Introduction
The monopisthocotylean ectoparasite Gyrodactylus von Nordmann, 1832 (Gyrodactylidae) is a species-diverse genus containing > 500 species found on 20 bony fish orders (Harris et al. Reference Harris, Shinn, Cable and Bakke2004; Kritsky et al. Reference Kritsky, Ali and Khamees2013; Rahmouni et al. Reference Rahmouni, Seifertová and Šimková2023). Owing to a direct life cycle and a relatively high host-specificity, infection and transmission of Gyrodactylus species are highly host-dependent (Bakke et al. Reference Bakke, Cable and Harris2007). Some species, such as G. salaris Malmberg, 1957 and G. cichlidarum Paperna, 1968, are harmful and are noted to cause substantial economic losses in aquaculture (Abdel-Latif and Khafaga Reference Abdel-Latif and Khafaga2020; Harris et al. Reference Harris, Bachmann, Bakke, Aas, Klemetsen, Einum and Skurdal2011).
The Qinghai-Tibet Plateau (QTP) is widely recognized as the “roof of the world” because of its average altitude exceeding 4,000 m (Peng et al. Reference Peng, Ho, Zhang and He2006). Schizothoracinae (Cypriniformes: Cyprinidae) and Triplophysa species (Cypriniformes: Nemacheilidae) are the dominant species of fish fauna in this region (Wu and Tan Reference Wu and Tan1991). The fish genus Triplophysa Rendahl, 1933 is one of the largest groups (with approximately 160 species) in the family Nemacheilidae, and 60% of the species are distributed in the rivers and lakes on the QTP and its adjacent regions of China (Sheraliev and Peng Reference Sheraliev and Peng2021; Zheng et al. Reference Zheng, Du, Chen and Yang2009).
In neighboring Central Asian countries bordering the QTP, 12 species of Gyrodactylus have been described thus far on Triplophysa species (Ergens and Allamuratov Reference Ergens and Allamuratov1972; Ergens and Karabekova Reference Ergens and Karabekova1980; Ergens and Kartunova Reference Ergens and Kartunova1991; Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009), including: G. parvus Bychowsky, 1936, G. kessleri Gvosdev & Martechov, 1953, G. luckyi Ergens, 1970 and G. incognitus Ergens & Gusev, 1980 collected from T. strauchi Kessler, 1874; G. afghanensis Ergens, 1979 and G. moraveci Ergens, 1979 from T. griffithi Günther, 1868; G. karatagensis Ergens & Allamaturov, 1972 and G. tibetanus Dzhalilov, 1980 collected from T. stoliczkae Steindachner, 1866; G. nemachili Bychowsky, 1936, G. paranemachili Ergens & Bykhovsky, Reference Ergens and Bykhovsky1967, G. pseudonemachili Ergens & Bykhovsky, Reference Ergens and Bykhovsky1967 and G. gvosdevi Ergens & Kartunova, Reference Ergens and Kartunova1991 from T. dorsalis Kessler, 1872.
Species identification of Gyrodactylus in earlier times is mainly based on the morphological features of the haptoral hard parts, especially the shape of the marginal hooks, which has proven to be a valuable character to distinguish closely related Gyrodactylus species (Malmberg Reference Malmberg1970; Ziętara and Lumme Reference Ziętara and Lumme2004). Gyrodactylus species on related hosts usually share marginal hooks resemblance (Huyse et al. Reference Huyse, Audenaert and Volckaert2003), such as rugiensis group species on marine gobies (Huyse and Volckaert Reference Huyse and Volckaert2002). For well over 25 years, Gyrodactylus has been identified using the molecular markers nuclear internal transcribed spacer region of ribosomal DNA (ITS rDNA), which encompasses ITS1-5.8S-ITS2. This indicates that ITS rDNA is an excellent tool for taxonomy and interspecific relationship inference (Cunningham Reference Cunningham1997; Lumme et al. Reference Lumme, Ziętara and Lebedeva2017; Pinacho-Pinacho et al. Reference Pinacho-Pinacho, Calixto-Rojas, García-Vásquez, Guzmán-Valdivieso, Barrios-Gutiérrez and Rubio-Godoy2021). Currently, there is no molecular data available for the description of Gyrodactylus species in Triplophysa hosts. In view of this, we applied morphometric and molecular methods to describe three new Gyrodactylus species on Triplophysa fish in Qinghai-Tibet Plateau, China.
Materials and Methods
Parasites collection and fixation
The fish Triplophysa orientalis Herzenstein, 1888 (one specimen) was collected from a wetland nearby Cuona Lake (Coordinates 32.14479N, 91.43786E; altitude 4553 m; temperature 3 °C), Anduo County, Naqu Prefecture, China on May 10, 2021. The first sampling site was in northern Tibet in the subcold monsoon climate zone, with an annual average temperature of –0.9 to –3.3 °C and a rainfall range of 100 to 200 mm (http://www.naqu.gov.cn/nqsrmzf/c100074/list_tt.shtml). Triplophysa sellaefer Fang, 1941 (10 specimens), T. scleroptera Herzenstein, 1888 (24 specimens) and T. robusta Kessler, 1876 (15 specimens) were collected from Lanzhou Reach of the Yellow River (Coordinates 36.42335N, 103.39658E; altitude about 2000 m; temperature 8 °C), Yongdeng County, Lanzhou City, China on October 14, 2021. The second sampling site was in the northeastern margin of QTP in the temperate continental climate zone, which has an annual average temperature of 10.9 °C and a rainfall of 300 mm (https://www.lanzhou.gov.cn/col/col5/index.html). The fish, upon collection, were anaesthetized with 0.02% MS-222, and their body surface were examined for gyrodactylids using a stereoscopic microscope Stemi SV6/AxioCam MRc5 (Zeiss, Oberkochen, Germany); worms collected using fine-pointed forceps. Gyrodactylids were fixed in 75% alcohol, haptors and bodies of some fixed specimens were cut using a sharp scalpel for morphological and molecular identification.
Morphological analyses
Specimens were fixed in glycerine and ammonium picrate (GAP) (Ergens Reference Ergens1969) and photographed using Axioplan 2 imaging and Axiophot 2 (Zeiss). The body and dorsal bar were measured according to Christison et al. (Reference Christison, Shinn and Van As2005); the hamulus, ventral bar, and marginal sickle were measured according to Shinn et al. (Reference Shinn, Hansen, Olstad, Bachmann and Bakke2004); and the marginal hook filament loop was measured according to Malmberg (Reference Malmberg1970). All the measurements were given in micrometers (μm). The type-material was remounted in Canada balsam and deposited in the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan City, Hubei province, China.
Principal component analysis was used to assess the morphological differences between the three new species using 11 haptoral sclerite morphological traits (hamulus total length, hamulus shaft length, hamulus point length, hamulus root length, hamulus aperture distance, marginal hook total length, marginal hook shaft length, marginal hook sickle length, marginal hook sickle proximal width, marginal hook sickle distal width and marginal hook aperture) (Shinn et al. Reference Shinn, des Clers, Gibson and Sommerville1996). The analyses were based on the covariance matrix and performed in the R package “vegan” using log-transformed data (R Core Team Reference R Core2017).
DNA extraction, amplification, and sequencing
Genomic DNA was extracted from the worm body using a Tissue Cell Genome Kit (TIANGEN, Beijing, China) following the manufacturer’s instructions. A partial sequence of the 18S rDNA gene was amplified using the forward primer PBS18SF (5′-CGC GCA ACT TAC CCA CTC TC-3′) and reverse primer PBS1863R (5′-CAA AGG GCA GGG ACG TAT TCA GCA CA-3′) (Gilmore et al. Reference Gilmore, Cone, Lowe, King, Jones and Abbott2012). The region of rDNA spanning the 5′ end of partial sequences of the internal transcribed spacer 1 (ITS1), 5.8S rDNA, ITS2, and the 5′ end of partial sequence of the 28S rDNA gene was targeted using primers BD1 (5′-GTC GTA ACA AGG TTT CCG TA-3′) and BD2 (5′-TAT GCT TAARTT CAG CGG GT-3′) (Luton et al. Reference Luton, Walker and Blair1992). The cox1 was amplified with cox1F (5′-TAG CNG CDG GNA THA CHA TG-3′) and cox1R (5′-GGD TTA CCD CGH CGW GTW TG-3′) primers (Jin et al. Reference Jin, Li, Cheng, Li, Wu, Zou and Wang2022). Polymerase chain reaction (PCR) amplification was conducted using LA Taq polymerase (Sangon Biotech, Shanghai, China) as follows: 5 min at 94 °C as the initial step; then 35 cycles of 30 s at 94 °C, 30 s at 50 °C, 1 min at 72 °C, and the final step being 7 min at 72 °C. After purification by SanPrep Column PCR Product Purification Kit (Sangon Biotech), PCR products were sequenced with the primers described previously that were produced by Sangon Biotech and assembled manually with the help of DNAStar’s SeqMan software (DNASTAR, Madison, WI).
Molecular analysis
The obtained sequences of 18S, ITS (ITS1-5.8S-ITS2), and cox1 were deposited in GenBank and compared using BLAST on the NCBI database (https://www.ncbi.nlm.nih.gov/).
ITS sequences ( Table 1) for molecular analysis were chosen based on similarity and hamulus resemblance, then imported into PhyloSuite (Xiang et al. Reference Xiang, Gao, Ivan, Lei, He, Zhang, Zou, Wang and Zhang2023 ; Zhang et al. Reference Zhang, Gao, Ivan, Zou, Zhang, Li and Wang2020) and aligned with other available Gyrodactylus spp. in GenBank by MAFFT 7.149 (Nakamura et al. Reference Nakamura, Yamada, Tomii and Katoh2018) using the “G-INS-I” strategy and normal alignment mode. Poorly aligned segments were trimmed using Gblocks (Talavera and Castresana Reference Talavera and Castresana2007); parameters were set according to the previous research (Jin et al. Reference Jin, Li, Cheng, Li, Wu, Zou and Wang2022). The uncorrected p-distance among the three new species was calculated based on the aligned ITS sequences using Mega X (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018). Gyrodactylus rugiensis Gläser, 1974 and G. rugiensoides Huyse & Volckaert, Reference Huyse and Volckaert2002 from subgenus G. (Paranephrotus) were used as outgroups. Phylogenetic analyses based on the ITS sequences were performed using Maximum Likelihood methods. Based on the Akaikeʼs information criterion, as implemented in ModelFinder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017), TVM+F+G4 was chosen as the best-fitting model for nucleotide evolution. Phylogenies were inferred using IQ-TREE (Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, Von Haeseler and Lanfear2020).
Table 1. List of Gyrodactylus ITS rDNA sequences used in this study

Results
Taxonomic summary
Family Gyrodactylidae van Beneden and Hesse, 1864.
Genus Gyrodactylus von Nordmann, 1832.
Subgenus G. (Limnonephrotus) Malmberg, Reference Malmberg1970.
Species group G. nemachili Prikrylova et al. 2008.
Gyrodactylus triplorienchili n. sp. (Fig. 1, Table 2)

Figure 1. Light micrographs (A-E) and line drawings (F-L) of Gyrodactylus triplorienchili n. sp. A, central hook complex; B, ventral bar; C, dorsal bar; D-E, marginal hook; F, hamulus-bar complex; G, hamulus; H, dorsal bar; I, ventral bar; J-K, marginal hook; L, male copulatory organ. Scale bars: A-D, F-L 10 μm; E 5 μm.
Table 2. Morphometric parameters of Gyrodactylus triplorienchili n. sp., G. yellochili n. sp. and G. triplsellachili n. sp
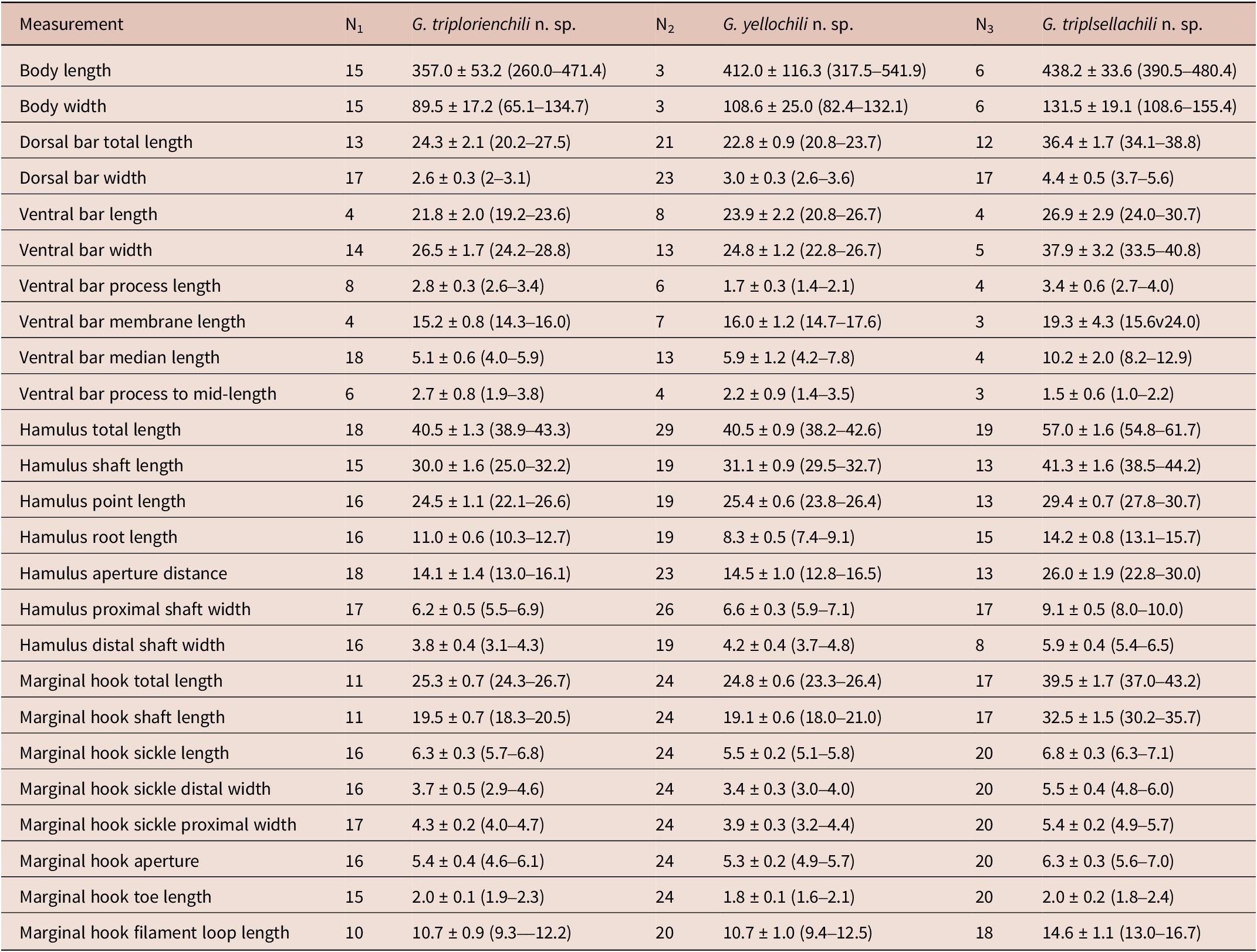
N, the number of gyrodactylid specimens measured.
Description: Morphological measurements were conducted on 22 specimens. Body length 357.0 (260.0–471.4; n = 15), width 89.5 (65.1–113.1; n = 15). Head had a pair of cephalic lobes and spike sensilla. Pharynx campaniform with anterior (smaller) and posterior pharyngeal bulb, total length 34.4 (32.2–35.5, n = 4), width 28.0 (23.7–33.3, n = 4). Male copulatory organ posterior to pharynx 18.1 (16.0–19.7, n = 5) in diameter, armed with a single large central spine with two medium spines on each side and eight smaller spines opposite to the central spine. Hamulus total length 40.5 (38.9–43.3; n = 18); hamulus shaft length 30.0 (25.0–32.2; n = 15), proximal shaft width 6.2 (5.5–6.9; n = 17), distal shaft width 3.8 (3.1–4.3; n = 16); hamulus point length 24.5 (22.1–26.6; n = 16); hamulus root heavily bent, length 11.0 (10.3–12.7; n = 16); hamulus aperture distance 14.1 (13.0–16.1; n = 18). Ventral bar width 26.5 (24.2–28.8; n = 14), total length 21.8 (19.2–23.6; n = 4); ventral bar process broad and triangular, length 2.8 (2.6–3.4; n = 8); process-to-mid length 2.7 (1.9–3.8; n = 6), median length 5.1 (4.0–5.9; n = 18); ventral bar membrane extended “V” shape, length 15.2 (14.3–16.0; n = 4). Dorsal bar straight without any decoration, total length 24.3 (20.2–27.5; n = 13), width 2.6 (2.0–3.1; n = 17). Marginal hook total length 25.3 (24.3–26.7; n = 11); shaft length 19.5 (18.3–20.5; n = 11); sickle length 6.3 (5.7–6.8; n = 16); sickle shaft slender, steeply sloping; sickle point exceeded the toe, slightly curved downward; sickle proximal width 4.3 (4.0–4.7; n = 17); distal width 3.7 (2.9–4.6; n = 16); sickle base flat with quadrate heel and long triangular toe, toe length 2.0 (1.9–2.3; n = 15), with spike on top; marginal hook aperture 5.4 (4.6–6.1; n = 16); filament loop length 10.7 (9.3–12.2; n = 10).
Type host: Triplophysa orientalis Herzenstein, 1888.
Type locality: A wetland near Cuona Lake (32.14479N, 91.43786E), Salween River, Anduo County, NaQu Prefecture at northern Tibet, China.
Infection site: Caudal fin.
Type material: The type materials were fixed in GAP, dehydrated in graded alcohol, and mounted in Canada balsam. Holotype (TO-GA 202101) and five paratypes (TO-GA 202102-202106) are deposited in the Museum of the Institute of Hydrobiology, Chinese Academy of Sciences, China.
Zoobank registration: urn:lsid:zoobank.org:act:DFF5B644-C63E-47A7-B9D5-D4F59CE4165D
Molecular marker: ITS rDNA (OP793877) comprises ITS1 (548 bp), 5.8S (157 bp) and ITS2 (390 bp). The partial 18S (OP793865) length 1200 bp. The partial cox1 (OP787151) length 1257 bp, with 99.6–100% sequence identity. A BLASTn search of the 5.8S sequence in GenBank revealed 100% identity with G. papernai Ergens & Bychowsky, Reference Ergens and Bykhovsky1967 (AF484533), G. nemachili (OQ641770) and G. tayshirensis Lebedeva, Ziętara, Mendsaikhan, Ermolenko & Lumme, Reference Lebedeva, Ziętara, Mendsaikhan, Ermolenko and Lumme2023 (OQ641774) from Nemacheilidae fish, G. mongolicus Ergens & Dulmaa, 1970 (OQ641768), G. cf. lagowskii (OQ672253) and G. cf. mantshuricusi (OQ672248) from Cyprinidae fish, G. zavkhanensis Lebedeva, Ziętara, Mendsaikhan, Ermolenko & Lumme, Reference Lebedeva, Ziętara, Mendsaikhan, Ermolenko and Lumme2023 (OQ641773) from salmonids, and G. gymnodiptychi Zhang, Hao, Arken, Rong, Tian, Kadir & Yue, Reference Zhang, Hao, Arken, Rong, Tian, Kadir and Yue2023 (MH445968) from Schizothoracinae fish. The ITS rDNA sequence exhibited 94.7% identity with G. papernai, and cox1 showed 85.1% identity with G. kobayashii Hukuda, 1940 (NC_030050).
Etymology: The specific epithet was derived from the first five letters of the generic name and the first five letters of the species name of the type host, “Triplophysa orientalis”, which ended with “chili” indicating the hamulus root inturning feature. The combined name was “triplorienchili”.
Remarks: Gyrodactylus triplorienchili n. sp. was the first Gyrodactylus species reported on Triplophysa species in Tibet. The most distinctive feature of this species was hamulus root inturning, which was typically appeared on nemachili group species (Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008) such as G. incognitus, G. paranemachili, G. pseudonemachili, G. karatagensis, G. gvosdevi and G. nemacheili that were collected from Triplophysa fish at high-altitude areas in central Asia (Ergens and Kartunova Reference Ergens and Kartunova1991), or G. tayshirensis, G. mongolicus, and G. pavlovskyi Ergens & Bychowsky, 1967 collected from Barbatula spp. in Mongolia; G. papernai Ergens & Bychowsky, Reference Ergens and Bykhovsky1967 and G. jiroveci Ergens & Bychowsky, Reference Ergens and Bykhovsky1967 collected from Barbatula spp. in Europe (Lebedeva et al. Reference Lebedeva, Ziętara, Mendsaikhan, Ermolenko and Lumme2023; Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008). The middle of the marginal hook sickles of G. nemacheili, G. jiroveci, G. incognitus, and G. pavlovskyi had a severe curvature, and the sickle point was comparatively longer than the shaft section. Whereas in G. triplorienchili n. sp., sickles were slender, which made it similar to G. nemacheili, G. paranemachili, G. karatagensis and G. pseudonemachili. However, G. triplorienchili n. sp. could be distinguished from those species listed previously by different sickle base and toe shape. Hamulus total length of G. triplorienchili n. sp. (38.9–43.3) was longer than G. nemacheili (36–38) and G. karatagensis (32–34), similar with G. paranemachili (36–42). The marginal hook total length of G. triplorienchili n. sp. (24.3–26.7) was longer than that of the G. nemacheili (20–22) and G. paranemachili (17–18) but shorter than that of the G. karatagensis species (31–33) (Ergens and Allamuratov Reference Ergens and Allamuratov1972; Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009).
Gyrodactylus yellochili n. sp. (Fig. 2, Table 2)

Figure 2. Light micrographs (A-E) and line drawings (F-L) of Gyrodactylus yellochili n. sp. A, central hook complex; B, ventral bar; C, dorsal bar; D-E, marginal hook; F, hamulus-bar complex; G, hamulus; H, dorsal bar; I, ventral bar; J-K, marginal hook; L, male copulatory organ. Scale bars: A-C, E-I, K-L 10 μm; D, J 5 μm.
Description: Morphological measurements were conducted on 32 specimens. Body length 412.0 (317.5–541.9; n = 3), width 108.6 (82.4–132.1; n = 3). Male copulatory organ 17.5 (15.8–19.2, n = 2) in diameter and armed with a single large central spine, two medium spines on each side, and six smaller spines opposite the central spine. Hamulus total length 40.5 (38.2–42.6; n = 29); shaft length 31.1 (29.5–32.7; n = 19), proximal shaft width 6.6 (5.9–7.1; n = 26), distal shaft width 4.2 (3.7–4.8; n =19); hamulus point length 25.4 (23.8–26.4; n = 19); hamulus root heavily bent, length 8.3 (7.4–9.1; n = 19); hamulus aperture distance 14.5 (12.8–16.5; n = 23). Ventral bar width 24.8 (22.8–26.7; n = 13), total length 23.9 (20.8–26.7; n = 8); ventral bar process broad and triangular, length 1.7 (1.4–2.1; n = 6); process-to-mid length 2.7 (1.9–3.8; n = 6), median length 5.9 (4.2–7.8; n = 13); ventral bar membrane extended in a “U” shape, length 16.0 (14.7–17.6; n = 7). Dorsal bar straight without any decoration, total length of 22.8 (20.8–23.7; n = 21), width 3.0 (2.6–3.6; n = 23). Marginal hook total length 24.8 (23.3–26.4; n = 24); shaft length 19.1 (18.0–21.0; n = 24); sickle length 5.5 (5.1–5.8; n = 24); sickle shaft slender, steeply sloping; sickle point exceeded the toe, slightly curved downward; sickle proximal width 3.9 (3.2–4.4; n = 24); distal width 3.4 (3.0–4.0; n = 24); sickle base flat with quadrate heel and long triangular toe, toe length 1.8 (1.6–2.1; n = 24); instep with a spike; marginal hook aperture 5.3 (4.9–5.7; n = 24); filament loop length 10.7 (9.4–12.5; n = 20).
Type host: Triplophysa scleroptera Herzenstein, 1888.
Other hosts: Triplophysa sellaefer Fang, 1941.
Type locality: Lanzhou Reach of the Yellow River (36.42335N, 103.39658E), Lanzhou City, China.
Infection site: Fins and skin.
Type material: Type materials were fixed in GAP, dehydrated in graded alcohol, and mounted in Canada balsam. Holotype (GQ-GH-202201) and five paratypes (GQ-GH-202202-202206) are deposited in the Museum of the Institute of Hydrobiology, Chinese Academy of Sciences, China.
Zoobank registration: urn:lsid:zoobank.org:act:41077872-68FE-462E-AEFF-D7785EE56516
Molecular marker: ITS rDNA (OP793878) comprises ITS1 (570 bp), 5.8S (157 bp) and ITS2 (413 bp). The partial 18S (OP793867) length 1242 bp. The partial cox1 (OP787152) length 1171 bp. The 5.8S sequence was identical to G. triplorienchili n. sp. and G. triplsellachili n. sp. A BLASTn search of the ITS rDNA and 18S sequence in GenBank revealed 96.9% and 99.9% identity with G. triplorienchili n. sp., respectively.
Etymology: The specific epithet was derived from the sampling location “Yellow river”, then ended with “chili”, which indicates the inturning hamulus root feature. Thus, the combined names were “yellochili”.
Remarks: Gyrodactylus yellochili n. sp. was the second Gyrodactylus species reported on Triplophysa fish in China. The sampling location of G. yellochili n. sp. was 1200 km distant away from G. triplorienchili n. sp. The two species could be easily confused because both have a slender marginal hook sickle, quadrate sickle heel, and long triangular sickle toe. The hamulus and marginal hook size in G. yellochili n. sp. was also similar to G. triplorienchili n. sp. (hamulus total length, 38.2-42.6 vs. 38.9-43.3; marginal hook total length, 23.3–26.4 vs. 24.3–26.7). However, G. yellochili n. sp. was shorter than G. triplorienchili n. sp. in hamulus root (7.4–9.1 vs. 10.3–12.7), marginal hook sickle (5.1–5.8 vs. 5.7–6.8) and ventral bar process (1.4–2.1 vs. 2.6–3.4). The marginal hook sickle point in G. yellochili n. sp. was blunt and not exceeding the sickle base toe. In G. triplorienchili n. sp., sickle point was sharp and exceeded the sickle base toe. Middle part of the ventral bar was smooth in G. yellochili n. sp., but it was pleated in G. triplorienchili n. sp.
Gyrodactylus triplsellachili n. sp. (Fig. 3, Table 2)
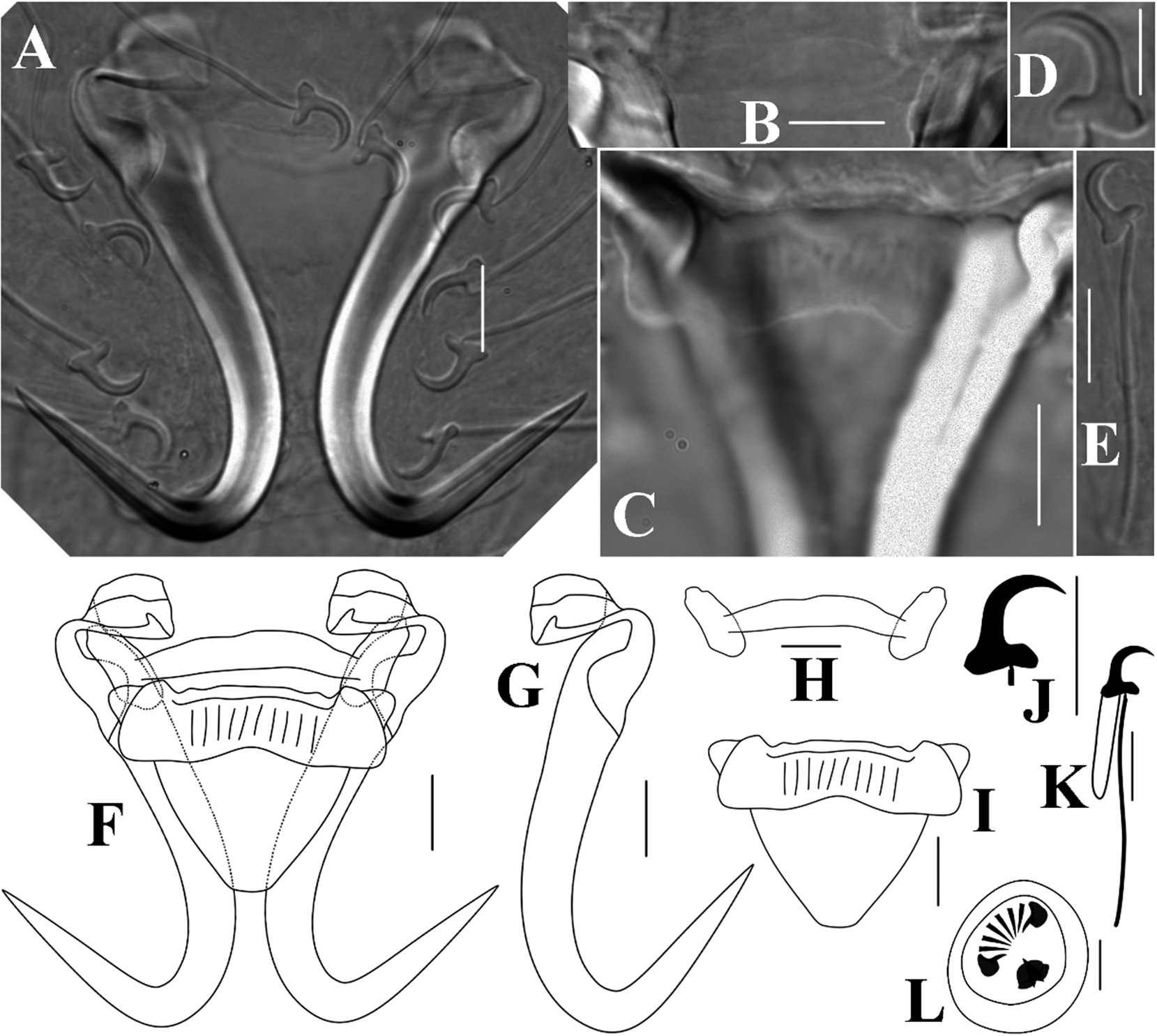
Figure 3. Light micrographs (A-E) and line drawings (F-L) of Gyrodactylus triplsellachili n. sp. A, central hook complex; B, ventral bar; C, dorsal bar; D-E, marginal hook; F, hamulus-bar complex; G, hamulus; H, dorsal bar; I, ventral bar; J-K, marginal hook; L, male copulatory organ. Scale bars: A-C, E-L 10 μm; D 5 μm.
Description: Morphological measurements were conducted on 22 specimens. Body length 438.2 (390.5–480.4; n = 6), width 131.5 (108.6–155.4; n = 6). Male copulatory organ diameter 26.6 (n = 1), armed with a single large central spine, two medium spines on each side, and six smaller spines opposite the central spine. Hamulus total length 57.0 (54.8–61.7; n = 19); shaft length 41.3 (38.5–44.2; n = 13), proximal shaft width 9.1 (8.0–10.0; n = 17), distal shaft width 5.9 (5.4–6.5; n = 17); hamulus point length 29.4 (27.8–30.7; n = 13); hamulus root heavily inturning, inturning part warping on each side, length 14.2 (13.1–15.7; n = 15); hamulus aperture distance 26.0 (22.8–30.0; n = 13). Ventral bar dumpy, width 37.9 (33.5–40.8; n = 5), total length 26.9 (24.0–30.7; n = 4); ventral bar process broad and triangular, length 3.4 (2.7–4.0; n = 4); process-to-mid length 1.5 (1.0–2.2; n = 3), median length 10.2 (8.2–12.9; n = 4); ventral bar membrane extended in a “V” shape, length 19.3 (15.6–24.0; n = 3). Dorsal bar slightly curved in the middle part, without any decoration, total length 36.4 (34.1–38.8; n = 12), width 4.4 (3.7–5.6; n = 17). Marginal hook huge, total length 39.5 (37.0–43.2; n = 17); shaft length 32.5 (30.2–35.7; n = 17); sickle length 6.8 (6.3–7.1; n = 20); sickle shaft thick, proximal part approximately perpendicular to the base and middle part rapidly curved downward; sickle point far exceeding the sickle base toe; sickle proximal width 5.4 (4.9–5.7; n = 20); distal width 5.5 (4.8–6.0; n = 20); sickle base flat with quadrate heel and trapeziform toe, toe length 2.0 (1.8–2.4; n = 20); marginal hook aperture 6.3 (5.6–7.0; n = 20); filament loop length 14.6 (13.0–16.7; n = 20).
Type host: Triplophysa sellaefer Fang, 1941.
Other hosts: Triplophysa robusta Kessler, 1876.
Type locality: Lanzhou Reach of the Yellow River (36.42335N, 103.39658E), Lanzhou City, China.
Infection site: Fins and skin.
Type material: Type materials were fixed in GAP, dehydrated in graded alcohol, and mounted in Canada balsam. Holotype (GQ-GC-202201) and five paratypes (GQ-GC-202202-202206) are deposited in the Museum of the Institute of Hydrobiology, Chinese Academy of Sciences, China.
Zoobank registration: urn:lsid:zoobank.org:act:FCE32B1F-BB26-4C00-946B-114F66BB7E7D
Molecular marker: ITS rDNA (OP793876) comprises ITS1 (571 bp), 5.8S (157) and ITS2 (409 bp). The partial 18S (OP793865) length was 1241 bp. The partial cox1 (OP787150) length was 1165 bp. The 5.8S sequence was identical to G. triplorienchili n. sp. and G. yellochili n. sp. The ITS rDNA and 18S sequence exhibited 95.4% and 99.8% identity with G. yellochili n. sp., respectively.
Etymology: The specific epithet was derived from the first five letters of the generic name and the first five letters of the species name of the type host “Triplophysa sellaefer”, then end with “chili”, which indicates the bent hamulus root feature. The combined names became “triplsellachili”.
Remarks: Gyrodactylus triplsellachili n. sp. was the third Gyrodactylus species collected from Triplophysa fish in China, the same location as G. yellochili n. sp. It could be easily distinguished from G. yellochili n. sp. by different opisthaptor size (hamulus total length, 54.8–61.7 vs. 38.2–42.6; marginal hook total length, 37.0–43.2 vs. 23.3–26.4). Both G. triplsellachili n. sp. and G. papernai had thick sickle shafts and elongated sickle point. Whereas in G. triplsellachili n. sp., sickle point smoothly curved and sickle base with quadrate heel and blunt toe. In G. papernai, sickle point sharply curved and sickle base with round heel and triangular toe (Fig. 4). Compared to other large opisthaptor size species in nemachili group such as G. papernai, G. triplsellachili n. sp. showed equal hamulus shaft length (38.5–44.2 vs. 38.0–43.0), but longer in marginal hook total length (37.0–43.2 vs. 26.5–31.5) (Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008).

Figure 4. Comparison of marginal hook sickle and hamulus-bar complex between Gyrodactylus triplorienchili n. sp., G. yellochili n. sp. and G. triplsellachili n. sp. and the morphologically similar species; G. nemacheili, G. pseudonemachili, G. pavlovskyi, G. paranemachili and G. karatagensis from Ergens and Kartunova (Reference Ergens and Kartunova1991); G. jiroveci and G. papernai and from Přikrylová et al. (Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008). Scale bars: 10 μm.
Morphometric analysis
Data for 11 morphometric features from 11 specimens of G. triplorienchili n. sp., 14 specimens of G. yellochili n. sp. and 10 specimens of G. triplsellachili n. sp. were included in the principal components analysis. The first principal component explained 87% of the data variability, to which the second component contributed 1.4%. The first component was associated with the total length of the hamulus root, marginal hook sickle, and marginal hook shaft length and marginal hook aperture. Symbols in the principal component analysis diagram indicated that the three new species could be well distinguished (Fig. 5).

Figure 5. Principal component analysis plot of the 11 measurements on the opisthaptoral hard parts among G. triplorienchili n. sp., G. yellochili n. sp. and G. triplsellachili n. sp.
Molecular analysis
The three new species formed a monophyletic group (99 of bootstrap) with G. papernai, G. jiroveci and G. pseudonemachili* (the asterisk represents that the name was previous attached to an ITS rDNA barcode AJ567674 but proved to be different from the type species G. pseudonemachili) from European Barbatula barbatula; G. pseudonemachili, G. nemachili, G. tayshirensis, G. mongolicus and G. zavkhanensis from Mongolian Oreoleuciscus spp. and Barbatula spp.; G. gymnodiptychi from Xinjiang region Gymnodiptychus dybowskii, all belonging to the nemachili group. G. triplorienchili n. sp. formed a sister clade (100 of bootstrap) with G. yellochili n. sp. and exhibited a relatively far phylogenetic relationship with G. triplsellachili n. sp. (Fig. 6A). The genetic distances among Gyrodactylus triplorienchili n. sp., G. yellochili n. sp. and G. triplsellachili n. sp. varied from 2.9 to 5.3% for ITS rDNA and 14.4 to 16.7% for cox1 (Fig. 6B).

Figure 6. Molecular analysis of the nemachili group species, i.e., phylogenetic position inferred using the ITS rDNA sequences (A); and genetic distance inferred using the ITS rDNA sequences (lower left) and cox1 (upper right) (B). Node numbers represent the bootstrap values. China-QTP and China-XJ represent the location Qinghai-Tibet Plateau and Xinjiang Region in China, respectively. Cox1 data for Gyrodactylus mongolicus (OQ661870) and G. pseudonemachili (OQ661866) sourced from Lebedeva et al. (Reference Lebedeva, Ziętara, Mendsaikhan, Ermolenko and Lumme2023).
Discussion
Three new Gyrodactylus species, including G. triplorienchili n. sp., G. yellochili n. sp., and G. triplsellachili n. sp. have been described on Triplophysa fish in the Qinghai-Tibet Plateau, China. These new species possessed the notable feature of “hamulus root inturning”, a typical characteristic in nemachili group species (Ergens and Bykhovsky Reference Ergens and Bykhovsky1967; Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008). Although G. yellochili n. sp. and G. triplsellachili n. sp. were collected from the same location (Yellow River), nevertheless, the latter had smaller opisthaptoral hard parts. Interestingly, G. yellochili n. sp. morphologically resembled G. triplorienchili n. sp., which was collected at a considerably higher altitude: i.e., both had long straight hamulus points, thin marginal hook sickle, and long triangular sickle toe. Multivariate analysis based on hamulus and marginal hooks suggested that the three new species could be effectively separated. The hamulus root and marginal hook sickle in G. triplorienchili n. sp. were longer than that of G. yellochili n. sp. Previous studies on specific Gyrodactylus species (Geets et al. Reference Geets, Appleby and Ollevier1999; Mo Reference Mo1991; Reference Mo1993) had documented a tendency for longer hamulus roots in colder water, whereas the marginal hook sickle was stable. For the two similar species described, the former feature variation might reflect difference in geographical and climatic conditions of their hosts. The varied features of the latter indicated that they belong to two distinct species. Morphological similarities among the three new species were further confirmed by molecular data, with an almost identical 18S sequence (99.8–99.9% similarity) among them. Genetic distance (p-distance for ITS rDNA) between G. triplorienchili n. sp. and G. yellochili n. sp. (2.9%) is closer than that between G. triplorienchili n. sp. and G. triplsellachili n. sp. (5.3%) but exceeding the 1% ITS rDNA sequence difference, which was suggested as a threshold for Gyrodactylus species delimitation (Ziętara and Lumme Reference Ziętara and Lumme2002).
The three new species showed relatively low interspecific differences of 2.9–5.3% (p-distance for ITS rDNA), which is below the difference among other nemachili group members found in Europe on Barbatula barbatula L. 6.5–8.2%, or higher than the difference between G. pseudonemachili and G. nemachili found in Mongolia on Barbatula sp. 2.3% (Fig 6B). In other Gyrodactylus species, sequence variation (Kimura 2P distances) within the parasite genus ranged from 2.7 to 56% and 1.5 to 38.7% for ITS1 and ITS2, respectively (Matejusová et al. Reference Matejusová, Gelnar, McBeath, Collins and Cunningham2001), and species differences (HKY distances for ITS1-ITS2) of the Gyrodactylus within host genus Pomatoschistus varied from 2.5 to 16.5% (Huyse and Volckaert Reference Huyse and Volckaert2002). The low interspecific genetic differences reflect the tight phylogenetic relationship among nemachili group species, and their host stone loaches (including Triplophysa and Barbatula species) show a similar pattern, which include numerous morphologically and molecular-related species, with of the majority of them being concentrated in a plateau in Central Asia and adjacent regions (Li et al. Reference Li, Wang, Jin, Li, Yan, Yan, Zhang, He and Song2017; Tang et al. Reference Tang, Liu, Mayden and Xiong2006; Wang et al. Reference Wang, Shen, Feng, Zhao, Song, Zhang, Yang and He2016, Reference Wang, Zhang, Yang, Liu and Du2020).
Compared to other species groups, such as wageneri group and rugiensis group (Huyse and Volckaert Reference Huyse and Volckaert2002; Malmberg Reference Malmberg1970) defined mainly based on the shape of the marginal hook sickle, nemachili group species showed higher shape consistency in hamulus, but less shape consistency in marginal hooks (Fig. 4). Sickle distal was smoothly curved in G. triplorienchili n. sp., G. yellochili n. sp., G. triplsellachili n. sp., G. nemachili, G. mongolicus, G. pseudonemachili, G. paranemachili and G. kataragensis. This sickles pattern can also be found in G. tokobaevi Ergens & Karabekova, Reference Ergens and Karabekova1980, G. aksuensis Ergens & Karabekova, Reference Ergens and Karabekova1980, G. llewellyni Ergens & Dulmaa, 1967, G. osoblahensis Ergens, 1963 and G. gracilihamatus Malmberg, 1964 parasitic on Cyprinidae fish (Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). Contrarily, the sickle point was sharply curved in G. jiroveci, G. zavkhanensis, G. papernai and G. pavlovskyi. Similarly, sickle pattern can also be found in the following eight species, parasitic on Cobitidae, Nemacheilidae, Cyprinidae, and Salmonidae: G. ajime Nitta, Reference Nitta2021, G. asiaticus Ergens, 1978, G. cobitis Bychowsky, 1933, G. dzhalilovi Ergens & Ashurova, 1984, G. mantschuricus Ergens & Yukhimenko, 1977, G. pewzowi Ergens, 1980 and G. tincae Malmberg, 1957 (Nitta Reference Nitta2021). Also, species within the nemachili group did not cluster based on their sickle shape; rather, they were scattered across the phylogenetic tree. Those molecularly closely related members such as G. triplorienchili n. sp. and G. yellochili n. sp., or G. nemachili, G. tayshirensis, G. mongolicus and G. zavkhanensis (fig. 6B) show greater morphology differences compared to the cryptic species such as G. pakan and G. teken (4.1–4.3% p-distance for ITS1-ITS2 rDNA) (Razo-Mendivil et al. Reference Razo-Mendivil, García-Vásquez and Rubio-Godoy2016), and G. sphinx and G. gerasevi (1.3% p-distance for ITS rDNA) (Dmitrieva et al. Reference Dmitrieva, Sanna, Vodiasova, Prokhorova, Casu, Burreddu, Piras, Garippa and Merella2022).
Most species within the nemachili group were found on stone loach (Přikrylová et al. Reference Přikrylová, Matějusová, Jarkovský and Gelnar2008). Although some members, such as G. mongolicus and G. pseudonemachili were collected from Oreoleuciscus humilis Warpachowski, 1889 (Cyprinidae), G. zavkhanensis was collected from Thymallus brevirostris Kessler, 1879 (Salmonidae) in Mongolia (Lebedeva et al. Reference Lebedeva, Ziętara, Mendsaikhan, Ermolenko and Lumme2023). G. gymnodiptychi was found on Gymnodiptychus dybowskii Kessler, 1874 (Cyprinidae) in Xinjiang, China (Zhang et al. Reference Zhang, Hao, Arken, Rong, Tian, Kadir and Yue2023). Considering that there are geographical distribution overlaps between the aforementioned host species and stone loach, the appearance of nemachili group species on non-stone loach host is likely due to host switching (Ziętara and Lumme Reference Ziętara and Lumme2002). Genetically, G. gymnodiptychi is relatively far from other members (see Fig 6B), suggesting that host switching is an important trigger for diversification in Gyrodactylus (Boeger et al. Reference Boeger, Kritsky and Pie2003). In addition, the “inturning hamulus root” feature can also be found in many Gyrodactylus under saline conditions, such as G. orecchiae Paladini, Cable, Fioravanti, Faria, Di Cave & Shinn, Reference Paladini, Cable, Fioravanti, Faria, Di Cave and Shinn2009 on Sparus aurata Linnaeus, 1758 (Sparidae) (Paladini et al. Reference Paladini, Cable, Fioravanti, Faria, Di Cave and Shinn2009), G. chileani Ziętara, Lebedeva, Muñoz & Lumme, Reference Ziętara, Lebedeva, Muñoz and Lumme2012 on Helcogrammoides chilensis Cancino, 1960 (Tripterygiidae) (Ziętara et al. Reference Ziętara, Lebedeva, Muñoz and Lumme2012), and G. amphibious Lebedeva, Muñoz & Lumme, Reference Lebedeva, Muñoz and Lumme2021, G. scartichthi Lebedeva, Muñoz & Lumme, Reference Lebedeva, Muñoz and Lumme2021, G. viridae Lebedeva, Muñoz & Lumme, Reference Lebedeva, Muñoz and Lumme2021 and G. zietarae Lebedeva, Muñoz & Lumme, Reference Lebedeva, Muñoz and Lumme2021 on clingfsh Sicyases spp. (Gobiesocidae) (Lebedeva et al. Reference Lebedeva, Muñoz and Lumme2021). They are not closely related to nemachili group species; therefore, it is possible that hamulus root inturning is a result of morphology convergence under extreme conditions.
Acknowledgements
The authors thank Mr. Fanglin Chen, Mr. Guangran Hu, and Mr. Hongpeng Lei for their assistance in collecting parasite samples.
Availability of data and material
Data are available from the authors upon reasonable request.
Author contribution
X.J.: fish examination, parasite collection, parasitologic analysis, manuscript writing; W.L., M.L., Z.D., and H.Z.: fish examination, parasite collection; W.L. and G.W.: designed the study; W.L., J.C. and K.A.: manuscript writing, review and editing. All authors have reviewed and approved the manuscript for publication.
Funding
This study was supported by the program for scientific research start-up funds of Guangdong Ocean University (060302022309) and the Earmarked Fund for CARS (CARS-45).
Competing interest
The authors declare no conflict of interest.
Ethical approval
The authors assert that the animal studies were conducted in accordance with the ethical standards and approved by the Animal Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
Declarations
Consent for participation
All authors have approved the final version of the manuscript and consent to participate in publishing this manuscript in this journal.
Consent for publication
The authors of this manuscript unequivocally state that this manuscript is original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part, and that all the authors consent to publishing this manuscript.










