A negative energy balance will result in weight loss if sustained over time(Reference Aragon, Schoenfeld and Wildman1). Despite the apparent simplicity of energy balance, i.e. energy intake (EI) v. energy expenditure (EE), most weight loss maintenance attempts are unsuccessful and weight loss recidivism is high(Reference Wadden, Neiberg and Wing2–Reference Dombrowski, Knittle and Avenell5). The development of strategies that promote successful weight loss and prevent weight regain therefore remains a priority. While a lack of sustained weight loss can in part be explained by a failure to adhere to dietary and physical activity guidelines(Reference Del Corral, Bryan and Garvey6, Reference Alhassan, Kim and Bersamin7), compensatory metabolic and behavioural responses to energy deficit also act to undermine weight loss and promote weight regain(Reference King, Hopkins and Caudwell8, Reference Melby, Paris and Foright9). A better understanding of the compensatory responses to energy deficit and surfeit is needed if more effective long-term weight maintenance strategies are to be developed. However, such strategies are complicated by the large inter-individual variability seen in body weight responses to weight loss interventions(Reference Williams, Ordovas and Lairon10–Reference Yancy, Olsen and Guyton12), and the lack of robust predictors of this response variability(Reference Rosenbaum, Agurs-Collins and Bray13).
The mechanisms that oppose negative energy balance are inter-related and complex, individually subtle and often difficult to quantify(Reference Melby, Paris and Foright9, Reference Rosenbaum, Agurs-Collins and Bray13). Metabolic and behavioural determinants of energy balance interact in a co-ordinated fashion during energy deficit and surfeit, but the mechanisms through which physiology drives behaviour are rarely acknowledged in the context of weight loss and weight regain(Reference Rosenbaum, Agurs-Collins and Bray13). Methodological limitations associated with the measurement of EI and EE have long frustrated energy balance research, and have limited our understanding of the putative signals that link physiology to behaviour. Such limitations have also contributed to debate over the primary cause of weight gain and secular trends in obesity prevalence(Reference Ng, Fleming and Robinson14). However, given the fundamental relationships between components of EE, body composition and EI, it might be argued that successful weight loss and weight loss maintenance strategies will only be developed if the inter-relationships between physiology and behaviour are explicitly acknowledged and incorporated in their design(Reference Millward15, 16).
To this end, there is renewed interest in integrative models of energy balance regulation that consider the dynamic relationships between body composition, EE and physiological function, and the way these interactions influence appetite and EI(Reference Hopkins and Blundell17–Reference Blundell, Caudwell and Gibbons20). Recent research has focused on the functional associations between components of body composition, EE and EI, and indicate that fat-free mass (FFM) and RMR are associated with a drive to eat that reflects the energetic demand of metabolically active tissue in weight-stable individuals(Reference Weise, Hohenadel and Krakoff21–Reference McNeil, Lamothe and Cameron26). However, it is unclear how changes in body composition and EE during weight loss influence appetite control. Therefore, the main aims of the present review are to (i) examine the metabolic adaptations that occur in response to negative energy balance, and (ii) to consider the putative or functional effects that these adaptations may have on appetite control and EI.
Energy balance: a dynamic regulatory system
It has been suggested that an energy deficit of 14644 kJ (3500 kcal) would lead to 1 pound of lost body weight (about 454 g)(Reference Wishnofsky27), but this simplistic approach is known to overestimate weight loss(Reference Hall, Sacks and Chandramohan28). The ‘3500 kcal per pound’ rule assumes that the composition of weight lost would be 100 % body fat (based on the assumption that the energy value of 1 g fat is 38 kJ (9 kcal) and adipocytes are composed of 85–90 % TAG) and fails to account for dynamic changes in the biological components of EE seen with weight loss (e.g. reductions in RMR and the energy cost of muscular activity). A concomitant reduction in EE during weight loss will attenuate the prescribed energy deficit and lead to lower than expected weight loss (as the actual energy deficit will be lower than that prescribed via dietary restriction)(Reference Hall29). Large inter-individual variability in weight loss and other physiological and behavioural responses are also apparent following lifestyle (diet or exercise)(Reference King, Hopkins and Caudwell8, Reference Yancy, Olsen and Guyton12), pharmacological(Reference Yancy, Olsen and Guyton12) and surgical(Reference de Hollanda, Ruiz and Jimenez30) weight loss interventions. The clinical significance(Reference Williamson, Atkinson and Batterham31) and statistical methods(Reference Atkinson and Batterham32) used to quantify such variability have been debated, but this inter-individual variability in treatment response appears to be a biological norm(Reference Hammond, Stotz and Brennan33).
Although adherence to a prescribed intervention is likely to contribute to such variability(Reference Alhassan, Kim and Bersamin7), metabolic and behavioural compensatory adaptations will also underlie differences in treatment response. Therefore, it should not be assumed that a linear relationship exists between the prescribed energy deficit and actual weight loss. Rather, energy balance should be viewed as a dynamic regulatory system in which perturbation to an individual component may produce co-ordinated responses in other components of the system that attenuate the gap between EI and EE. For instance, compensatory responses to negative energy balance such as a greater than predicted decreases in RMR(Reference Hopkins, Gibbons and Caudwell11, Reference Müller, Enderle and Pourhassan34, Reference Nymo, Coutinho and Torgersen35) and increased muscular efficiency(Reference Leibel, Rosenbaum and Hirsch36, Reference Rosenbaum, Vandenborne and Goldsmith37) have been observed (relative to that predicted based on changes in metabolically active tissue). Additionally, increases in EI have also been reported following energetic restriction(Reference Mars, de Graaf and de Groot38, Reference O'Connor, Scisco and Smith39). Thus, the apparent simplicity of energy balance belies a dense and complex network of inter-related biological, nutritional, psychological and behavioural determinants of EI and EE(16), and multiple regulatory systems and feedback loops that operate concurrently to influence energy homeostasis (see Fig. 1). It is also tempting to try to explain overconsumption and weight gain solely in terms of a failure in innate biological or homeostatic regulation. However, such biological reductionism fails to adequately acknowledge the importance of psychological and behavioural aspects of energy balance during weight gain or loss(Reference Blundell40). Psychological factors such as cognitive restraint remain robust predictors of EI when considered alongside physiological determinants of EI (such as body composition and RMR), and indeed, have the potential to play a mediating role between physiological and behavioural outcomes(Reference Hopkins, Finlayson and Duarte41).
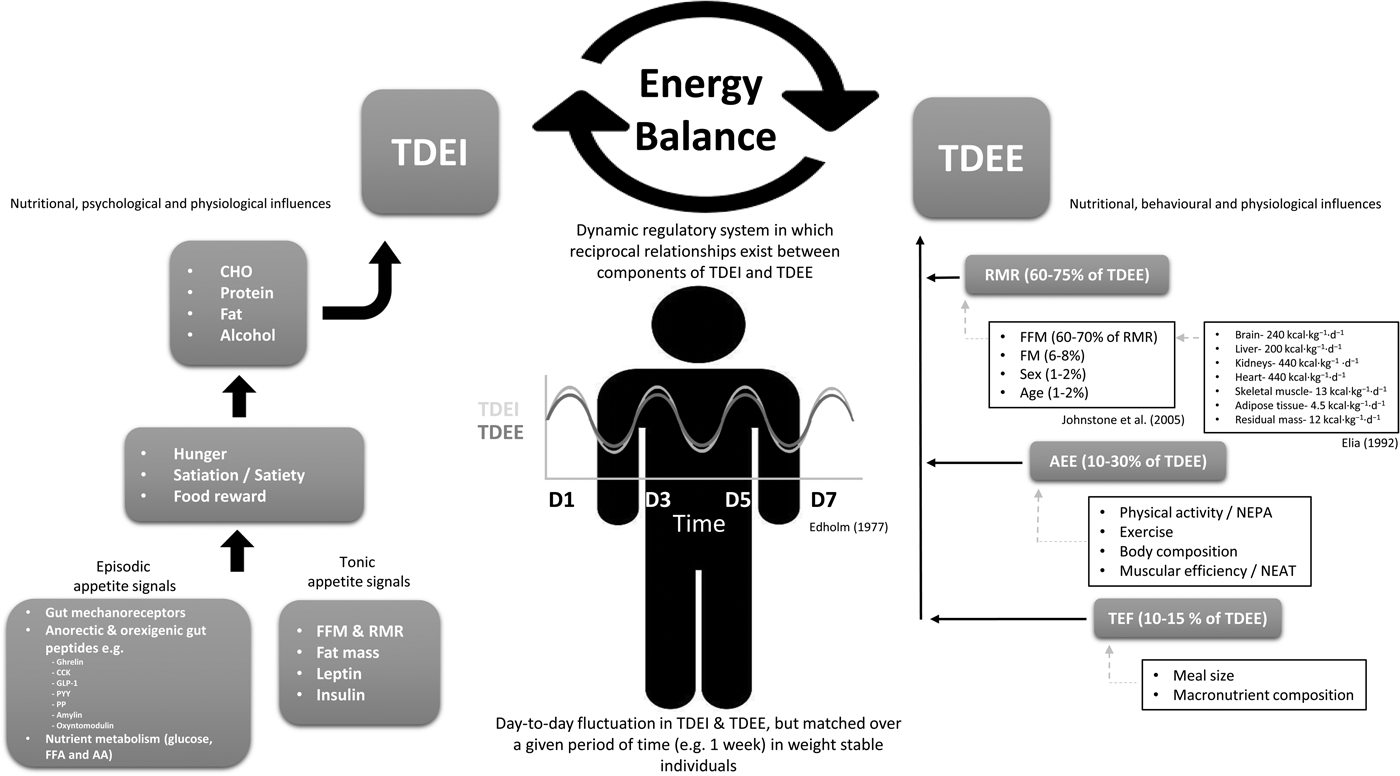
Fig. 1. Schematic overview of energy balance and the nutritional, psychological, behavioural and physiological influences on total daily energy intake and energy expenditure. Reference values for organ and tissue contribution to metabolic rate taken from Elia(Reference Elia42), while determinants of RMR taken from Johnstone et al.(Reference Johnstone, Murison and Duncan43). TDEI, total daily energy intake; TDEE, total daily energy expenditure; CHO, carbohydrate; NEPA, non-exercise physical activity; NEAT, non-exercise activity thermogenesis; CCK, cholecystokinin; PP, pancreatic polypeptide; PYY, peptide YY; GLP-1, glucagon-like peptide-1; FFA, free-fatty acid; AA, amino acid; FFM, fat-free mass; AEE, activity energy expenditure; TEF, thermic effect of food.
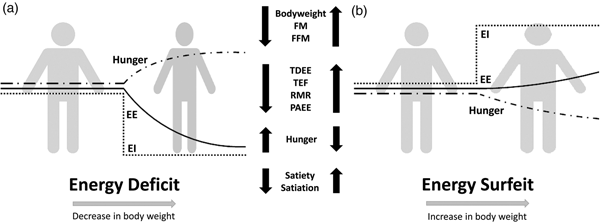
Compensation to energy imbalance appears asymmetrical, with the forces that resist weight loss stronger than those that resist weight gain (Fig. 2)(Reference Hopkins, Beaulieu and Myers44). This asymmetry may help account for the apparent ease with which people gain weight but typically fail to sustain weight loss over the long term(Reference Kelly, Yang and Chen45). However, studies examining compensation to controlled over rather than underfeeding are less common(Reference Cuthbertson, Steele and Wilding46), and considerable inter-individual variability in the magnitude of weight gain and extent of compensation also exists with overfeeding(Reference Leaf and Antonio47). While EE and its components may change in response to energy imbalance in a quantitatively important manner, changes in appetite and EI may have a greater capacity to perturb energy balance and body composition(Reference Stubbs, Hopkins and Finlayson48). For example, Polidori et al.(Reference Polidori, Sanghvi and Seeley49) recently estimated that the increase in appetite following a 52-week placebo-controlled trial using canagliflozin (a sodium glucose co-transporter inhibitor) was approximately three times greater than the corresponding change in EE (about 418·4 (100 kcal) v. about 125·5 kJ (30 kcal)/d per kg weight lost). Elevations in EE may also provide ‘limited auto-regulatory capacity’ to dissipate excess EI during periods of energy surfeit, with Siervo et al.(Reference Siervo, Fruhbeck and Dixon50) reporting that total daily EE increased by just 11·4 % after progressive overfeeding (3 weeks at 120 %, 3 weeks at 140 % and 3 weeks at 160 % of baseline intake). Such findings would point to the relative importance of appetite and EI as the primary means to compensate for energy deficit and surfeit in human subjects.
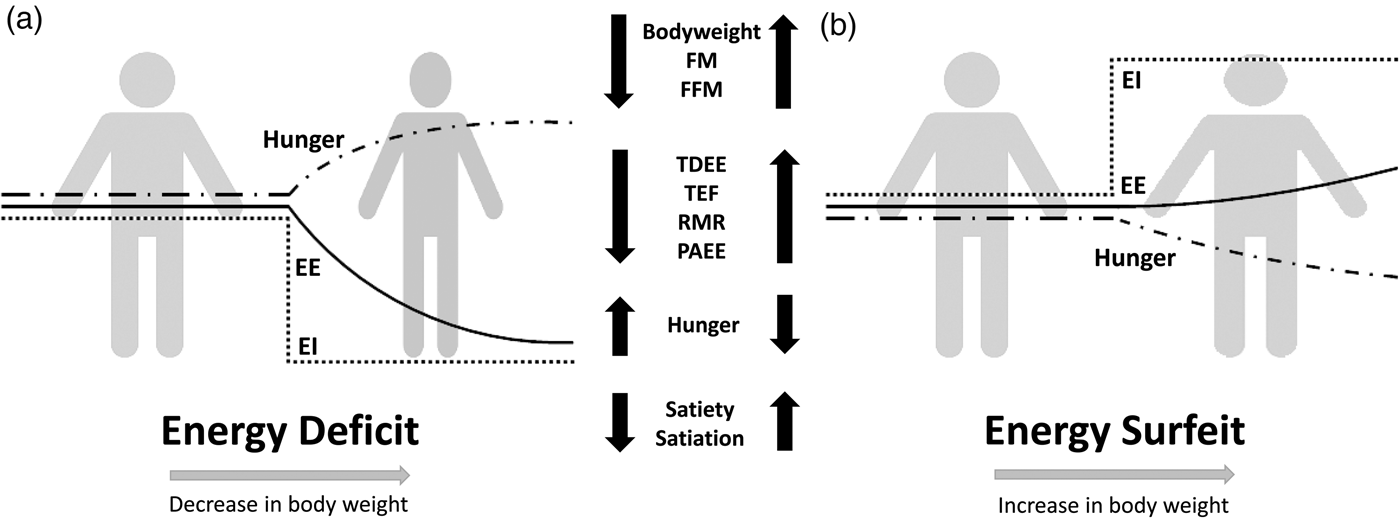
Fig. 2. Overview of physiological and behavioural responses during: (a) energy deficit and (b) energy surfeit. In (a) and (b) it is possible to observe an asymmetrical response between periods of energy deficit and surfeit in which there is a greater force resisting weight loss than weight gain. Figure adapted from Melby et al.(Reference Melby, Paris and Foright9). EI, energy intake; EE, energy expenditure; TDEE, total daily energy expenditure; PAEE, physical activity energy expenditure; TEF, thermic effect of food; FFM, fat-free mass; FM, fat mass.
In relation to our understanding of the peripheral physiological mechanisms involved in the regulation of appetite, there is a growing number of gut peptides purported to play unique roles in hunger and satiety signalling(Reference Cummings and Overduin51). However, not all of these peptides have a close association with the temporal profiles of hunger and fullness(Reference Gibbons, Caudwell and Finlayson52), and eating behaviour reflects the combined influence of multiple hormonal and metabolic stimuli (as depicted in the satiety cascade(Reference Blundell53)). An area that has been a target of recent interest is the role of body composition and EE in appetite control, with studies demonstrating that FFM and RMR play important roles in the tonic drive to eat in weight-stable individuals(Reference Hopkins, Finlayson and Duarte41, Reference Stubbs, Hopkins and Finlayson48, Reference Blundell, Finlayson and Gibbons54). It remains unclear though how the functional relationships between body composition, EE and EI operate during periods of negative energy balance and weight loss. Decreases in body composition and EE will influence energy balance by reducing total daily EE during weight loss, but such responses may also promote changes in EI that further attenuate the energy gap. As such, a clear understanding of these adaptations and their impact on bodyweight and appetite regulation during negative energy balance could be a key factor in improving weight maintenance.
Metabolic adaptations in response to negative energy balance
Resting energy expenditure
RMR, used interchangeably with resting EE and BMR in this review, represents the energy requirements to fuel the body's basic functions in a resting state. It is thought to account for up to 70 % of total daily EE, depending on physical activity and exercise levels(Reference MacLean, Bergouignan and Cornier55, Reference Trexler, Smith-Ryan and Norton56). It has been postulated that changes in RMR could influence weight loss and maintenance success since associations between a lower RMR and future weight gain have been observed(Reference Piaggi, Thearle and Bogardus57, Reference Ravussin, Lillioja and Knowler58), although this hypothesis has been questioned(Reference Anthanont and Jensen59). RMR is primarily determined by the quantity of FFM, which accounts for 63 %(Reference Johnstone, Murison and Duncan43) and up to 75 % of variability between individuals(Reference Stubbs, Hopkins and Finlayson48). Factors such as fat mass (FM), age and sex also contribute to the between-subject variability in RMR(Reference Johnstone, Murison and Duncan43), but an unknown component typically remains in models examining between-subject differences in RMR.
There is a decrease in RMR during periods of negative energy balance that occurs primarily as a result of losses of metabolically active tissue(Reference Johnstone, Murison and Duncan43, Reference Stubbs, Hopkins and Finlayson48, Reference Muller, Enderle and Bosy-Westphal60). However, during the first stages of fasting/starvation (i.e. about 2 d), a transient increase in RMR (5–10 %) can be observed(Reference Elia, Wood and Khan61), possibly due to an increase in gluconeogenesis as this is a more energy-demanding pathway(Reference Eriksson, Olsson and Bjorkman62). There also appears to be an additional down-regulation in EE not explained by changes in FFM or FM(Reference Dulloo, Jacquet and Montani63–Reference Westerterp65), even after adjusting for losses in organ mass(Reference Bosy-Westphal, Kossel and Goele66). This phenomenon has been termed adaptive thermogenesis, and is usually defined as a greater than predicted decrease in EE after adjusting for changes in body composition(Reference Leibel, Rosenbaum and Hirsch36). A 5–10 % lower than predicted decrease in RMR has been observed following weight loss which could subvert continued weight loss or weight maintenance(Reference Müller, Enderle and Pourhassan34, Reference Nymo, Coutinho and Torgersen35, Reference Westerterp65, Reference Doucet, St-Pierre and Almeras67), although the existence and functional significance of adaptive thermogenesis has been questioned(Reference Muller and Bosy-Westphal64, Reference Flatt68). It is worth noting that studies examining the presence of adaptive thermogenesis typically only adjust for changes in FFM as a single homogenous tissue compartment assume that losses in FFM are uniform across its constituent components (e.g. skeletal muscle and organs) and that tissue hydration loss remains constant during underfeeding. Reductions in organ masses during weight loss have been reported(Reference Müller, Enderle and Pourhassan34, Reference Gallagher, Kelley and Thornton69, Reference Pourhassan, Bosy-Westphal and Schautz70), and it may be that after accounting for changes in more energy-demanding structures such as the heart and kidneys, which expend approximately 1841 kJ (440 kcal)/kg in contrast to 54·4 kJ (13 kcal)/kg for skeletal muscle(Reference Wang, Ying and Bosy-Westphal71), adaptive thermogenesis becomes negligible. For instance, after 10 % weight loss, it was observed that from a total decrease in RMR (−570·7 kJ (−136·4 kcal) = 7·7 %), 40 % was attributed to adaptive thermogenesis (about 230·1 kJ (55 kcal)) after accounting for changes in organ mass(Reference Bosy-Westphal, Kossel and Goele66). Reduced sympathetic nervous system output, impaired thyroid activity (lower free triiodothyronine) and a fall in insulin secretion have been suggested as possible mechanisms for adaptive thermogenesis(Reference Muller, Enderle and Bosy-Westphal60, Reference Muller and Bosy-Westphal64, Reference Tremblay, Royer and Chaput72), but the underlying causes remain to be fully understood. From a biological standpoint, it makes sense that the body reacts in order to reduce the energy gap induced by ‘voluntary starvation’, becoming more efficient in response to food restriction. However, it is not fully understood whether adaptive thermogenesis is a permanent consequence of weight loss or is reversed after a period of weight stability at a newly reduced body weight(Reference Nymo, Coutinho and Torgersen35, Reference Zinchenko and Henselmans73).
Non-resting energy expenditure
Thermic effect of feeding
The thermic effect of feeding, representing the energy expended above RMR due to the energy cost of digestion and storage of food(Reference Westerterp74), represents 10–15 % of an individual's total EE(Reference MacLean, Bergouignan and Cornier55). During periods of negative energy balance, there is some evidence that the thermic effect of feeding decreases for the same given meal(Reference Roberts, Fuss and Heyman75, Reference Luscombe, Clifton and Noakes76). For instance, after an 11-week hypoenergetic diet (protein supplement modified fast) that led to about 12 kg weight loss, five obese women showed a decrease of about 19·1 % in the thermic effect of feeding in response to a fixed meal (60 % of RMR) over a 300 min period(Reference Bessard, Schutz and Jequier77). However, after removing one outlier (57·5 % reduction), only a 9·8 % decrease was observed (i.e. 20·9 kg (5kcal)–41·84 kJ (10 kcal)). Furthermore, after 20 d energy deficit (3179·8 kJ (760 kcal) daily) in ten young and nine older men, a decrease of 13 and 23·6 %, respectively, was observed after measuring the thermic effect of feeding for 4 h in response to a meal comprising 25 % of total daily EI(Reference Roberts, Fuss and Heyman75). Even though there may be a decrease in the thermic effect of feeding during periods of negative energy balance, the extent to which changes in the thermic effect of feeding contribute to resistance to weight loss and promote weight gain is unclear. Furthermore, it should be noted that depending on meal composition, measuring the thermic effect of feeding for <6 h may give an incomplete estimate(Reference Reed and Hill78).
Non-exercise activity thermogenesis and non-exercise physical activity
As with the thermic effect of feeding, changes in physical activity (or the EE associated with such activity) during periods of negative energy balance are yet to be fully understood. In such situations, it is important to distinguish between metabolic and behavioural adaptations. For instance, non-exercise activity thermogenesis refers to the EE of a determined activity(Reference Levine, Eberhardt and Jensen79). However, quantifying the number of steps or the amount of time spent in sedentary or vigorous activities, for example, refers to non-exercise physical activity. This is an important distinction because non-exercise activity thermogenesis is not a volitional component, while non-exercise physical activity levels could be influenced by behaviour change interventions.
An increase in non-exercise activity thermogenesis has been observed during periods of overfeeding with weight gain ranging from 1·4 to 7·2 kg after 8 weeks in an energy surplus of 4184 kJ (1000 kcal)/d(Reference Levine, Eberhardt and Jensen79). A decrease in non-exercise physical activity and non-exercise activity thermogenesis during periods of energy deficit has also been observed(Reference Weigle80, Reference Racette, Schoeller and Kushner81), but a recent systematic review indicated that the majority of evidence does not support a significant reduction in non-exercise physical activity with weight loss(Reference Silva, Judice and Carraca82). It could be that as observed by Levine(Reference Levine, Eberhardt and Jensen79) during phases of overfeeding where some individuals were more resistant to body fat storage, large variability between individuals is present during periods of underfeeding making individuals more resistant or responsive to weight loss. In line with this, Reinhardt(Reference Reinhardt, Thearle and Ibrahim83) reported that the change in EE following a 24 h period of either fasting or overfeeding (200 %) was associated with weight loss during a subsequent 6-week period of dietary energy restriction. A smaller reduction in 24 h EE during fasting, and a larger response to overfeeding, was found to be associated with greater weight loss over the 6 weeks. These findings led the authors to suggest that individuals could be categorised as displaying either ‘thrift’ or ‘spendthrift’ EE phenotypes, with spendthrift individuals losing more weight during the intervention as they displayed an attenuated reduction in EE during weight loss. An increase in muscular efficiency (i.e. lower EE for the same activity) has also been observed following weight loss(Reference Müller, Enderle and Pourhassan34–Reference Rosenbaum, Vandenborne and Goldsmith37). For instance, a 26·5 % increase in muscular efficiency during a graded cycle ergometer protocol was observed after 10 % weight loss(Reference Rosenbaum, Vandenborne and Goldsmith37). However, whether between-subject differences in muscular efficiency following weight loss contribute to resistant or susceptible weight loss phenotypes is unclear.
Impact of metabolic adaptations on energy intake
While a compensatory change in one component of total daily EE during negative energy balance may have limited impact on subsequent weight loss, compensation in multiple components of total daily EE may exert stronger influence. It is also plausible that changes in EE may be accompanied, or indeed, trigger responses in eating behaviour. Marked weight loss is associated with both a decrease in EE and an increase in orexigenic drive(Reference Doucet and Cameron84, Reference Keys, Brožek and Henschel85). In the past it was thought that the long-term metabolic influences on EI were mainly due to changes in FM and peripheral leptin concentrations(Reference Perry and Wang86). A reduction in leptin is thought to promote increased hunger and EI via a down-regulation in pro-opiomelancortin and α-melanocyte-stimulating hormone expression, and an up-regulation in neuropeptide Y and agouti-related protein expression(Reference Sainsbury and Zhang87). There is also limited evidence in human subjects that changes in fasting leptin concentrations are associated with changes in subjective appetite(Reference Doucet, Imbeault and St-Pierre88–Reference Heini, Lara-Castro and Kirk90) and food reward(Reference Hopkins, Gibbons and Caudwell91) during dietary and exercise-induced weight loss, respectively. Exogenous leptin administration in a weight-reduced state also reverses the adaptive suppression of multiple metabolic, autonomic and neuroendocrine functions(Reference Rosenbaum, Hirsch and Gallagher92, Reference Rosenbaum, Goldsmith and Haddad93), and potentially improves satiety(Reference Kissileff, Thornton and Torres94).
Changes in appetite-related peptides during weight loss may also act as physiological cues for increased EI during energy deficit. Decreased concentrations of anorexigenic hormones and increased concentrations of orexigenic hormones following short-term energy deficit (2–7 d), which would favour an increase in EI, have been observed with and without concomitant reductions in body weight(Reference Mars, de Graaf and de Groot38, Reference Chin-Chance, Polonsky and Schoeller95–Reference Dubuc, Phinney and Stern98). Furthermore, an increase in the orexigenic hormone ghrelin(Reference Cummings, Weigle and Frayo99), and a reduction in the fasting(Reference Sumithran, Prendergast and Delbridge100–Reference Essah, Levy and Sistrun104) and postprandial(Reference Sumithran, Prendergast and Delbridge100, Reference Valderas, Irribarra and Boza103–Reference Adam, Jocken and Westerterp-Plantenga105) concentrations of the anorexigenic hormones cholecystokinin, peptide YY and glucagon-like peptide-1 have been reported following longer term dietary weight loss. Limited evidence also suggests that these changes in appetite-related peptides may persist in the weight-reduced state(Reference Sumithran, Prendergast and Delbridge100, Reference Nymo, Coutinho and Eknes106, Reference Iepsen, Lundgren and Holst107), with Sumithran et al.(Reference Sumithran, Prendergast and Delbridge100) reporting that 8 % weight loss, induced by a very low energy diet, led to persistent changes circulating appetite-related hormones and increased hunger 12 months after weight loss. However, persistent changes in appetite-related peptides during weight loss maintenance are not always reported(Reference Adam, Lejeune and Westerterp-Plantenga108). Taken together, these metabolic responses to weight loss appear to create a ‘biological pressure’(Reference MacLean, Blundell and Mennella109) that promotes increased EI and weight regain. However, it is now increasingly recognised that the energetic demand of metabolically active tissue(Reference Javed, He and Davidson110) and metabolic processes also creates a functional drive to eat(Reference Blundell, Caudwell and Gibbons20). This tonic drive from metabolic energy needs acts alongside the tonic inhibition arising from leptin and insulin and the acute modulating influence of episodic gut peptides in the overall expression of appetite and food intake.
Functional associations between body composition, energy expenditure and food intake
A conceptual model highlighting a drive to eat based on energy needs has previously been proposed(Reference Blundell, Goodson and Halford111), but only now are studies beginning to fully recognise EE and its main determinants (e.g. body composition and activity-related EE) as important excitatory features of homeostatic appetite control. Interestingly, previous research had already reported that lean tissues were associated with EI and hunger(Reference Lissner, Habicht and Strupp22, Reference Cugini, Salandri and Cilli112). Almost 30 years ago, Lissner et al. (Reference Lissner, Habicht and Strupp22) observed that EI was associated with lean mass, but not FM, while Cugini et al. (Reference Cugini, Salandri and Cilli112) reported 10 years later the potential role of FFM in the control of appetite by observing that hunger sensations were positively associated to FFM, but negatively to FM (a finding consistent with other research demonstrating an inhibitory effect of FM on appetite through the action of leptin that promotes a reduction in hunger and EI(Reference Zanchi, Depoorter and Egloff113–Reference Schwartz, Seeley and Zeltser115)).
More recently, several studies have observed associations between FFM and EI, with higher levels of FFM associated with greater EI in individuals at or close to energy balance(Reference Blundell, Caudwell and Gibbons20, Reference Weise, Hohenadel and Krakoff21, Reference Hopkins, Finlayson and Duarte23, Reference Caudwell, Finlayson and Gibbons24, Reference Blundell, Finlayson and Gibbons54, Reference Piaggi, Thearle and Krakoff116, Reference Cameron, Sigal and Kenny117). For instance, after 12 weeks of imposed aerobic exercise (five sessions per week), a positive association was observed between self-selected meal size and daily EI with FFM both at baseline and post-intervention in fifty-eight individuals (β = 0·33, P < 0·01 and β = 0·28, P < 0·02, respectively). Interestingly, there were no correlations between meal size or EI and FM or BMI. This result is confirmed by the findings of Cameron(Reference Cameron, Sigal and Kenny117), in which after adjusting for age, sex, height and physical activity, FFM (β = 21·9, P = 0·007) and skeletal muscle (β = 25·8, P = 0·02), but not FM, were predictors of EI in 304 post-pubertal adolescents. Additionally, Weise et al. (Reference Weise, Hohenadel and Krakoff21) observed an association between FFM index and daily EI in 184 individuals. The relationship between FFM and EI seems to be mediated by RMR(Reference Hopkins, Finlayson and Duarte41) (Fig. 3), suggesting that the association between FFM and EI is primarily due to the energetic demand (EE) that it creates in terms of energy turnover. Additionally, Piaggi et al. (Reference Piaggi, Thearle and Krakoff116) observed that the association between FFM and EI was mediated by total daily EE (P = 0·01, partial R 2 = 7 %), indicating EE per se may exert influence over food intake. However, given skeletal muscle's role as an endocrine organ, specific molecular signalling pathways linking FFM to appetite and EI cannot be ruled out.

Fig. 3. Path diagram for the mediation model with the standardised parameter coefficients for the direct effects of fat mass and fat-free mass on RMR and RMR on energy intake, the indirect effect of fat mass and fat-free mass on energy intake mediated by RMR and the squared multiple correlations (R 2) for RMR and energy intake (adapted from Hopkins et al.(Reference Hopkins, Finlayson and Duarte18)). ** P<0·01, *** P<0·0001; NS, non-significant.
Do changes in fat-free mass or energy expenditure act as an orexigenic signal during weight loss?
While the aforementioned studies indicate robust associations between FFM, RMR and EI under conditions of energy balance, these data are typically cross-sectional in nature and do not provide evidence of the mechanisms that drive EI during weight loss or gain. While evidence is limited at present, associations between changes in FFM and EI have been reported during periods of weight change. For example, during Ancel Key's Minnesota semi-starvation experiment(Reference Keys118), a group of thirty-two healthy individuals went through a period of 24 weeks of semi-starvation (about 25 % weight loss), followed by 12 weeks of controlled re-feeding and 8 weeks of ad libitum re-feeding. Twelve of these participants completed all phases of this intervention. During the 8 weeks of ad libitum re-feeding a significant hyperphagic response was observed (n 12), which only abated after FFM was completely restored. Interestingly, there was evidence of ‘fat overshoot’ in which FM increased significantly above baseline values. This observation is not exclusive to this intervention. For instance, after losing approximately 12 % of initial bodyweight, Nindl et al. (Reference Nindl, Friedl and Frykman119) also observed a hyperphagic response in ten healthy young men until FFM levels were restored. However, even though this restoration of FFM was noted at week 5, it was accompanied by an above baseline increase in FM. This happens because after a period of underfeeding, restoration seems to be faster for FM than for FFM. Additionally, in a more recent intervention(Reference Vink, Roumans and Arkenbosch120), after 5 weeks of a very-low-energy diet or 12 weeks of a low-energy diet, there was a significant association between percentage of FFM loss during the weight loss phase and weight regain (r 0·325, P = 0·018).
Although there is a renewed interest in the role of FFM and its associated energetic demand on food intake, the idea that lean tissue exerts influence over appetite and food intake has been previously suggested, e.g. the protein-stat(Reference Millward121) and aminostatic(Reference Mellinkoff, Frankland and Boyle122) theories of appetite regulation, respectively. Millward's protein-stat theory suggests lean mass, and in particular skeletal muscle, is tightly regulated and that food intake (dietary protein) is directed to meet the needs of lean tissue growth and maintenance(Reference Millward121). This theory is based on the existence of an ‘aminostatic’ feedback mechanism in which food intake is adjusted in response to amino acid availability to meet the protein demands of lean tissue growth and maintenance. When coupled with the metabolic demand for fuel, Millward suggests that appetite control allows substrate intake to match overall nutrient demand(Reference Millward121). However, evidence to date to support such a feedback mechanism remains limited. As noted by Stubbs et al.(Reference Stubbs, Hopkins and Finlayson48), there are also some interesting parallels between the differential recovery trajectories of FM and FFM and the hyperphagia seen during the Minnesota study, and the changes in whole body ‘catch-up growth’ in undernourished children (i.e. repletion of body weight for a given growth trajectory). When a child's individualised pattern of growth is impeded by malnutrition (or infection), a period of catch-up growth is typically observed in body weight for height and height for age(Reference Ashworth and Millward123). Of note though, catch-up growth in body weight for height occurs before any catch-up growth in height for age is seen, and the catch-up growth in body weight for height is accompanied by a marked increase in appetite and EI that subsequently declines once a normal body weight for height is achieved(Reference Ashworth and Millward123).
These data suggest that while FFM may influence the control of EI due to its effect on energy requirements, it is also possible that there could be feedback signalling between deficits in FFM and appetite control (as a means of increasing EI in attempt to restore FFM levels). However, a challenge in this area is to reconcile the differing relationships between FFM and EI under conditions of energy balance and energy deficit (see Stubbs et al.(Reference Stubbs, Hopkins and Finlayson48) or Dulloo et al. (Reference Dulloo, Miles-Chan and Schutz124) for a detailed discussion), and to identify the signalling pathways that link EE and EI. Notwithstanding, these data linking FFM and EE to hunger and EI may have relevance in the design of weight loss and weight loss maintenance strategies, with emphasis placed on the importance of preserving FFM during periods of energy restriction. Preservation of FFM during periods of energy restriction (via greater protein intakes(Reference Helms, Zinn and Rowlands125, Reference Jäger, Kerksick and Campbell126), slower weight loss rates(Reference Vink, Roumans and Arkenbosch120) and performing exercise(Reference Stiegler and Cunliffe127) for example) might help offset the increase in orexigenic drive seen with weight loss, but to date, this remains speculative and more data are needed in order to fully comprehend the impact of metabolic adaptations on appetite and EI during periods of negative energy balance.
Cross-talk between energy expenditure and energy intake: implications for weight loss?
Given the apparent cross-talk between components of EE and EI, it is plausible to suggest that some individuals may demonstrate co-ordinated adaptive metabolic (EE) and behavioural (EI) responses during energy deficit that act synergistically to attenuate perturbations to energy balance. In other words, people who show greater than predicted decreases in RMR may also present with a greater hyperphagic response following negative energy balance. In a particular case, even though average weight loss was small (−1·3 kg, range = −7·7 to +3·8 kg), Hopkins et al.(Reference Hopkins, Gibbons and Caudwell11) observed a negative association between the extent of adaptive thermogenesis and ad libitum EI (r −0·45; R 2 = 0·20, P = 0·01). These findings support those of Tremblay et al.(Reference Tremblay, Royer and Chaput72) who showed a strong positive association between adaptive thermogenesis and hunger (r 0·73, P < 0·05) after reanalysing the data from a previous study where fifty-four overweight women followed an energy-restricted diet (about −2929 kJ (−700 kcal)/d) for 4 months(Reference Gilbert, Drapeau and Astrup128) leading to a mean weight loss of about 5 % (−4 kg). These responses would favour the defence of body weight rather than promoting weight loss, and may contribute to the inter-individual variability seen in weight loss. While the underlying mechanisms still need to be determined, common biological signals such as leptin have been causally implicated in adaptive thermogenesis and compensatory appetite responses following energy deficit, and support the previously mentioned distinction between resistant and susceptible individuals suggested by Reinhardt et al. (Reference Reinhardt, Thearle and Ibrahim83). Regarding the thermic effect of feeding, some authors have observed associations between this EE component and appetite or EI(Reference Ravn, Gregersen and Christensen129). Since protein has a greater thermic effect of feeding (20–30 % in comparison to 0–3 % for fat and 5–10 % for carbohydrates)(Reference Tappy130) and impact on satiety(Reference Astrup131) in comparison to the remaining macronutrients, this component of total EE might be associated with appetite control. However, a meta-analysis failed to support any link between the thermic effect of feeding and satiety(Reference Ravn, Gregersen and Christensen129).
It could be postulated that some individuals could be more resistant to weight loss (and prone to weight gain), presenting greater co-ordinated behavioural and metabolic responses that oppose weight loss and weight loss maintenance. If a ‘weight loss resistance’ phenotype exists, it could potentially be characterised by a greater than predicted decrease in RMR, as well a smaller thermic effect of feeding for the same meal and EE for the same activity (i.e. greater muscular efficiency). Additionally, these responses could act in a synergistic way with greater increases in hunger and appetite, as well as lower satiety and satiation, prompting an individual to regain lost weight. However, more data incorporating a multi-component analysis assessing changes in body composition, EE, appetite and EI are needed to fully comprehend the cross-talk in the energy balance system and determine whether distinct phenotypes are present. Identification of inter-individual variability in compensation during the initial stages of an intervention may act as a marker of longer term success, but whether the identification of such phenotypes leads to more personalised and efficacious weight loss interventions remains unclear.
Conclusions
Even though the regulation of energy balance appears simple when considered in relation to thermodynamic theory, i.e. energy in v. energy out, energy balance is a highly complex dynamic system involving multiple feedback signals from individual components of EE and EI. Under conditions of energy deficit, and to a lesser extent energy surfeit, individual components of energy balance can act in a co-ordinated fashion to resist perturbations elsewhere in the system. The strength of these metabolic and behavioural compensatory responses appears to be individually subtle, and in part, underlie the heterogeneity seen in body weight responses to weight loss interventions. The potency of such compensatory mechanisms means that effective strategies that promote sustained weight loss and weight loss maintenance have proved remarkably elusive to date. While it is clear that individuals differ in the susceptibility to weight loss (and their subsequent ability to sustain this lower body weight), robust predictors of treatment response remain elusive.
While biological reductionism and a failure in innate biological regulatory mechanisms often dominates discussions around the putative causes of weight gain, psychological and behavioural aspects of energy balance are of equal importance when trying to account for overconsumption. Indeed, there is a renewed interest in integrative models of energy balance regulation that consider the dynamic relationships between body structure, physiological function and the way these interactions influence key psychological and behavioural determinants of energy balance such as appetite. Recent research has focused on the functional associations between components of body composition, EE and EI, and indicates that FFM and RMR are associated with a tonic drive to eat that reflects the energetic demands of metabolically active tissue. Future research should examine how the functional relationships between body composition, appetite and EI operate during periods of negative energy balance, and the implications that changes in body composition and EE have on appetite control and EI.
Acknowledgements
The authors would like to acknowledge Professor John Blundell and Professor R. James Stubbs for their theoretical input when writing this paper.
Financial Support
None.
Conflicts of Interest
None.
Authorship
All authors contributed to the writing and editing of the manuscript.