INTRODUCTION
Microbiological risk assessments have been developed to establish food safety priorities, strategies and practices [1]. The determination of the infectious dose of a foodborne pathogen is one of the most important and fundamental steps in evaluating outbreaks of foodborne infections. However, only a few studies have evaluated the infectious dose of foodborne pathogens, such as Salmonella, Escherichia coli O157, enterotoxigenic E. coli, Vibrio parahaemolyticus and Campylobacter jejuni. In some of these studies, volunteers ingested pathogens, such as Salmonella, at various doses, and the ensuing symptoms were then observed [Reference Levine2–Reference Szita, Kali and Redey4]. As expected, large doses of pathogens produced overt disease. However, the level of contamination by foodborne pathogens that was determined or estimated in some actual outbreaks has been much lower. In a Salmonella outbreak [Reference Vought and Tatini5], ice cream was contaminated with Salmonella at a concentration of <0·38 c.f.u./g. The infectious dose, based on the consumption of a single ice cream cone, appeared to be no more than 28 c.f.u. There is, therefore, a serious discrepancy between studies in volunteers and actual foodborne infections. Microbiological analysis performed under actual conditions of foodborne infection is required to elucidate the true infectious dose of the foodborne pathogen.
In this study, we assessed the contamination levels of foodborne pathogens in vehicle food items consumed during 17 outbreaks. We also estimated the doses of the ingested pathogens in terms of the contamination level of the pathogens in food together with the amount of food ingested by the patients. Because knowledge of the dose of ingested pathogens is very limited, the data obtained from this study will be of particular value in the development of dose–response models for risk analysis of foodborne infections.
METHODS
The pathogen levels in outbreak-implicated vehicle food items were analysed by the prefectural governments in Japan between 2004 and 2006 (Table 1). Diluted food samples were analysed using a most probable number (MPN) method (outbreak nos. 3, 5, 8, 9, 12, 13, 16, 17) or a plating method (outbreak nos. 1, 2, 4, 6, 7, 10, 11, 14, 15). Food samples were homogenized in 9× vol. saline.
Table 1. Contamination level of pathgens in food associated with outbreaks
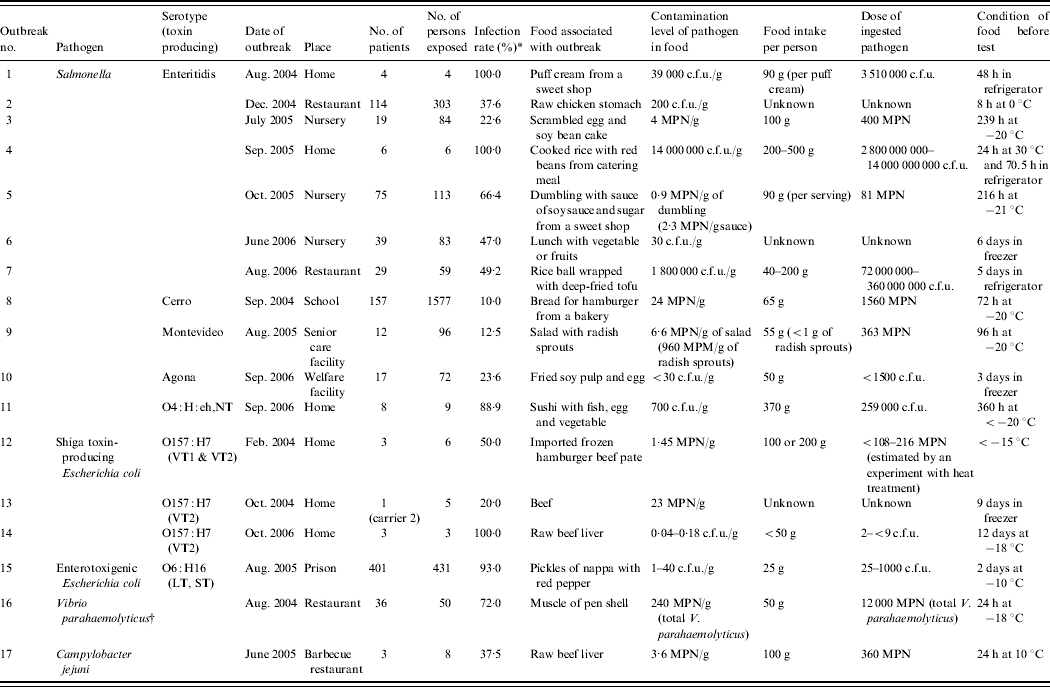
MPN, Most probable number; VT, Verotoxin; LT, heat-labile enterotoxin; ST, heat-stable enterotoxin.
* No. of person exposed/no. of patients×100.
† TDH-producing V. parahaemolyticus O3;K6 was isolated from samples of patients. The number of total V. parahaemolyticus in food was measured.
In the MPN method, the homogenates were incubated in either three or five tubes containing enrichment broth, followed by plating onto selective agar media. To detect Salmonella, the homogenate was incubated in buffered peptone water (BPW, Oxoid, UK) at 35–37°C for 20–24 h, and 1 ml of culture in BPW was transferred to 10 ml Rappaport–Vassiliadis broth (Oxoid) and incubated at 42°C for 20–24 h. For the second enrichment, the culture was plated onto DHL (Eiken Chemical Co., Japan), MLCB (Eiken), SS (Nissui Pharmaceuticals, Japan) or XLT4 (Merck, Germany) agar media. After incubation at 35–37°C for 20–24 h, the suspected colonies of Salmonella were confirmed by examining their biochemical characteristics in triple sugar iron medium (TSI, Eiken) and lysine indole motility medium (LIM, Eiken). Serotypes were tested using Salmonella antisera (Denka Seiken, Japan). To detect E. coli O157, the homogenate was incubated in modified EC broth with novobiocin at 42°C for 20–24 h. The culture was plated onto CHROMagar O157 medium (CHROMagar, France) and sorbitol MacConkey agar with cefixim and tellurite (CT-SMAC, Eiken). After incubation at 35–37°C for 20–24 h, the suspected colonies of E. coli O157 were tested for agglutination with O157 antiserum, for the verotoxin (VT) gene by PCR assay (O157 typing set; Takara Bio, Japan), and for VT production by agglutination using a reversed passive latex agglutination kit (VTEC-RPLA, Denka Seiken). To detect V. parahaemolyticus, homogenates were incubated in alkaline peptone water (Nissui) at 35°C for 18 h. The culture was plated onto TCBS agar medium. After incubation at 35°C for 18 h, the suspected colonies of V. parahaemolyticus were confirmed by examining their biochemical characteristics in TSI and LIM, and on the basis of their growth in 0, 3, 7 and 10% salt, along with oxidase production. In order to detect Campylobacter, homogenates were incubated in Preston broth (Oxoid) at 37°C for 48 h in a microaerobic atmosphere with BBL CampyPak (BD, USA). The culture was plated onto CCDA agar medium (Oxoid) and Skirrow's agar medium (Nissui). After incubation at 37°C for 48 h in a microaerobic atmosphere, the suspected colonies were examined using Gram stain, oxidase test, and hippurate hydrolysation test.
In the plating method, 0·1 ml of the food homogenate and tenfold serially diluted samples were spread onto various selective agar media. To detect Salmonella, the following media were used for plating: XLD, DHL, SS, CHROMagar Salmonella, and MLCB. After incubation at 35–37°C for 18–24 h, the suspected colonies were confirmed by examining their biochemical characteristics in TSI and LIM. Serotypes were tested using Salmonella antisera (Denka Seiken). To detect E. coli O157, the food homogenate and dilutions were plated onto CHROMagar O157 medium and CT-SMAC. After incubation at 35–37°C for 20–24 h, the suspected colonies of E. coli O157 were tested for agglutination with O157 antiserum, for the VT gene by PCR assay (O157 typing set), and for VT production by agglutination using a reversed passive latex agglutination kit (VTEC-RPLA). To detect enterotoxigenic E. coli, the food homogenate and diluted samples were plated onto desoxycholate agar medium (Nissui). After incubation at 35°C for 20 h, the suspected colonies were confirmed by examining their biochemical characteristics in TSI and LIM. Heat-stable enterotoxin and heat-labile enterotoxin genes were tested using a PCR assay (Primer set ELT-1&2, Primer set ESH-1&2; Takara Bio).
The numbers of patients and people involved with the food, the amount of food ingested by the patients, and the method of food storage prior to testing are described in Table 1. The infection rate was obtained by dividing the number of patients by the number of persons exposed.
RESULTS AND DISCUSSION
The contamination levels of food pathogens were analysed in 17 outbreaks of foodborne infections in Japan between 2004 and 2006, including 11 outbreaks of Salmonella, three outbreaks of E. coli O157, one outbreak of enterotoxigenic E. coli, one outbreak of V. parahaemolyticus, and one outbreak of C. jejuni (Table 1). The infectious dose specific to each outbreak was determined using existing data, except for two outbreaks of Salmonella infection and one outbreak of E. coli O157 infection.
Salmonella
Seven (outbreak nos. 1–7) of the 11 outbreaks of Salmonella were caused by the serotype Enteritidis. The remaining four outbreaks were caused by individual strains of serotypes, i.e. Agona, Cerro, Montevideo and O4:H:eh,NT. There are some reports on the infectious dose of serotype Enteritidis [Reference D'Aoust, Maurer, Doyle and Beuchat6, Reference Hennessy7], but reports on other serotypes are limited. In this study, the ingestion doses of serotypes Montevideo (outbreak no. 9), Agona (outbreak no. 10), Cerro (outbreak no. 8) and O4:H:eh,NT (outbreak no. 11) were 363 MPN, <1500 c.f.u., 1560 MPN, and 259 000 c.f.u., respectively. The infection rate of serotype O4:H:eh,NT (88·9%) was higher than that of the serotypes Agona (23·6%), Cerro (10·0%) and Montevideo (12·5%). Because the samples from these outbreaks had been stored in a freezer, the amount of Salmonella in these food samples did not increase during storage.
In outbreaks nos. 1 and 4 caused by S. Enteritidis, the infection rates were 100%. In outbreak no. 1, the contamination level was 39 000 c.f.u./g in puff cream and the ingestion dose was 3 510 000 c.f.u.. In outbreak no. 4, the contamination level was 14 000 000 c.f.u./g in cooked rice with red beans and the ingestion dose was 14 000 000 000 c.f.u.. The food samples obtained from outbreak nos. 1 and 4 were stored for 2 days in refrigerators, and later at around 30°C for 24 h, followed by storage in a refrigerator before testing. On the basis of the report that the S. Enteritidis count does not change at 10°C for 72 h even after incubation in enrichment broth [Reference Szczawinski, Klusek and Szczawinska8], we concluded that the ingestion dose in outbreak no. 1 appears to be an actual number. However, it was expected that Salmonella in cooked rice with red beans, implicated in outbreak no. 4, would significantly grow at 30°C for 24 h. The growth of Salmonella in food like cooked rice with red beans was not clarified, but an increase by >1000-fold is possible [Reference Szczawinski, Klusek and Szczawinska8]. In outbreak no. 7, the contamination levels and the ingested Salmonella dose were 1 800 000 c.f.u./g and 72 000 000–360 000 000 c.f.u., respectively. The infection rate, however, was <50%.
On the other hand, the samples of outbreak nos. 2, 3, 5, 6, 8–11 were stored at 0°C or in a freezer. Konuma [Reference Konuma9] reported that freezing at about −15 to −25°C either causes no decrease or causes 1 log reduction in S. Enteritidis population in five kinds of food, e.g. ground beef, radish sprouts, canned corn, fish meat and sweet bean paste for up to 14 days. On the basis of this report, it is considered that the Salmonella numbers did not increase much and decreased in these food samples prior to testing. In outbreak nos. 3, 5, and 8–10, the ingested Salmonella dose was <1560 MPN or c.f.u., and the infection rates were between 10·0% and 66·4%. In outbreak no. 5, the ingestion dose was 81 MPN, but the infection rate was 66·4%. It appears that the infection rate was high as most of these patients were very young children who are sensitive to pathogens. In outbreak no. 11, the dose of ingested Salmonella was estimated to be 259 000 c.f.u. from the Salmonella level in the frozen food, and the infection rate was high (89%).
E. coli O157
In E. coli O157 outbreak no. 14, which was associated with raw beef liver, the infection rate, the contamination level, and the ingestion dose were 100% (3/3), 0·04–0·18 c.f.u./g and 2 to <9 c.f.u., respectively. The food samples had been stored in a freezer for 12 days before testing. In E. coli O157 outbreak no. 13, which was associated with beef, a 6-year-old child was infected and two adults were diagnosed as carriers. In E. coli O157 outbreak no. 12, which was associated with hamburger patties, 50% of the people who consumed this meat became infected. Hamburger patties from the same lot were cooked in the same way to measure the number of surviving pathogens. The contamination level in the cooked patties and the ingestion dose were 1·45 c.f.u./g and 108–216 c.f.u., respectively. It has been reported that the infectious dose of E. coli O157 is low, <100 c.f.u. or MPN [Reference Meng, Doyle and Beuchat10, Reference Tuttle, Gimez and Doyle11]. The ingestion doses determined in this study are in agreement with previous reports.
Enterotoxigenic E. coli
In enterotoxigenic E. coli outbreak no. 15, which occurred in a prison in Japan, each prisoner had ingested 25 g pickles. The infection rate, the contamination level, and the ingestion dose were 93% (401/431), 1–40 c.f.u./g, and 25–1000 c.f.u., respectively. Because there is little data available on the infectious dose of enterotoxigenic E. coli [Reference Smith, Fratamico, Fratamico, Bhunia and Smith12], it is not clear how to interpret these findings. Most people infected with even a small amount of E. coli O157 and enterotoxigenic E. coli exhibit symptoms. Although some people appear to be susceptible to infection with small doses of Salmonella, it appears that the large doses make people ill at a high rate.
V. parahaemolyticus
In outbreak no. 16 of the TDH-producing V. parahaemolyticus O3:K6 infection, which was associated with pen shells, the total number of V. parahaemolyticus, but not specifically the TDH-producing V. parahaemolyticus, was counted. The infection rate, total V. parahaemolyticus contamination level, and the ingestion dose were 72%, 240 MPN/g, and 12 000 MPN, respectively. The Japanese government regulations indicate the safe level of total V. parahaemolyticus in seafood for raw consumption to be <100 MPN/g [Reference Tsurumi13]. The number of V. parahaemolyticus in pen shells was higher than that suggested by the Japanese regulations.
Campylobacter
In an outbreak of C. jejuni infection, which was associated with raw beef liver, the infection rate, contamination level, and ingestion dose were 37·5%, 3·6 MPN/g and 360 MPN, respectively. C. jejuni is found at high concentrations in the bile of cattle infected with the pathogen, and contaminates the liver [Reference Enokimoto14]. The consumption of raw beef liver is associated with foodborne infection. The test sample was stored at 10°C for 24 h before testing. Because Campylobacter is unable to grow in an aerobic atmosphere and survives under low temperature for a short time such as 24 h, the number of pathogens ingested seems accurate. The infectious dose in experimental human infection is reported to be as low as 500 bacteria [Reference Black15, Reference Robinson16].
In this study, the ingestion dose of foodborne pathogens was estimated by using existing data on the outbreaks. Although the storage condition of the food samples before testing may have had some effects on the number of pathogens in the tested samples, it appears that relatively small doses of Salmonella, E. coli O157, enterotoxigenic E. coli and C. jejuni caused foodborne infections. In this study, the infectious doses of the pathogens were utilized in microbiological risk assessments. However, infectious doses vary by the characteristics of the exposed populations, such as age, immunity, illness, and the characteristics of the pathogens, such as virulence, resistance to environmental factors, and activities. Thus, further investigations to analyse the vehicle food items in the outbreaks are needed to identify the actual infectious dose of foodborne pathogens.
ACKNOWLEDGEMENTS
This research was supported by Research on Food Safety in Health and Labour Science Research Grant, Japan, from 2004–2006. We thank Gaku Takahashi (Chiba City Government, Japan), Hiroshi Ueyama (Edogawa-ku, Tokyo Metropolitan Government, Japan), Hajime Amano (Yokosuka City Government, Japan), Hiromi Nagaoka (Shizuoka Prefectural Government, Japan), Hiroshi Kanazawa (Shizuoka City Institute of Environmental Sciences and Public Health, Japan), Machi Inada (Nara Prefectural Government), Kazumi Horikawa (Fukuoka Institute of Health and Environmental Sciences, Japan), Yoshimasa Makimoto (Fukuyama City Public Health Center, Japan), Kikuyo Ogata (Oita Prefectural Institute of Health and Environment, Japan), Keiko Inoue (Oita City Public Health Center, Japan), Setsu Watanabe (Miyagi Prefectural Institute of Public Health and Environment, Japan), Jun Kudaka (Okinawa Prefectural Institute of Health and Environment, Japan), and other participants in this study in city and prefecture governments for their cooperation in collecting data on the outbreaks.
DECLARATION OF INTEREST
None.



