INTRODUCTION
Gastrointestinal illness (GI) is an important global public health issue [Reference Bern1, Reference Kosek, Bern and Guerrant2]. In developed countries, although GI is typically mild and self-limiting, the associated burden is considerable due to high morbidity [Reference Mead3–Reference Majowicz5]. Quantifying disease burden and understanding the distribution within communities is important for resource planning and the design of preventive strategies. Estimating the burden of acute GI also may provide a basis for estimating the burden of foodborne illness [Reference Flint6]. Numerous developed countries have conducted studies on the burden of GI in the general population [Reference Herikstad7–Reference Hall15] and there are on-going international initiatives to estimate the global burden of acute GI and foodborne illness [Reference Flint6, Reference Scallan16]. While differences in study methodologies and case definitions make comparisons between studies difficult, standardization of approaches has allowed some international comparisons to be conducted [Reference Scallan16]. To estimate the burden of GI in Canada, the Public Health Agency of Canada (formerly Health Canada) developed the National Studies on Acute Gastrointestinal Illness (NSAGI) initiative in 2000. Population-based studies, designed to describe self-reported, acute GI in selected Canadian populations, are part of this initiative. In March 2002, the first such population study was piloted in the city of Hamilton (Ontario, Canada) [Reference Majowicz14]. Acute GI burden was estimated for the province of British Columbia between June 2002 and June 2003 [Reference Thomas17]. Since the province of Ontario represents about 40% of the Canadian population, the objectives of the study presented here were to estimate the burden, severity, and demographic distribution of acute GI in Ontario, Canada, and to describe health-care-seeking behaviour in individuals with acute GI.
METHODS
Study design and data collection
A retrospective, cross-sectional telephone survey of randomly selected English-speaking residents of Ontario, Canada was conducted between May 2005 and April 2006. The province of Ontario is located in central Canada, and has an estimated population of ~12·5 million, representing about 40% of the Canadian population. The population is concentrated in the southern part of the province, which consists of large urban centres surrounded by suburban and rural areas. The northern part of the province has a more widely distributed population.
Two-step sampling was used. Residential telephone numbers with area codes exclusively in Ontario were randomly sampled without replacement from a commercial telephone number database. One individual from each residence was randomly selected to participate by selecting the individual whose birthday was the next chronologically. Proxy respondents were used when the selected participant was aged <12 years, when the selected participant was aged between 12 and 18 years and their parent or guardian felt that the child would not be able to answer the questions themselves, or when the selected participant was aged >18 years and had a developmental or intellectual disability such that their parent or guardian felt that they would not be able to answer the questions themselves.
Telephone interviews were conducted by trained interviewers from the Centre for the Evaluation of Medicines (St Joseph's Hospital, Hamilton, Ontario). Residential telephone numbers were attempted five times, on different days and at different times of the day. Once an individual at a given residential telephone number was identified, five attempts were made to contact that individual. All surveys were administered in English, and data were entered using Computer Assisted Telephone Interviewing (CATI).
The final sample size of 2090 was calculated to allow a point prevalence of illness of 10% to be estimated with an allowable error of ~1·3%. Interviews were conducted over a 12-month period, with an approximately equal number of interviews completed each month.
The survey was developed by selecting questions from a pilot survey of the burden of acute GI conducted in Hamilton, Ontario in 2001 [Reference Majowicz14]. Questions solicited information on the presence or absence of vomiting or diarrhoea in the 28 days prior to the interview, the number of individuals residing in the household, demographic characteristics (age, education, total household income, gender), whether the respondent resided on a livestock farm, residential postal code, and perceived risk of illness. For individuals experiencing vomiting or diarrhoea in the previous 28 days, additional questions were asked related to secondary symptoms, perceived causes of vomiting or diarrhoea, whether other individuals in the household had experienced vomiting or diarrhoea concurrently, medical history of potential predisposing causes of illness (medical conditions or medication use), health-care-seeking behaviour, and medications taken for the illness.
The survey was pre-tested until no new changes were noted and the average time to administer was 7 min (15 min for cases, 6 min for non-cases). Ethical approval was obtained from the Research Ethics Board of McMaster University, Hamilton, Ontario.
Case definition
The case definition corresponded to that used in the pilot study [Reference Majowicz14] and the NSAGI study in British Columbia [Reference Thomas17], and is consistent with international studies using telephone survey methods [Reference Scallan16]. Individuals who reported experiencing vomiting or diarrhoea in the 28 days prior to interview were included as cases. ‘Vomiting’ was described as forcible expulsion of the contents of the stomach out of the body through the mouth, and ‘diarrhoea’ was described as stool with abnormal liquidity or any loose stool. Individuals who reported any pre-existing illnesses or conditions, as diagnosed by a medical doctor, in which vomiting or diarrhoea is a common symptom were excluded as cases but were retained in the non-case group.
Analysis
The 28-day period prevalence was defined as the number of respondents reporting at least one episode of acute GI within the 28 days preceding the interview, divided by the number of survey respondents. The point prevalence was defined as the number of individuals experiencing acute GI on the day of the interview divided by the number of survey respondents. Ninety-five percent confidence intervals (95% CI) for prevalence estimates were calculated using a binomial distribution.
The incidence rate (also called incidence density) was calculated according to the definition and formula described by Rothman & Greenland [Reference Rothman and Greenland18]. Individuals experiencing more than one episode of acute GI during the 28-day period were included only once in the numerator. Unadjusted incidence rate included all cases. Adjusted incidence rate was calculated to account for episodes of acute GI that began prior to the 28-day period but were ongoing at the start of this period. The average duration of illness (x) was used to determine the probable proportion of cases that began prior to the start of the 28-day period using the formula: [x – 1]/[28+(x – 1)], with the assumption that cases occurred evenly throughout the 28-day period. Incidence rates were adjusted by subtracting this proportion from both the numerator (number of new acute GI cases) and denominator (total population at risk).
Data were analysed in Excel 2000 (Microsoft Corporation, Redmond, WA, USA) and SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA). Individuals responding ‘don't know/not sure’ or who refused to answer a question were excluded from the analysis of that question. Descriptive statistics were used to depict disease severity, secondary symptoms, and treatments.
Demographic variables were tested for bivariable associations with acute GI using logistic regression, with the binary outcome corresponding to the case or non-case status of the individual. Gender, age, total household income, and education were coded as categorical variables. The number of individuals in the household, residence on a livestock farm, and study month also were tested as independent variables. The urban/rural status of each individual was obtained from their residential postal code using a Postal Code Conversion File (Statistics Canada, 2006), and was assessed as an independent variable. Multivariable logistic regression was performed by entering all independent variables in a model and removing them sequentially based on the Wald χ2 test until all variables remaining in the model were significant at P⩽0·05. Two-way interactions between variables significant in the final model were tested for statistical significance.
For cases, bivariable associations with accessing health care were tested using logistic regression. The dependent variable was binary and corresponded to whether or not the case visited a doctor or nurse practitioner for their illness. The independent variables evaluated were age (categorical), gender, duration of illness, and symptoms of illness. Variables were considered statistically significant at P⩽0·05.
RESULTS
Response rate and representativeness of respondents
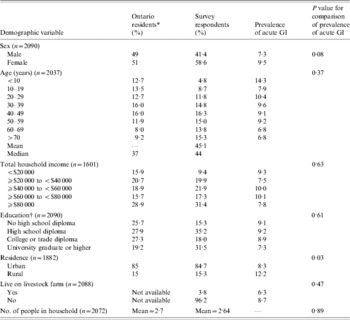
A total of 5714 telephone calls were made to obtain 2090 completed interviews, yielding a 36·6% response rate. Compared to demographic information for Ontario residents obtained from the 2001 Canadian Census (www.statcan.ca), survey respondents were older, had a higher total household income, were more likely to be female, and had a higher level of education (Table 1).
Table 1. Comparison of the demographic characteristics of all Ontario residents and 2090 randomly selected respondents to a telephone survey of acute gastrointestinal illness (GI) conducted in Ontario, Canada, 2005–2006 and bivariable associations between demographic factors and acute GI during the 28 days prior to interview
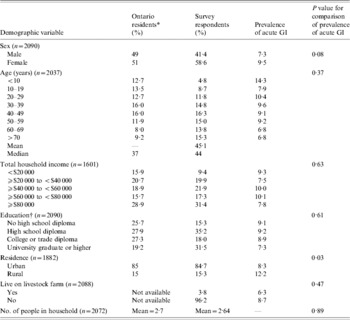
* Data obtained from 2001 Canadian Census (www.statcan.ca).
† The education variable in the Canadian census excludes individuals aged 1–19 years. For comparative purposes, survey respondents aged <20 years were not included in educational demographical descriptions in this table. For statistical comparison, education level corresponded to the respondent if aged >19 years; else for proxy.
Burden of illness
Of the 2090 respondents, 213 (10·2%) reported an episode of acute GI in the 28 days prior to interview. Of these, 34 (16·0%) had been diagnosed by a medical doctor with, or were taking medication for, a pre-existing condition for which vomiting or diarrhoea is a common symptom. These conditions included Crohn's disease, irritable bowel syndrome, lactose intolerance, and pregnancy. These 34 respondents were excluded from the case group. This yielded a total of 179 cases of self-reported acute GI, and translated into a period prevalence of 8·56% (95% CI 7·36–9·76). The point prevalence was 2·34% (95% CI 1·70–2·99). The average duration of illness of 4 days was used to estimate that 9·68% of the acute GI events that had probably begun prior to the start of the 28-day observation period. Thus, the adjusted annual incidence rate was 1·17 (95% CI 0·99–1·35) episodes of acute GI per person-year.
Demographic associations
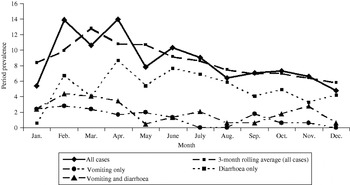
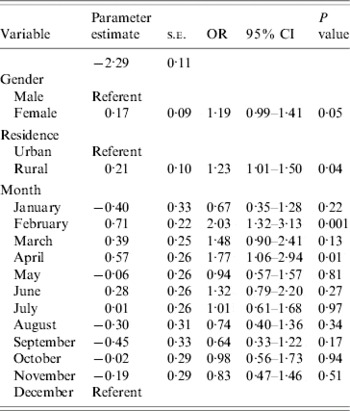
Bivariable associations between demographic factors and acute GI are shown in Table 1. Rural residents were significantly more likely to have experienced acute GI (P=0·03) and there was a higher period prevalence in females that approached statistical significance (P=0·08). Study month was significantly associated with acute GI (P=0·02), with a higher period prevalence between February and April (Fig.). The higher prevalence was primarily associated with cases experiencing diarrhoea with no vomiting. The final multivariable regression model contained the variables of gender, rural vs. urban residence, and study month (Table 2). No two-way interaction terms between these variables were statistically significant.
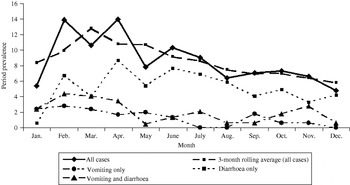
Fig. Twenty-eight-day prevalence of acute gastrointestinal illness by study month, and by primary symptom, in randomly selected residents of Ontario, Canada (n=2090).
Table 2. Multivariable associations with acute gastrointestinal illness during the 28 days prior to interview in randomly selected residents of Ontario, Canada (n=1882, respondents who did not provide information on gender or residence were excluded)
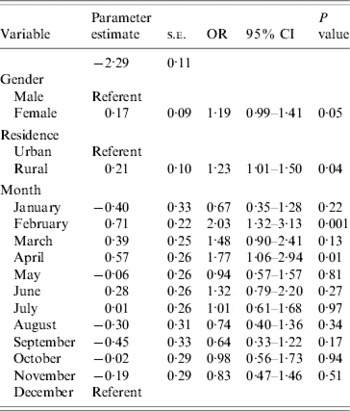
s.e., Standard error; OR, odds ratio; CI, confidence interval.
Symptoms, duration, and severity of illness
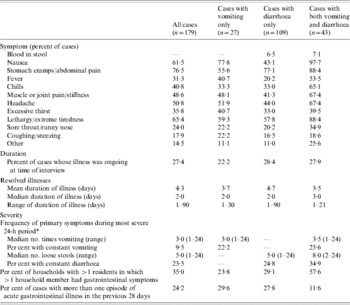
Of the 179 acute GI cases, 15% (n=27) experienced vomiting only, 61% (n=109) experienced diarrhoea only and 24% (n=43) respondents experienced both vomiting and diarrhoea (Table 3). The most common secondary symptoms were stomach cramps or abdominal pain, lethargy or extreme tiredness, nausea, and headache. For those cases experiencing diarrhoea, blood in the stool was not commonly reported as a symptom (6·7%).
Table 3. Symptoms, duration, and severity of acute, self-reported gastrointestinal illness in Ontario, Canada, May 2005–April 2006 (n=179)
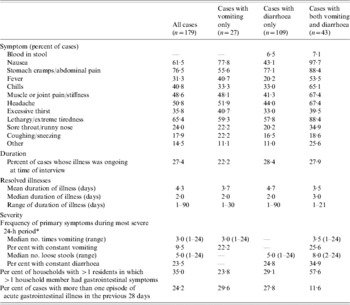
* Some individuals reported their vomiting or diarrhoea was ‘constant, all day long’; these individuals were coded as experiencing 24 bouts of vomiting or loose stool in the 24-h period.
Duration and severity of illness are described in Table 3. At the time of their interview, 27% of the cases (n=49) were still experiencing symptoms of their illness. For those whose illness had resolved, the mean duration was 4·26 days. However, the distribution of the duration of illness was right-skewed; the majority of illnesses lasted ⩽3 days. Seventy-eight percent of households had more than one resident. In these households, 35% of cases indicated that someone else in the household had similar symptoms at the same time. About one quarter of respondents (24·2%) with acute GI reported more than one episode during the 28-day period, where a new episode was defined as vomiting or diarrhoea separated from another such illness by at least 7 days.
Health-care-seeking behaviour
A physician or nurse practitioner was visited by 22% (n=40) of cases, and of these 33% (n=13) were asked to provide a stool sample, for which there was full compliance. Respondents who visited a health-care provider were asked to provide the reason for their decision to access health care. The most common reasons provided for seeking health care was because they perceived that their symptoms had persisted for a long time (55%), because they felt sick enough to go (38%) or because of their primary symptom. There were no significant associations between seeking health care and age, gender, or duration of illness. The only symptoms associated with seeking health care were the presence of blood in the stool [odds ratio (OR) 9·62, 95% CI 2·36–39·19] and experiencing excessive thirst (OR 2·14, 95% CI 1·04–4·37).
Cases who did not visit a health-care provider were asked the reason they decided not to seek health care. Of the 139 cases who did not visit a health-care provider, the predominant reason was the perception that their illness was not serious enough or that they were not sick long enough to warrant a visit (86%, n=120). Other reasons included a belief that their illness was caused by flu or a cold (6·5%, n=9), or a known cause other than flu or cold (2·2%). About 6% (n=8) cited long waiting times or not having an available health-care provider in their area or at the time of their illness as a reason for not seeking medical assistance.
Almost half of the cases that did not access a health-care provider used anti-diarrhoeal products or medications to reduce nausea or vomiting. As expected, anti-diarrhoeal product use was more common when the symptoms included diarrhoea, with or without vomiting, and medications to reduce nausea were more common when symptoms included vomiting, with or without diarrhoea. Rehydration therapies were used by 5·8% of cases who did not visit a health-care provider, with use more common in individuals experiencing both vomiting and diarrhoea. While not common, some individuals who did not visit a health-care provider reported self-administering antibiotics (2·2%).
DISCUSSION
The purpose of this study was to estimate the burden, severity, and demographic distribution of acute GI in Ontario, Canada and to describe health-care-seeking behaviour related to acute GI. Overall, acute GI represented a significant health burden in Ontario: the monthly prevalence was 8·6% and ~1·2 episodes occurred per person-year. If extrapolated to the population of Ontario (2006 population of ~12·5 million), the monthly prevalence corresponds to over a million cases of acute GI in Ontario every month. One-quarter of cases reported more than one episode of acute GI during the 28 days prior to the interview, and about one third of the households in which there was more than one person reported that additional persons in the household had similar symptoms at the same time as the selected case.
The incidence rate reported in this study (1·2 episodes per person-year) is similar to that reported in the pilot (1·3 episodes per person-year [Reference Majowicz14]) as well as to that reported in a similar study conducted in the province of British Columbia, Canada, in 2002–2003 (1·3 episodes per person-year [Reference Thomas17]). Observing similar incidences over study times and populations supports the conclusion that the incidence of acute GI in Canada is probably just over one episode per person-year. Moreover, the 28-day prevalence reported here (8·6%) is consistent with reports from other countries where similar methodologies have been used (Australia, 6·4%; Ireland, 3·4%, United States, 7·6%) [Reference Scallan16]. We used a more liberal definition of GI than some of these international studies. However, comparisons of results with the definition used in the previous Canadian studies vs. other definitions showed minimal differences in the observed prevalence [Reference Majowicz14, Reference Thomas17].
It is possible that the rate reported here is an overestimate of the true incidence of acute GI in Ontario, since estimates of incidence obtained retrospectively have been shown to be 2·8 times higher than those obtained prospectively [Reference Wheeler10]. This discrepancy has been attributed to recall bias, with respondents telescoping past illness events into the observation period [Reference Wheeler10, Reference Scheaffer, Mendenhall and Ott19], resulting in an overestimate of the true rate of disease. However, the accuracy of prospectively obtained estimates has not been determined, and it is plausible that such estimates may actually underestimate the true rate of disease. Since reporting an illness episode in past prospective studies led to a request to submit a stool sample, participants may under-report illness events to avoid having to do so [Reference Wheeler10]. However, it is possible that retrospectively obtained rates like the ones observed here may overestimate the true incidence, potentially by 2–3 times; if that is the case, the true rate in Ontario may be closer to 0·4–0·6 episodes per person-year. Further research into the accuracy and validity of both retrospective and prospective estimates is needed to address this issue. This could include prospective cohort studies which compare data collection that is, or is not, linked to sample collection, retrospective studies that employ different observational periods to calculate annual incidence, and community incidence calculations that multiply reported cases of GI by an estimate of under-reporting.
Here, females were 1·2 times more likely to experience acute GI than males, adjusting for urban/rural status and study month. This is consistent with other studies [Reference Hoogenboom-Verdegaal11, Reference Scallan13, Reference Majowicz14, Reference Hall20], in which higher rates were observed in females than males. The reasons for this increase in females may be due to biological differences, or differences in routes of exposure such as food preparation [Reference Kagan, Aiello and Larson21]. However, given that several studies observe this increased risk in females, in-depth evaluation of the specific reasons for such an increase is needed in order to implement appropriate prevention efforts aimed at decreasing the burden of disease in this sub-population.
We observed that those who lived in a rural setting were 1·2 times more likely to experience acute GI than those who lived in an urban setting, adjusting for gender and study month. This is consistent with other, pathogen-specific work done within the study area, where increased rates of E. coli O157:H7 infection [Reference Michel22] and cryptosporidiosis [Reference Majowicz23] have been observed in rural areas vs. urban areas. It is probable that risks specific to rural areas, such as exposure to livestock, manure, and untreated drinking water, contribute to this increased risk in rural areas of Ontario.
In this study, respondents interviewed in February and April were 2·0 and 1·8 times more likely, respectively, to report acute GI than those interviewed in December. This was somewhat unexpected; since the temporal distribution of GI is reported to be bimodal, with bacterial gastroenteritis tending to peak in the summer [Reference Michel22, Reference Gurwith and Williams24] and viral gastroenteritis in the winter in temperate climates [Reference Gurwith and Williams24–Reference Cook26], we anticipated higher rates in the winter and summer, rather than the winter and spring. Possible explanations for this observation may include increased illness in spring due to foreign travel, or a secondary peak in viral GI in the spring. To explore the possibility that the seasonal distribution was related to a viral aetiology, we attempted to categorize cases according to the following syndromic definitions: Salmonellosis – diarrhoea and fever with duration of ⩾3 days; ETEC infection – diarrhoea of ⩾3 days' duration with no fever or vomiting; Norovirus infection – vomiting or diarrhoea of 1–2 days' duration (data not shown). Based on these rough syndromic definitions, we calculated that there were 16 cases fitting the Salmonella profile, 21 fitting the ETEC profile, and 101 fitting the viral profile. Therefore, there were an insufficient number of cases within profiles to evaluate seasonality. However, this potential use of syndromic surveillance should be explored in future studies, in particular the sensitivity and specificity of such syndromic definitions for these types of data.
Contrary to other studies [Reference Herikstad7, Reference de Wit9, Reference Hoogenboom-Verdegaal11, Reference Scallan13, Reference Majowicz14, Reference Hall20], we did not observe an association between age and the prevalence of acute GI in either the univariate or multivariate analysis. In the univariate analysis, although the two highest prevalences occurred in those aged <10 years and those aged between 20 and 29 years (14·3% and 10·4%, respectively), the association between age and illness was non-significant (P=0·37). This lack of observed association may be due to sample size, rather than a true lack of association in the population, since other studies have observed higher rates in children and young adults [Reference Herikstad7, Reference Scallan13, Reference Majowicz14, Reference Hall20].
Just over one-fifth of cases sought medical care for their illness. Of those who sought care, one-third were asked to give a stool sample, of which all respondents reported submitting the requested sample. These values are consistent with estimates from the province of Ontario generated from a stochastic model that utilized data from the pilot study [Reference Majowicz27], which estimated that 24% of cases seek medical care, of which 26% are asked to give a stool, and 80% submit the requested stool. Reasons for the slight discrepancies between the estimates include different study populations and time-frames. However, given the values observed here, we would expect that for every stool sample submitted in Ontario, there are about 14 community cases of acute GI.
In Ontario, there is universal health care under which residents are not required to pay for medical care. Despite this, only 22% of cases of acute GI sought medical care for their illness. The overwhelming reason for not seeking care was the belief that their illness was not serious enough or that they were not sick long enough to warrant a visit (86%). Despite the existence of universal health care, ~6% cited long waiting times or not having an available health-care provider in their area or at the time of their illness as a reason for not seeking medical assistance. Given the large proportion of cases who do not seek care (a prerequisite for being captured in surveillance data), it is important to understand how the aetiology and risk factors for illness differ in those who seek care vs. those who do not. In Canada, further research evaluating this phenomenon is needed to enable more accurate interpretation of provincial and national surveillance data.
A substantial proportion of cases who did not seek health care self-medicated for their illness. This highlights that there is still a burden and cost associated with cases who do not seek formal medical care. Additionally, 2% of those who did not seek medical care took antibiotics for their illness; in all of these cases, diarrhoea was their only primary symptom. Information on the source of these antibiotics was not collected. However, it is possible that antibiotics were acquired from friends or family members or were left over from previous illnesses where the entire course of antibiotic treatment was not completed. Regardless of the source, this use of antibiotics for diarrhoeal illness without accompanying medical care is of concern, since the use of antibiotics may exacerbate certain infectious diarrhoeal diseases, such as STECs, increasing the risk of adverse health outcomes [Reference Tarr, Gordon and Chandler28]. Education to this effect may be warranted.
As with other such studies, low response rate was the main limitation of the study presented here. However, the response rate in this study was comparable to the other two Canadian studies, where the response rates were 36·6% [Reference Majowicz14] and 44·3% [Reference Thomas17], and is in the range of response rates reported from other similar international studies [Reference Herikstad7, Reference Wheeler10, Reference Roderick12, Reference de Wit29, Reference Tijssen30]. Provided non-respondents do not differ from respondents with respect to any potential confounders, the effect of non-response will be minimal. Here, respondents were older, had a higher total household income, were more likely to be female, and had a higher level of education than Ontario residents. These differences were expected and resulted from the sampling strategy employed. Another limitation of this study was the administration of the survey in English only. However, language would only be an important source of bias if the magnitude and distribution of acute GI in those who do not speak English were distinct from those who do. There may be plausible reasons to suspect this is true (e.g. new immigrants) and thus the results of this study may not be valid for those who do not speak English.
This study demonstrates that acute GI represents a significant health burden in Ontario. In Ontario, 1·2 episodes occur per person-year, with higher rates in females and residents of rural areas. The burden of illness was consistent with estimates from a pilot study in a single Ontario city and with estimates from the province of British Columbia, confirming that the burden of GI is similar between Canadian populations over varying geography. The demographic associations also are consistent among populations and this information should be used to investigate attribute-specific aetiologies and risk factors and to design targeted intervention strategies. Temporally, acute GI peaked in the winter and spring, a phenomenon which should be investigated further.
ACKNOWLEDGEMENTS
The authors thank Carol Tinga for input to question development; Kathy Gaebel and the Centre for the Evaluation of Medicines for their expertise in survey administration and for conducting the interviews; Dr Paul Sockett and Dr Mohamed Karmali at the Public Health Agency of Canada for their support; Kate Thomas for her input on an earlier draft of this manuscript; and the Public Health Agency of Canada for funding this research. Financial support for this project was provided by the Public Health Agency of Canada.
DECLARATION OF INTEREST
None.