Depression is a major cause of disability, with depressive disorders thought to affect over 260 million people worldwide(Reference James, Abate and Abate1). This is particularly evident in adolescents and young adults, with unipolar depression being the most common contributor to disability between the ages of 10 and 24 years(Reference Gore, Bloem and Patton2). Maintaining a healthy diet, including a diet high in fruit and vegetable intake, may reduce risk of depression and is associated with a decrease in depressive symptoms(Reference Khalid, Williams and Reynolds3–Reference Lassale, Batty and Baghdadli9). Meta-analysis of randomised controlled trials consisting of whole of diet interventions has shown significantly reduced depressive symptoms with dietary intervention(Reference Firth, Marx and Dash10). Additionally, intervention studies focusing on specific food groups such as fruits, vegetables and wholegrains show potential to reduce severity of depressive symptoms(Reference Opie, O’Neil and Itsiopoulos11,Reference Agarwal, Mishra and Xu12) . These plant foods are often high in dietary fibre.
Observational studies have shown that adults with low-fibre diets have greater severity of depressive symptoms(Reference Gopinath, Flood and Burlutksy13–Reference Miki, Eguchi and Kurotani19). This association is seen with total fibre along with fruit, vegetable and cereal fibre. However, only one study to date has focused on adolescence(Reference Kim, Choi and Lee20) despite evidence that up to 50 % of adult mental health disorders first emerge during this period(Reference Belfer21,Reference Kim-Cohen, Caspi and Moffitt22) .
Understanding and treating mental health concerns in adolescence is particularly important due to the long-term ongoing effects associated with depression(Reference Thapar, Collishaw and Pine23). Having juvenile or adolescent depression has been associated with higher odds of adult anxiety, depression, mania and schizophreniform disorder(Reference Kim-Cohen, Caspi and Moffitt22). Even subthreshold depressive symptoms in adolescence have been associated with higher rates of major depression and suicidal tendencies in adulthood(Reference Fergusson, Horwood and Ridder24). Proximal correlates of adolescent depression include impaired school performance and attendance, and a higher likelihood of engaging in unhealthy behaviours such as smoking and binge eating(Reference Fröjd, Nissinen and Pelkonen25,Reference Glied and Pine26) . These factors associated with depression may have detrimental consequences for future employment and health. It has been suggested that early treatment of depression, including during adolescence, may improve outcomes(Reference Hetrick, Parker and Hickie27,Reference Hetrick, Cox and Witt28) .
The mechanisms by which dietary fibre may impact depression are currently unclear, but adiposity may be involved. Waist circumference and abdominal adiposity have been linked to depressive symptoms(Reference Zhao, Ford and Li29), and increased risk of mental disorders in adulthood has been associated with being overweight in childhood(Reference Sanderson, Patton and McKercher30). These relationships between adiposity and depression are likely to be bidirectional. Depression has been associated with cravings for foods high in sugar and saturated fat which may contribute to increases in adiposity(Reference Parker and Crawford31,Reference Whitaker, Sharpe and Wilcox32) . On the other hand, the release of pro-inflammatory cytokines with adiposity may play a role in the link between overweight and obesity and depression(Reference Miller, Freedland and Carney33–Reference Visser, Bouter and McQuillan35). Inflammation is also a potential mediator of the association between dietary fibre and depressive symptoms. Studies have linked a high dietary fibre intake with decreased chronic inflammation(Reference Wannamethee, Whincup and Thomas36–Reference Parikh, Pollock and Bhagatwala38) and the presence of elevated inflammation with depression(Reference Martinac, Babic and Bevanda39–Reference Dowlati, Herrmann and Swardfager43). An alternative explanation for a relationship between dietary fibre and depression may be the effects of other nutrients obtained from foods high in dietary fibre.
The direction of the relationship between dietary fibre intake and depression is unclear. Higher intake of fat and sugar dense foods which are likely to be low in dietary fibre can be seen in those with depression(Reference Parker and Crawford31,Reference Whitaker, Sharpe and Wilcox32) . However, there is evidence that people with previous depression may have healthier diets than those without previous depression(Reference Jacka, Cherbuin and Anstey44). Our hypothesis is that the population-averaged effect is in the direction from diet to depression.
Thus far, the association of dietary fibre intake with depression is an understudied area, particularly in adolescents. In our study, we aimed to seek evidence for the beneficial effect of dietary fibre intake on depressive symptoms in adolescents at 14 and 17 years of age and to investigate the impact of adiposity and inflammation on that relationship. The confounding effect of other nutrients present in high-fibre foods was considered.
Methods
Population
The Raine Study recruited 2900 pregnant women (termed Generation 1) from King Edward Memorial Hospital in Perth, Western Australia. The resulting 2868 babies (Generation 2) have been regularly followed up from birth. Detailed cohort information can be found elsewhere(Reference Straker, Mountain and Jacques45,Reference White, Eastwood and Straker46) . There were 1864 adolescents participating in the 14-year follow-up and 1726 in the 17-year follow-up. After excluding those with incomplete data on all covariates except inflammation, 1260 adolescents in the 14-year follow-up were included in principle analyses and 653 from the 17-year follow-up (Fig. 1). Of these adolescents, 531 had complete data at age 14 and 17 years after excluding those with moderate/extreme depressive symptoms at the 14-year follow-up and were used in secondary analysis. A subset of participants restricted to those with data on the inflammatory marker high-sensitivity C-reactive protein (hs-CRP) included 718 adolescents at the 14-year follow-up and 547 at the 17-year follow-up.

Fig. 1. Number of participants in the Raine Study 14- and 17-year follow-ups and number with complete data on depressive symptoms, diet and confounders included in the analysis.
Ethics
The present study was conducted according to the guidelines in the Declaration of Helsinki. Ethics approval for the Raine Study was granted by the committees of King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, Perth, Western Australia. The adolescent and their primary caregiver provided informed written consent.
Diet measurement
Dietary fibre intake and other dietary data were collected using the 212 item Commonwealth Scientific and Industrial Research Organisation (CSIRO) FFQ. The CSIRO FFQ is a semi-quantitative self-report FFQ measuring usual diet over the past year. Studies have validated the CSIRO FFQ for use in the Australian population and in Raine Study adolescents specifically(Reference Ambrosini, de Klerk and O’Sullivan47–Reference Lassale, Guilbert and Keogh49). Comparison of the CSIRO FFQ to a 3-d food record in the Raine Study at the 14-year follow-up found a moderate positive correlation for dietary fibre (r 0·43 in males and r 0·29 in females, P < 0·001 for both)(Reference Ambrosini, de Klerk and O’Sullivan47). Participants were asked to estimate frequency and quantity of intake of foods based on provided standard serving size examples (e.g. one serve of pasta is one cup of cooked pasta). The serving size and frequency of consumption of each food item were used by the CSIRO to calculate estimated daily nutrient intakes. Dietary fibre is defined as fibre obtained from whole foods (overall fibre), from cereal and grains (cereal fibre) and from fruits or vegetables (fruit and vegetable fibre). Intakes of all fibre types were divided into quartiles for analysis as decided a priori. Available nutrients associated with depressive symptoms in the literature and in this sample include potassium, Mg, Fe, Cu, Zn, thiamine, riboflavin, vitamin B6 and folate(Reference Woo, Lynn and Lau16,Reference Kim, Choi and Lee20,Reference Zhang, Ding and Chen50–Reference Jacka, Maes and Pasco52) . Dietary patterns termed ‘Healthy’ and ‘Western’ have been determined previously in the Raine Study as described elsewhere(Reference Ambrosini, Oddy and Robinson53). In brief, the 212 foods measured in the questionnaire were merged into thirty-eight food groups which were used in factor analysis to identify data-driven dietary patterns. The Healthy pattern includes consumption of high-fibre foods such as wholegrain cereals, fresh fruit, legumes and vegetables, along with steamed, grilled or canned fish. The Western pattern includes takeaway foods, red and processed meats, full-fat dairy products and other refined foods(Reference Ambrosini, Oddy and Robinson53) (see reference for full list of components).
Inflammation measurement
Serum levels of hs-CRP were used as a marker of inflammation. Participants with hs-CRP concentrations over 10 mg/l were excluded as these concentrations represent acute inflammation and the present study aimed to examine the impact of chronic inflammation (<10 mg/l). Twelve-hour fasting blood samples were taken by a trained home-visiting phlebotomist early in the morning and analysed at the PathWest Laboratory at the Royal Perth Hospital. hs-CRP was measured on an Architect c16000 analyser with an intra-assay CV of 15·96 % and a lower detection threshold of 0·15 mg/l. Values of hs-CRP were log transformed due to skewness.
Adiposity measurement
Participant height (to the nearest 0·1 cm) and waist circumference (to the nearest 0·1 cm) were measured by trained researchers at each time point and used to calculate waist:height ratio (WHtR, waist circumference (cm)/height (cm)). Studies have found that BMI is a poor indicator of body fat percentage(Reference Nuttall54), including in children and adolescents(Reference Widhalm and Schönegger55). For this reason, we chose to use WHtR as a marker of adiposity, as it has been shown to be more effective in predicting adiposity than BMI or waist circumference alone in adolescents(Reference Brambilla, Bedogni and Heo56).
Depression measurement
Depressive symptoms were assessed with the Beck Depression Inventory for Youth (BDI-Y) at the 14- and 17-year follow-ups. The BDI-Y is a self-report scale consisting of twenty questions regarding negative feelings in the previous 2 weeks(Reference Beck, Beck and Jolly57). Responses are scored as 0–3 with the possible answers of: never (0), sometimes (1), often (2) and always (3). Scores are summed, with higher total scores indicating more severe depressive symptoms(Reference Steer, Kumar and Beck58). The minimum valid completion of the BDI-Y questionnaire defined in the handbook is eighteen out of twenty questions(Reference Beck, Beck and Jolly57). Multiple imputation (predictive mean matching) was used for thirty participants who had two or fewer questions unfilled. Raw scores in the range of 0–60 are converted to T-scores standardised by age and sex. The present study uses the T-score conversion recommendations for 15–18- year-olds for consistency(Reference Beck, Beck and Jolly57). Possible scores for girls range from 38 to 97 and scores for boys range from 41 to 100. Adolescents are categorised into groups of severity of depressive symptoms using recommended cut-offs (<55: average, 55–59: mildly elevated, 60–69: moderately elevated and ≥70: extremely elevated). The groups were combined to create a binary outcome variable of those with average or mildly elevated depressive symptoms (<60) and those with moderately elevated or extremely elevated depressive symptoms (≥60), labelled as none/mild (0) and moderate/extreme (1). Research using the BDI-Y in adolescent girls indicates that this cut-off is indicative of clinically meaningful symptoms(Reference Stapleton, Sander and Stark59). Additionally, known case analysis of the adult version of the questionnaire (BDI-II) in adolescents found that cut-off scores approximately equivalent to moderate depressive symptoms had the highest clinical efficiency at identifying those who met diagnostic criteria for major depressive disorder(Reference Krefetz, Steer and Gulab60,Reference Kumar, Steer and Teitelman61) .
Confounders
Confounders plausibly associated with dietary fibre intake and depression included sex, follow-up year (as a marker of age), energy intake, binge drinking, leisure time physical activity, parental education, family income, parental history of mental health problems and family functioning. Energy intake was obtained from the CSIRO FFQ. Dietary misreporting was calculated with the Goldberg method(Reference Goldberg, Black and Jebb62), using energy intake and BMR as described elsewhere(Reference Appannah, Pot and O’Sullivan63).
Lifestyle
At both ages, data on binge drinking and leisure time physical activity were measured from a self-report survey. Adolescents were asked if they had ever drunk more than six standard drinks on the same day, with a ‘yes’ response categorised as having engaged in binge drinking on one or more occasions. Leisure time physical activity was defined as the average number of occasions per week adolescents reported spending in vigorous physical activity (causing out of breath and sweating) outside of school hours.
Parental factors
Details of family income were provided by the primary caregiver at the 14- and 17-year follow-ups. Parental education was represented by the level of educational attainment of the highest educated parent as of the 8-year follow-up, the most proximal survey of parental education to the 14- and 17-year follow-ups. Parents were asked to report in the questionnaires if they had ever been treated for an emotional or mental health problem (other than postnatal depression, yes/no). Family functioning, a measure of family communication and support, was calculated from the McMaster Family Assessment Device general functioning subscale as completed by the primary caregiver(Reference Epstein, Baldwin and Bishop64). Scores were averaged with higher scores on the general functioning subscale representing poorer family functioning. Better family functioning has been associated with greater dietary fibre intake in this population previously(Reference Swann, Breslin and Kilpatrick65) and was chosen as a proxy for family environmental factors that may influence both diet and mental health.
Analysis
Odds of moderate/extreme depressive symptoms by quartile of dietary fibre intake were determined using mixed-effects logistic regression. Although dietary fibre intake differs by sex in this population(Reference Swann, Breslin and Kilpatrick65), there was no significant interaction between sex and fibre intake (P > 0·36) and therefore sex-stratified analysis was not performed. Significant interactions were found between sex and WHtR, exercise and follow-up year. However, addition of these interaction terms to the model had no effect on the OR of depressive symptoms by fibre intake. Additionally, it was not the aim of the paper to examine how the impact of lifestyle factors differs by sex, and therefore they were not included in the final models.
The principle analysis consisted of three models. Model 1 was adjusted for sex, energy intake and dietary misreporting. Model 2 was adjusted for factors in model 1 plus WHtR (per increase of 0·1). Model 3 adjusted for factors included in model 2, plus follow-up year, binge drinking, physical activity, parental education, family income, parental history of mental health problems and family functioning. All variables except sex and parental education were included as time-varying factors across the two follow-ups. A secondary analysis was restricted to those with data at both the 14- and 17-year follow-ups. In this secondary analysis, adolescents who reported moderate/extreme depressive symptoms at the 14-year follow-up were excluded to obtain a population with incident depression at age 17 years.
Additional mixed-effects logistic regression analyses were conducted to estimate odds of depressive symptoms by quartiles of dietary fibre intake in a subset of the population with hs-CRP measurements. hs-CRP was added to the final model to test for a mediation effect of inflammation.
The suitability of adjustment for alternative nutrients associated with depression was determined by calculating Pearson’s correlation coefficients between the candidate nutrients and variance inflation factors (VIF) for the fibre coefficient. VIF can be used to measure multicollinearity or the level of correlation between variables in regression analysis. Research suggests that VIF as low as two can have an impact on results(Reference Graham66). Nutrients considered were K, Mg, Fe, Cu, Zn, thiamine, riboflavin, vitamin B6 and folate. As correlation coefficients and VIF were high for all nutrients under consideration (online Supplementary Tables S1 and S2), further analysis adjusted for Healthy and Western dietary patterns instead. Although multicollinearity exists between dietary fibre and the Healthy and Western dietary patterns, it is less severe than for individual nutrients and therefore dietary pattern adjustment was the preferred method. VIF for dietary fibre, the Healthy pattern and the Western pattern were 3·3, 2·8 and 1·4, respectively. Statistical analysis was performed with R version 3.5.0(67) and R studio version 1.2.1335(68).
Results
Characteristics of participants overall and with and without moderate/extreme depressive symptoms at ages 14 and 17 years are shown in Table 1. The proportion of adolescents who were female was higher at the 17-year follow-up compared with the 14-year follow-up (54 v. 48 %). A higher percentage of those with moderate/extreme depressive symptoms was female at both ages (80 % at age 14 years and 63 % at age 17 years). Those with moderate/extreme depressive symptoms appeared to have poorer lifestyle habits, with lower physical activity and higher rates of binge drinking. They were more likely to have lower family incomes and non-formally educated parents with a history of mental health problems. Mean dietary fibre intakes were lower in adolescents with moderate/extreme depressive symptoms at both 14 and 17 years (Table 1). The subset of the population with inflammatory data had characteristics similar to those with complete data for all variables (online Supplementary Table S3).
Table 1. Characteristics of included participants from the 14- and 17-year follow-ups of the Raine Study overall and by level of depressive symptoms* (Numbers and percentages; mean values and standard deviations)
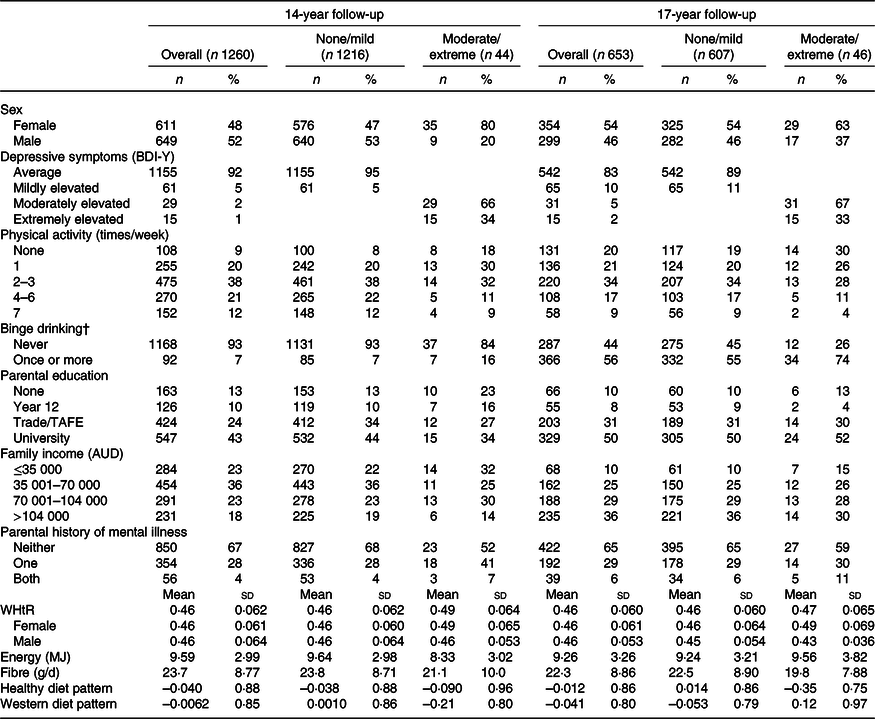
BDI-Y, Beck Depression Inventory for Youth; TAFE, technical and further education; WHtR, waist:height ratio.
* Percentages were rounded to the nearest 1 %.
† Classified as consuming more than six drinks on one occasion.
Table 2 shows the proportion of adolescents with moderate/extreme depressive symptoms at the 14- and 17-year follow-ups overall and by quartile of dietary fibre intake. The proportion of adolescents with moderate/extreme depressive symptoms overall was higher at age 17 years (7 %) than at age 14 years (3 %). The proportion of adolescents who had moderate/extreme depressive symptoms was lowest in the fourth (highest) quartile of dietary fibre intake at both 14 and 17 years.
Table 2. Proportion of participants with none/mild and moderate/extreme depressive symptoms overall and across quartiles of dietary fibre intake at the 14- and 17-year follow-ups of the Raine Study*
(Percentages)

* Moderate/extreme depressive symptoms determined by a Beck Depression Inventory for Youth T-score of >60.
Table 3 shows the OR of moderate/extreme depressive symptoms by quartile of overall fibre, cereal fibre, and fruit and vegetable fibre intake relative to the first quartile. In the first model, odds were significantly lower in the fourth quartile of both overall and fruit and vegetable fibre intake and in the third and fourth quartiles of cereal fibre intake compared with the first quartile. After adjusting for WHtR in model 2, there was negligible change in OR for any fibre type. In model 3 (further adjusted for lifestyle and family factors), significantly lower odds of moderate/extreme depressive symptoms remained in the fourth quartile of overall fibre intake compared with the first quartile (Table 3). The expanded results of model 3 in each fibre type are shown in online Supplementary Table S4. In models for overall fibre, cereal fibre, and fruit and vegetable fibre, male was associated with lower odds of moderate/extreme depressive symptoms. For all fibre types, having engaged in binge drinking one or more times and having poorer family functioning were associated with higher odds of moderate/extreme depressive symptoms (online Supplementary Table S4).
Table 3. Risk of moderate/extreme depressive symptoms per quartile of overall, cereal, and fruit and vegetable fibre intake relative to the first quartile (n 1913)*
(Odds ratios and 95 % confidence intervals)

* Comprising 1260 observations from the 14-year follow-up and 653 observations from the 17-year follow-up. Model 1 adjusted for sex, energy intake and dietary misreporting. Model 2 adjusted for model 1 + waist:height ratio. Model 3 adjusted for model 2 + binge drinking, physical activity, parental education, family income, follow-up year, parental history of mental health problems and family functioning.
OR in paired analysis restricted to those without moderate/extreme depressive symptoms at the 14-year follow-up are shown in Table 4. As in the principle analysis, odds of moderate/extreme depressive symptoms were significantly lower in the fourth quartile of overall dietary fibre intake compared with the first quartile in all models (Table 4).
Table 4. Risk of moderate/extreme depressive symptoms per quartile of overall dietary fibre intake relative to the first quartile in paired analysis restricted to those without moderate/extreme depressive symptoms at age 14 years (n 1062)*
(Odds ratios and 95 % confidence intervals)

* Comprising 531 observations from the 14-year follow-up and 531 observations from the 17-year follow-up. Model 1 adjusted for sex, energy intake and dietary misreporting. Model 2 adjusted for model 1 + waist:height ratio. Model 3 adjusted for model 2 + binge drinking, physical activity, parental education, family income, follow-up year, parental history of mental health problems and family functioning.
Further analysis was performed to adjust for Healthy and Western dietary patterns. Following adjustment for the dietary patterns, the associations with cereal and grain fibre and fruit and vegetable fibre were attenuated in all models, although OR remained consistently lower in the fourth fibre intake quartile (online Supplementary Table S5). For overall fibre, lower OR for moderate/extreme depressive symptoms in the fourth quartile of intake persisted but became non-significant in model 3 (Table 5).
Table 5. Adjusted risk of moderate/extreme depressive symptoms per quartile of overall fibre intake relative to the first quartile in all models further adjusted for dietary patterns (n 1913*)
(Odds ratios and 95 % confidence intervals)
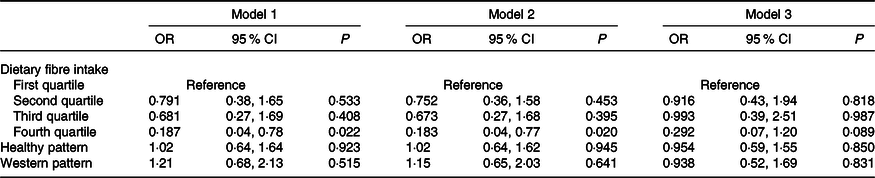
* Comprising 1260 observations from the 14-year follow-up and 653 observations from the 17-year follow-up. Model adjusted for sex, energy intake, dietary misreporting, waist:height ratio, binge drinking, physical activity, parental education, family income, follow-up year, parental history of mental health problems, family functioning, and healthy and Western dietary patterns.
Table 6 shows the results of the repeat analysis of odds of moderate/extreme depressive symptoms and overall fibre performed in the subset of participants with hs-CRP measurements. In model 3, odds of moderate/extreme depressive symptoms were significantly lower in the fourth quartile of dietary fibre intake compared with the first. The addition of hs-CRP to the final model had little impact on the OR of moderate/extreme depressive symptoms.
Table 6. Risk of moderate/extreme depressive symptoms per quartile of overall dietary fibre intake relative to the first quartile in the subset with data on inflammatory markers (n 1265*)
(Odds ratios and 95 % confidence intervals)

hs-CRP, high-sensitivity C-reactive protein.
* Comprising 718 observations from the 14-year follow-up and 547 observations from the 17-year follow-up. Model 1 adjusted for sex, energy intake and dietary misreporting. Model 2 adjusted for model 1 + waist:height ratio. Model 3 adjusted for model 2 + binge drinking, physical activity, parental education, family income, follow-up year, parental history of mental health problems and family functioning.
Discussion
Our findings suggest that adolescents with the highest intakes of overall dietary fibre have significantly lower odds of clinically relevant moderate/extreme depressive symptoms compared to adolescents with the lowest fibre intakes, before adjustment for overall diet. Although participant numbers were low, similarly lower odds of depressive symptoms were seen in analysis restricted to adolescents without moderate/extreme depressive symptoms at the 14-year follow-up. However, attenuation of the association of dietary fibre with depressive symptoms following adjustment for Healthy and Western dietary patterns suggests that the effect of dietary fibre does not occur independent of other dietary nutrients. Adjusting for hs-CRP in a subset of adolescents with inflammatory data did not alter the results.
Our study is novel in that it is the first to our knowledge to investigate the relationship of dietary fibre and depressive symptoms in an adolescent population including both females and males. Our finding that the odds of moderate/extreme depressive symptoms were lower in adolescents with the highest dietary fibre intakes before adjustment for dietary factors is consistent with previous research in adult populations(Reference Gopinath, Flood and Burlutksy13–Reference Miki, Eguchi and Kurotani19) and in adolescent girls(Reference Kim, Choi and Lee20). However, in all such observational studies, there is a possibility of confounding by overall diet. Due to its plant food origins, consumption of dietary fibre occurs with a variety of other nutrients, which may confound the relationship between dietary fibre and depressive symptoms. To our knowledge, ours is only the second study to attempt to isolate the effect of dietary fibre through adjusting for other dietary factors, with the first by Miki et al. examining depressive symptoms in an adult Japanese population(Reference Miki, Eguchi and Kurotani19). Miki et al. adjusted for intakes of folate, vitamin B6, vitamin B12, n-3 PUFA, Mg and Zn and found that the association between vegetable and fruit fibre and lower odds of depressive symptoms remained significant after nutrient adjustment(Reference Miki, Eguchi and Kurotani19).
In contrast, we chose to look at the confounding effect of previously determined dietary patterns instead of individual nutrients. Analysis of dietary patterns has been suggested as a superior method to consider the impacts of overall diet compared with individual nutrient analysis(Reference Hu69). Dietary pattern adjustment allowed us to investigate the effects of overall diet while minimising the complications of the high levels of collinearity between dietary fibre and individual nutrients(Reference McGee, Reed and Yano70). The dietary patterns used in our study were developed with factor analysis based on the CSIRO FFQ at the 14-year follow-up. The Healthy pattern includes consumption of high-fibre foods such as wholegrain cereals, fresh fruit, legumes and vegetables(Reference Ambrosini, Oddy and Robinson53). Therefore, adjusting for these patterns may underestimate the effect of fibre. Even with this in mind, substantially lower odds for moderate/extreme depressive symptoms remain in the fourth quartiles of fibre intake, though not statistically significant, suggesting that dietary fibre intake itself still contributes to lower odds of moderate/extreme depressive symptoms.
These results of diet-adjusted analysis suggest that the association between a higher dietary fibre intake and lower odds of moderate/extreme depressive symptoms may be more reflective of a high-fibre diet with all its accompanying nutrients than of an independent effect of fibre. Other possible pathways from dietary fibre intake to depressive symptoms likely occur in conjunction with overall diet.
Given the links between adiposity and depression(Reference Zhao, Ford and Li29), it is plausible that the association between a diet high in fibre and moderate/extreme depressive symptoms in our study is mediated by a reduction in adiposity. In univariable analysis, we showed that a higher fibre intake was associated with a smaller WHtR and a smaller WHtR associated with lower OR of moderate/extreme depressive symptoms. Despite this, adjustment for adiposity using WHtR did not impact on the relationship between dietary fibre intake and depressive symptoms in a multivariable model. This suggests that in adolescents, pathways other than adiposity may be involved. Sensitivity analysis adjusting for BMI instead of WHtR showed little difference in results. It may be that a more precise measure of adiposity such as fat mass or body fat percentage is required to fully capture the impact of adiposity on depressive symptoms.
Dietary fibre may impact depressive symptoms independent of overall diet through the gut microbiota. Dietary fibre remains undigested until it reaches the intestines where it is often fermented by bacteria. A high fibre intake can encourage the growth of beneficial bacteria(Reference Xu and Knight71,Reference Tap, Furet and Bensaada72) . These bacteria can produce neurotransmitters like gamma amino butyric acid and serotonin, and serotonin’s precursor tryptophan(Reference Lyte73,Reference O’Mahony, Clarke and Borre74) , low concentrations of which have been linked to the pathophysiology of depression(Reference Bell, Abrams and Nutt75,Reference Luscher and Fuchs76) . Additionally, fermentation of dietary fibre by the microbiota creates SCFA(Reference Cummings77). SCFA can inhibit histone deacetylases(Reference Sealy and Chalkley78), resulting in increased gene expression, including brain-derived neurotrophic factor(Reference Kim, Leeds and Chuang79). In a previous study of adolescents, concentrations of brain-derived neurotrophic factor, along with other neurotrophins, were significantly lower in clinically depressed adolescents than in the controls(Reference Pallavi, Sagar and Mehta80). In the same study, lower concentrations of brain-derived neurotrophic factor in the depressed adolescents were associated with greater severity of depressive symptoms. Evidence suggests that SCFA can also activate G protein-coupled receptors in the brain(Reference Tarini and Wolever81–Reference Scheppach, Pomare and Elia83), resulting in norepinephrine release(Reference Kimura, Inoue and Maeda84,Reference Inoue, Kimura and Wakabayashi85) which can have antidepressant effects(Reference Moret and Briley86).
Another potential mediator of a relationship between a high-fibre diet and depressive symptoms is inflammation. Studies have shown that healthy dietary patterns and a high dietary fibre intake associate with lower inflammation(Reference Wannamethee, Whincup and Thomas36–Reference Parikh, Pollock and Bhagatwala38,Reference Silveira, Oliveira and Andrade87) , and that high inflammation is associated with the presence of depressive symptoms(Reference Martinac, Babic and Bevanda39–Reference Dowlati, Herrmann and Swardfager43). Dietary fibre may reduce inflammation through mechanisms such as lowering circulating levels of inflammatory lipopolysaccharide by reducing intestinal membrane permeability(Reference Peng, Li and Green88) and pH(Reference den Besten, van Eunen and Groen89). High levels of lipopolysaccharide and inflammatory cytokines can alter production and re-uptake of neurotransmitters and the function of the hypothalamic–pituitary–adrenal axis(Reference Leonard and Maes90,Reference Krishnan and Nestler91) , which may contribute to depression(Reference Werner and Coveñas92). Despite this biological plausibility, our results suggest that reduced hs-CRP, as a marker of inflammation, does not mediate the relationship between dietary fibre and depressive symptoms in adolescents.
It is worth noting that the only modifiable lifestyle factor significantly associated with odds of moderate/extreme depressive symptoms in the final model was binge drinking. This is consistent with previous research linking excessive alcohol consumption to an increased risk of depression in adolescents and young adults(Reference Fergusson, Boden and Horwood93). Definitions of excessive, risky or binge drinking vary and have changed over time. Our definition of binge drinking (>6 standard drinks on the same day) differs from the current (2009) Australian National Health and Medical Research Council recommendation of <4 standard drinks per d to reduce alcohol-related health risks. Therefore, our study may underestimate rates of binge drinking. However, the National Health and Medical Research Council guidelines are designed for adults, with no level of alcohol consumption deemed safe for people under the age of 18 years, and therefore there is no formal definition of adolescent binge drinking. The other factors significantly associated with moderate/extreme depressive symptoms were female sex and poorer family functioning.
Strengths and limitations
In the present study, we used prospective data from two time points in adolescence which is a major strength, as adolescence is an age group where research has so far been lacking. The comprehensive range of data collected from both the adolescents and their parents in the Raine Study allowed adjustment for many potential confounders and testing of mediators. However, the presence of missing data meant that some participants had to be excluded from the study, and the proportion of adolescents with moderate/extreme depressive symptoms was low (n 90, 4·7 %). To investigate whether the exclusion of some participants biased the results, we performed a sensitivity analysis using inverse probability weighting. The results of the weighted analysis were consistent with the main analysis, providing some reassurance that exclusion bias did not greatly impact the results. Another strength of the study was the use of two time points, with the same measurements of diet (CSIRO FFQ) and depressive symptoms (BDI-Y) used at each age group. The CSIRO FFQ measures diet from the previous year while the BDI-Y determines depressive symptoms over the 2 weeks prior to follow-up, allowing the examination of the effect of habitual diet on current depressive symptoms. However, due to its retrospective nature the FFQ may be subject to recall bias. As the calculation of the dietary fibre content of diet is dependent on the portion sizes provided by the participant, a reference standard serving size was given for each food item in the questionnaire. To improve accuracy, the primary caregiver assisted in the completion of the FFQ at age 14 years, while it was completed by the adolescent alone at age 17 years. As such, there may have been inconsistencies in reporting between the two follow-ups.
The present study is observational; therefore, we could not confirm any biological causal mechanisms for the association of higher dietary fibre intake with lower odds of moderate/extreme depressive symptoms. Additionally, we cannot entirely rule out an effect of reverse causality between a high-fibre diet and depressive symptoms. However, our results of paired analysis show that even after excluding participants who had moderate/extreme depressive symptoms at the 14-year follow-up, the highest dietary fibre intakes were associated with lower odds of incident moderate/extreme depressive symptoms at the 17-year follow-up. Participant numbers were low (n 531, 31 with moderate/extreme depressive symptoms at 17 years), however, and therefore these results cannot be used as strong evidence. We chose not to perform longitudinal analysis using dietary fibre intake at 14 years as the exposure and depressive symptoms at 17 years as the outcome because of a lack of biological plausibility for dietary fibre intake to impact depressive symptoms 3 years later. The BDI-Y is a non-diagnostic self-report questionnaire and as such we cannot determine the association between dietary fibre intake and diagnosed clinical depression.
It is difficult if not impossible to isolate the impacts of a single nutrient on depressive symptoms. As dietary fibre is primarily sourced from fruits, vegetables and grains, there is high multicollinearity between fibre and vitamins and minerals that are also present in those foods. As such, we cannot exclude a confounding effect of other nutrients present in a fibre-rich diet. Development of a high-fibre dietary pattern may be of benefit for future research regarding the effects of dietary fibre as a component of healthy diet, rather than as an isolated nutrient.
The findings of our study suggest that a good quality, high-fibre diet, rather than a high fibre intake alone, may have potential to improve symptoms of depression in adolescents independent of adiposity and inflammation. However, our results cannot confirm causality, and further research such as randomised controlled trials is required to confirm the potential of dietary fibre to impact depressive symptoms. While studies exist on other aspects of diet and mental health in adolescents, to our knowledge, this is the first study to examine the association between dietary fibre intake and depressive symptoms in a sex-mixed adolescent cohort. More research in adolescent age groups is important due to the high incidence of mental disorders and associated potential lifelong impacts on health and lifestyle. Further research to determine the utility of dietary fibre in reducing depressive symptoms should focus on a high-fibre diet rather than simply consuming an allocated quantity of dietary fibre daily. Furthermore, increasing physical activity and discouraging excessive alcohol consumption may be additional effective targets for reducing or preventing depressive symptoms in adolescents.
Acknowledgements
We are grateful to the Raine Study participants and their families, and we thank the Raine Study research staff for cohort coordination and data collection. The core management of the Raine Study is funded by the University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. We thank the National Health and Medical Research Council of Australia (NHMRC) for their long-term contribution to funding the study over the last 30 years and the Telethon Kids Institute for long-term support of the Raine Study.
This work was supported by the Heart Foundation Beyond Blue Strategic Research Program (Oddy et al. ID G08P4036 2009-2012). The Raine Study received funding from the Raine Medical Research Foundation at The University of Western Australia, the NHMRC, the Telstra Research Foundation, the Western Australian Health Promotion Foundation and Australian Rotary Health Research Fund. We acknowledge the Telethon Kids Institute, the Commonwealth Scientific and Industrial Research Organization, the NHMRC Program Grant ID #003209 and Project Grant #211912 for supporting the 14-year follow-up. Data collection and biological specimens at the 17-year follow-up were funded by the NHMRC Program Grant ID 353514 and Project Grant #403981. T. A. M. is supported by an NHMRC Research Fellowship (ID 1136046) and A. L. is funded by an NHMRC Career Development Fellowship (#1148793).
Authors’ contributions were: O. G. S. – formulating the research question, study design, analysis, interpretation, drafting and preparation of final manuscript; M. B. – formulating the research question, study design, analysis, interpretation, drafting and review of final manuscript; M. K. and W. H. O. – formulating the research question, study design, interpretation, drafting and review of final manuscript; T. A. O., T. A. M., L. J. B. and A. L. – study design, interpretation and review of final manuscript.
All authors declare that there are no potential or existing conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003426












