
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.



