Colorectal cancer (CRC) is the second most common type of cancer worldwide in both men and women and accounts yearly for 9 % of all new cases of cancer(Reference Parkin, Bray and Ferlay1). Most CRC are sporadic, with dietary factors being among the most important risk factors. Latest epidemiological and preclinical studies revealed that an increased intake of dietary fibre can influence normal human gut function as well as intestinal flora and may modulate colon cancer risk(2). Dietary fibre exerts several effects in the gastrointestinal tract, but the precise mechanisms for this protective role are not clearly understood. The mechanisms by which dietary fibre may reduce the risk of CRC include stool bulking (diluting faecal content and shortening transit time), binding luminal potential carcinogens like secondary bile acids and lowering faecal pH(Reference Hamer, Jonkers and Venema3). A further mechanism by which dietary fibre could improve colonic health is by acting as a substrate for luminal health-promoting bacteria species to increase fermentation products mainly SCFA, particularly acetate, propionate and butyrate. Butyrate is considered as the major energy source for normal, non-transformed colonocytes and has received much attention as a potential chemopreventive agent by inhibiting promotion and progression or by removing degraded cells from the tissue(Reference Young, Hu and Le Leu4).
Butyrate inhibited histone deacetylases resulting in histone hyperacetylation, which can lead to transcriptional modulation and silencing of genes that are involved in control of cell cycle progression, differentiation, apoptosis and cancer development(Reference Hamer, Jonkers and Venema3, Reference Williams, Coxhead and Mathers5).
Under physiological conditions, butyrate affects human colon cells not separately but rather in conjunction with other fermentation products, which are formed simultaneously by microbial degradation of dietary fibre. Detailed evidence is limited on how fermentation products from dietary fibre sources such as grains interact with human intestinal cells and how these fermentation supernatants (fs) affect cells in comparison to analogous SCFA concentrations or butyrate alone(Reference Beyer-Sehlmeyer, Glei and Hartmann6, Reference Munjal, Glei and Pool-Zobel7). Fermentation products can be generated in vitro using batch models that simulate the physiological conditions of the human gastrointestinal tract. This provides an experimental approach to compare the biological activities of foodstuffs rich in dietary carbohydrates in terms of their fermentation products and interactions of these products(Reference Wang and Gibson8, Reference Aura, Härkönen and Fabritius9). Wheat grain contains high amounts of dietary fibre and other physiologically beneficial substances. In wheat grains, aleurone is a unicellular layer that forms the outer part of the endosperm. The aleurone layer makes up 7–9 % of the kernel and 45–50 % of the bran fraction and contains the major portion of the physiologically relevant substances found in the whole grains in a concentrated form, namely dietary fibre, minerals, B-vitamins, proteins and secondary plant compounds(Reference Amrein, Gränicher and Arrigoni10, Reference Buri, von Reding and Gavin11). Several epidemiological and experimental studies indicated that wheat bran as a source of dietary fibre is one of the most effective parts of the whole grain in protecting against CRC(Reference Ferguson and Harris12, Reference Lupton and Turner13). The main components of the dietary fibre fraction are predominantly arabinoxylans, followed by cellulose, β-glucans and starch, whereas the ratio of insoluble to soluble fibre is in the order of 9:1(Reference Buri, von Reding and Gavin11). Reddy et al. (Reference Reddy14) showed that wheat bran, but not oat or maize bran, significantly decreased the levels of several tumour promoters in the colon. It was assumed that this protective mechanism is most likely multifactorial, because differences could be caused by the slower degradation of wheat bran by colonic microflora or additional effects of phytoprotectants, although there is only few data to support this(Reference Lupton and Turner13). In the aleurone layer, ferulic acid is the major phenolic compound largely ester linked to arabinoxylans(Reference Buri, von Reding and Gavin11, Reference Ferguson and Harris12). The protective potential of this natural antioxidant depends on the fermentability, because digestive processes increase the antioxidative activity of wheat and wheat-based products(Reference Baublis, Lu and Clydesdale15). In human subjects, more than 95 % of the enzymatic release of ferulic acid occurs in the colon(Reference Baublis, Lu and Clydesdale15). Thus, antioxidative compounds may enhance chemopreventive effects of fermentation products of dietary fibre.
The first goal of the present work was to use a modified ‘batch’ model simulating the whole digestive tract to characterise and compare fermentation products of three wheat sources, namely aleurone, bran and flour, on parameters of chemoprevention, more precisely enhancement of health-promoting metabolites and reduction in tumour-promoting faecal products. Secondly, the effect of fs and relevant concentrations of SCFA (acetate, propionate and butyrate) as well as deoxycholic acid (DCA) on cell growth of HT29 colon tumour cells was studied. Beyond this, fermented European (EU) and American (US) aleurone were used to determine modulation of cell cycle and apoptosis, because dysfunction of the balance between cell growth and death in colonic epithelium is associated with cancer promotion. Understanding how this balance is influenced by interaction of specific dietary components, especially fermentation products, could lead to improved treatment and prevention strategies for cancer since there is considerable evidence from animal experiments that prebiotics are chemoprotective in the later phases of carcinogenesis(Reference Reddy14).
Materials and methods
Dietary fibre sources
Three different wheat fractions (aleurone, bran and flour) of two varieties (EU and US) were used as dietary fibre sources. All were from Kampffmeyer Food Innovation GmbH (Hamburg, Germany). To guarantee the stability of the wheat samples, aliquots were prepared and stored in air- and light-proof flasks at 4°C.
Analysis of dietary fibre sources
Analysis of dietary fibre was performed according to standard method AOAC Official Methods of Analysis no. 985.29 and no. 991.43, respectively. Arabinoxylans were analysed according to Hollmann et al. (Reference Hollmann and Lindhauer16). Analysis of crude ash, β-glucans and protein was performed according to standard method ICC no. 104/1, no. 166 and no. 167, respectively. Concentration of starch was determined according to standard method EWG, VO 228/67. d-glucose was analysed by high-performance anion-exchange chromatography on a DIONEX BioLC system (DIONEX Corporation, Sunnyvale, CA, USA) according to Hollmann et al. (Reference Hollmann and Lindhauer16). Analysis of crude fat was performed by Soxhlet extraction of lipids with petroleum ether (flash point 40–60°C) for 4 h and gravimetric determination of the dried residue.
Digestion and fermentation of wheat components
Aleurone (EU and US), flour (EU) and bran (EU) were digested and fermented in vitro according to the described procedures of Aura et al. (Reference Aura, Härkönen and Fabritius9) and Glei et al. (Reference Glei, Hofmann and Kuster17) with some modifications. Two fermentation series (each n 6) were used to study the effects of fermented wheat components. In fermentation I, EU aleurone, EU flour and EU bran were digested and fermented in vitro, whereas in fermentation II, EU aleurone and US aleurone were used.
Briefly, for simulation of the whole digestive tract, samples were weighed (0·5 g fermentable sample), mixed thoroughly with 10 ml PBS (0·1 m, pH 7·0), and 0·85 % (w/v) saline solution (6·25 ml) was added to the samples. A sample without wheat was used (blank – faeces control) as a control. All incubation steps were performed at 37°C in a shaking water-bath under the same conditions. Salivary α-amylase (Sigma A-0521) diluted to 1000 U/ml with 20 mm sodium phosphate buffer was added (17·36 U/sample), and the samples were incubated for 5 min. HCl solution (150 mm, 2·8125 ml) was added to lower pH to 2·0. Pepsin (Sigma P-7012, 1·11 mg) dissolved in 0·9375 ml of 20 mm HCl per sample or blank was added and incubated for 2 h. Intestinal extract was produced by dissolving 3 mg ox gall (Fluka-Sigma 70 168) and 2·6 mg pancreatin (Sigma P-1750) in 5 ml sodium bicarbonate buffer (11 mm, pH 6·5). Intestinal extract was added to each sample or blank, the pH was adjusted to 6·5 using NaOH solution (10 m) and the suspension (25 ml) was filled into a dialysis tube (Roth, 4596.1, molecular weight cut-off 1000 Da). The tubes were placed into glass bottles filled with 2 litre dialysis buffer (13·61 g potassium phosphate and 1·88 g sodium bicarbonate dissolved in distilled water, pH 6·5) and incubated for 6 h at 37°C under semi-anaerobic conditions. Semi-anaerobic conditions in the glass bottles were achieved by removing a part of the air with an injected cannula (0·5 bar for 1 min). Subsequently, the bottles were filled with a fermentation gas mixture (86 % N2, 10 % CO2 and 4 % H2) via the cannula (0·8 bar for 1 min). After 15 min (seven cycles repeated), the cannulae were removed. At the end of the incubation, the suspension was transferred from the dialysis tube into a 500 ml glass bottle and the pH was measured for each sample. The in vitro fermentation was performed as described by Glei et al. (Reference Glei, Hofmann and Kuster17) with slight modifications. All fermentations were conducted under anaerobic conditions at 37°C for 24 h. The fermentation process was stopped by placing the suspensions on ice and the pH was measured. Each sample was transferred to 50 ml tubes and centrifuged (4200 g, 4°C) for 30 min. The supernatants were transferred into new 50 ml tubes and centrifuged again (4200 g, 4°C) for 15 min and stored at − 80°C. The fs were pooled after finishing one fermentation series to minimise effects of individual nutrition. Before sterilisation of the fs, the samples were thawed quickly, aliquoted in 2 ml tubes and centrifuged (16 000 g, 4°C). Afterwards, the fs were sterilised by filtration (pore size, 0·22 μm) to get final fs for use in the cell culture experiments.
Determination of pH and analysis of fermentation products
The pH was determined with a pH meter Hydrus 300 (Fisherbrand, Schwerte, Germany).
SCFA concentrations were determined by GC combined with MS (GC–MS) as described by Wang et al. (Reference Wang, Zhu and Li18) with some modifications. In short, SCFA were extracted from diluted fs (1:50 in fermentation buffer) via liquid–liquid with tert-butyl methyl diethyl ether using 2-ethyl butyric acid as the internal standard. About 1 ml of the combined organic phase was derivatised with 20 μl trimethylsulfonium hydroxide solution (Sigma 92 723) in methanol (10 % v/v) for 20 min at room temperature. GC–MS was performed with a Star 3400cx and Saturn 2000 (Varian, Palo Alto, CA, USA). About 2 μl aliquots of the derivatisated samples were injected onto a Stabilwax-DA column (Restek, Bellefonte, PA, USA, 30 m × 0·25 mm internal diameter × 0·25 μm) using helium gas as carrier. The chromatographic conditions were as follows: 60°C held for 2 min, +10°C/min until 150°C held for 1 min and +50°C/min until 240°C held for 10 min. For the detection of the SCFA, the mass spectrometer was used in full scan mode.
Bile acids were determined similar to the procedure of Burkard et al. (Reference Burkard, von Eckardstein and Rentsch19). Briefly, after dilution of the fs (1:10 in fermentation buffer), solid phase extraction was carried out using 101 sorbent cartridges (Separtis, Grenzach-Wyhlen, Germany) and cholic acid-d4 as an internal standard. The eluted bile acids were separated on an RP-C8 column (100 × 4 mm, 5 μm, MZ-Analysentechnik, Mainz, Germany), using 10 mm ammonium acetate buffer (pH 5) containing 0·012 % formic acid (eluent A) and acetonitrile (eluent B) as a mobile phase at a flow rate of 0·5 ml/min. The eluents were linearly changed from 70 % A and 30 % B to 30 % A and 70 % B within 38 min, held for 10 min and finally adjusted to the original ratio of 70 % A and 30 % B for 15 min for equilibration. The detection of bile acids was performed with a tandem mass spectrometer API 4000™ (Applied Biosystems/MDS SCIEX, Toronto, Ont., Canada), equipped with an ESI ionisation source operating in the negative mode at − 4·5 kV at 450°C. Bile acids were quantified by external calibration in the MRM mode using the Analyst 1.4 (Applied Biosystems/MDS SCIEX).
The ammonia concentration of the resulting fs was determined colorimetrically by the Berthelot reaction, in which ammonia is converted into a blue-coloured indophenol ion(Reference Chaney and Marbach20). For this, the fs were diluted 1:1000 in aqua bidest, and 2 ml solution 1 (0·1 g/l phenol and 0·5 mg/l sodium nitroprusside dissolved in aqua bidest) and 2 ml solution 2 (0·05 g/l NaOH and 4 mg/l sodium hypochlorite dissolved in aqua bidest) were added. Subsequently, samples were incubated for 10 min at 60°C and thereafter cooled down to room temperature. The samples (200 μl/cavity) were transferred into ninety-six-well plates, and the extinction at 630 nm was measured in triplicates (GENios, Tecan Germany GmbH, Crailsheim, Germany).
Preparation of synthetic mixtures and of aqueous extracts of wheat fractions
Sodium butyrate and sodium acetate were obtained from Merck-Schuchardt (Hohenbrunn, Germany) whereas sodium propionate and DCA were from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). All chemicals were of the highest commercially available grade of purity. SCFA and DCA were weighed and dissolved in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Eggenstein, Germany), supplemented with 10 % fetal calf serum to obtain corresponding mixtures containing identical concentrations and ratios of SCFA and DCA as the particular fs. Mixtures or individual substances were used for cell culture incubation experiments.
Aleurone, flour and bran of both varieties (EU and US) were weighed and mixed thoroughly for 30 s with supplemented DMEM (100 g/l) according to Glei et al. (Reference Glei, Matuschek and Steiner21).
Cell culture
The human colon adenocarcinoma cell line HT29 (American Type Culture Collection (ATCC) no. HTB-38) was used for all cell culture experiments(Reference Kiefer, Beyer-Sehlmeyer and Pool-Zobel22) (passages 12–47). In regular intervals, a mycoplasma test (MycoAlert™ Detection Kit) was performed and contamination with mycoplasma was excluded.
Determination of cell growth
HT29 cells were incubated with fs 24 h after seeding in ninety-six-well plates. Concentrations between 2·5 and 20 % of fs and corresponding concentrations of synthetic mixtures as well as individual substances were tested. After 24, 48 or 72 h of incubation, the amount of DNA, which is related to the number of cells, was quantified as described previously(Reference Glei, Hofmann and Kuster17). The results were calculated on the basis of the medium control that was set to equal 100 %. In comparison to the fs, the aqueous extracts of unfermented wheat samples (0·05–40 g/l) were analysed for their influence on cell number. The effective mean doses (EC50) of fs and aqueous extracts that inhibited growth by 50 % were determined and expressed as percentage (%) and gram/litre (g/l), respectively.
Determination of genotoxicity
To determine the genotoxic potential of the fs, 1 × 106 cells were seeded in six-well plates 24 h before the experiment. The cells were incubated with different concentrations (5 and 10 %) of fs aleurone or fs blank for 1 h (short term) or 24 h (long term). Subsequently, the cells were trypsinised and dissolved in PBS. Hydrogen peroxide (150 μm; 5 min at 4°C) was used as positive control. Viabilities and cell numbers were determined with a CASY-cell counter (CASY®-TT, Innovatis AG, Reutlingen, Germany), and 0·4 × 106 cells were mixed with 0·7 % low-melting agarose (Biozym, 850.110) dissolved in PBS and distributed onto microscopical slides followed by application of another layer of low-melting agarose. The further steps were carried out as described elsewhere(Reference Glei, Hofmann and Kuster17, Reference Glei, Matuschek and Steiner21).
Cell cycle analysis
For analysis of cell cycle parameters, the cells were seeded in six-well plates (1·5 × 106 cells/well) and grown for 24 h before exposition to fs (5 and 10 %) or corresponding synthetic SCFA mixtures (10 %) and butyrate (10 %) for 24 or 48 h. Subsequently, the cells were trypsinised and dissolved in PBS. Viabilities and cell numbers were determined with a CASY-cell counter and 2 × 106 cells were stained with nuclear isolation medium-4,6-diamidino-2-phenylindole dihydrochloride (NIM-DAPI) (0·6 % Nonidet P40 and 10 μg/ml DAPI dissolved in PBS). After incubation for 10 min, intercalated DAPI was quantified by flow cytometry, whereas counted cells were allocated to specified phases of cell cycle using cytometry analysis software (Cell Lab Quanta™ SC-MPL 1.0, Beckman Coulter, Krefeld, Germany).
Detection of apoptosis
Cells were seeded in six-well plates (1·5 × 106 cells/well) and grown for 1 d before exposition to fs (10 %) or corresponding concentrations of synthetic SCFA mixtures and butyrate for 24 and 48 h. Subsequently, the cells were trypsinised and dissolved in PBS. Viabilities and cell numbers were determined with a CASY-cell counter, and 1 × 106 cells were stained with annexin V-FITC as well as 7-amino-actinomycin D in annexin V-binding buffer using the annexin V-FITC/7-amino-actinomycin D kit (Beckman Coulter). After incubation for 15 min, apoptosis was quantified by flow cytometry and counted cells were classified using cytometry analysis software (Cell Lab Quanta™ SC-MPL 1.0, Beckman Coulter). Enzyme activity of caspase-9, caspase-8 and caspase-3 was determined to obtain more detailed information on the induction of apoptosis. Therefore, cells were treated as described above, and 2·4 × 106 cells were lysed for 20 min at 4°C in a cell lysis buffer (250 mm HEPES, 25 mm 3-[(3-cholamidopropyl)-dimethylamino]-2-hydroxy-l-propanesulfonate (CHAPS), 25 mmthreo-1,4-dimercapto-2,3-butanediol (DTT), 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 100 μg/ml pefabloc SC, 1 mm phenylmethylsulfonylfluoride (PMSF) and 1 mm sodium orthovandate). After centrifugation at 16 000 g for 15 min at 4°C, half of each lysate was incubated with 50 nm caspase inhibitors (caspase-3: Ac-DEVD-CHO; caspase-9: Ac-LEHD-CHO; caspase-8: Ac-IETD-CHO, Axxora GmbH, Lörrach Germany) for 10 min, followed by incubation with 25 μm caspase substrate (caspase-3: Ac-DEVD-AMC; caspase-9: Ac-LEHD-AMC; caspase-8: Ac-IETD-AMC, Axxora GmbH) for 2 h at 37°C for all cell lysates. Modulation of caspase activity was detected by fluorimetrical analysis with Ex/Em 380/465 nm (SpectraFluor Plus, Tecan Germany GmbH, Crailsheim, Germany). For interpretation of all results, fold changes were calculated on the basis of the medium control that was set to 1·0.
Statistical evaluation
Means and standard deviations were calculated from at least three independent experiments. Differences were calculated by one- or two-way ANOVA, including Bonferroni post test with selected pairs, using GraphPad Prism Version 4 for Windows (GraphPad Software, San Diego, CA, USA). The one-way ANOVA was done to define differences within one group if more than two concentrations were used. The two-way ANOVA was used to define differences between two groups if more than two concentrations were used. Otherwise comparisons of two groups were done with Student's t test. The statistical analyses used depended on the respective experimental design and are specified in the legends to the figures and tables.
Results
Composition of dietary fibre sources
Generally, no large differences were seen in the composition of both wheat varieties (EU and US). In flour, starch was the major component (66 %), followed by protein (16 %), dietary fibre (15 %), crude fat (3 %) and crude ash ( < 2 %). Starch is the primary compound of the endosperm, which is the main part of the whole grain. Isolation of bran from whole grains resulted in increased amounts of all components except from starch (14 %). The bran fraction and the concentrated aleurone fraction primarily contained dietary fibre (56 and 47 %). Additionally, substantial amounts of protein (17 and 21 %), fat (5 and 6 %) and ash (6 and 10 %) were present. Therefore, isolation of aleurone from bran resulted in an accumulation of protein, fat and ash. Furthermore, while levels of total dietary fibre were slightly reduced, water-soluble fibres were enriched in the aleurone fraction (3 % in bran and 5 % in aleurone). Arabinoxylans were the predominant fibres in the aleurone fraction with a quantity of nearly 55 % of total dietary fibres, whereas less amounts of β-glucans were present (9 %).
Fermentation products in fermentation supernatants of different wheat samples
Table 1 compares the concentrations of various fermentation products and the measured pH in the fs of both fermentation series (I and II). Fermentation of the wheat samples resulted in significantly decreased pH (5·15–5·80) compared with fs blank (6·41–6·45). Lowered pH reflects the increased total concentration of the three SCFA acetate, propionate and butyrate. The fermented wheat samples contained two- to threefold higher amounts of SCFA than the fs blank. In particular, butyrate was increased three- to fivefold during fermentation, whereas the ratio of SCFA shifted from acetate to butyrate compared with fs blank. The analytical measurements of bile acids revealed low concentrations, and only CA and DCA were detectable. Both levels of the primary bile acid, CA, and particularly the secondary bile acid, DCA, were reduced compared to fs blank (1·3–2·2-fold and 3·5–13·8-fold lower concentrations, respectively). By fermentation of the wheat samples, more ammonia was produced in correlation to contained protein amounts of the raw material.
Table 1 Comparison of pH, SCFA concentrations, ratio of SCFA as well as concentrations of bile acids and ammonia in fermentation supernatants (fs) after in vitro fermentation of different wheat fractions
(Mean values and standard deviations)

CA, cholic acid; DCA, deoxycholic acid; EU, European; US, American.
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·001; unpaired t test).
† Because fs from six fermentations were pooled, only one determination in triplicate could be conducted. A statistical analysis was therefore not possible.
Modulation of cell growth by fermentation supernatants (series I) and corresponding SCFA
The fermentation series I was used to analyse the effect of different wheat fractions on cell growth. Treatment of HT29 cells with the fs of different wheat samples (aleurone, flour and bran) reduced the cell number in a time- and dose-dependent manner. After 24 h, the calculated EC50 (effective concentrations leading to a reduction of 50 % in cell number) was not detectable for any fs, whereas after 48 h, EC50 ranged from 10·2 to 19·0 % (Table 2). After 48 and 72 h of treatment, fermented wheat samples reduced cell growth more effectively than fs blank. Significant differences between wheat fractions were seen for aleurone or flour and bran (P < 0·05) in lower concentrations (5 and 10 %). To evaluate which compounds are responsible for the cytotoxic effects of fs, synthetic mixtures of SCFA (acetate, propionate and butyrate), butyrate and propionate as well as butyrate alone mimicking the concentration in the fs were analysed for growth-inhibitory activities (Table 2). The growth of cells was efficiently retarded in HT29 cells by the SCFA mixtures, as well as mixtures of butyrate and propionate or butyrate as single substance of fermented wheat samples. Nevertheless, all synthetic mixtures were less effective than the fs, but the general profile of response was similar. No differences were seen between synthetic mixtures of fermented aleurone or flour. Higher concentrations of butyrate in fs flour (30 mm) compared with fs aleurone (24 mm) did not result in an additional influence on cell growth. However, butyrate was still primarily responsible for the cytotoxic effect of fs. Acetate and propionate only slightly affected cell growth. Opposed to this, the inhibitory effect of fs blank was not only caused by the contained SCFA (Table 2). Fs blank significantly affected cell growth more than the synthetic mixtures.
Table 2 EC50 (%)* of fermentation supernatants (fs; series I) and corresponding synthetic mixtures SCFA (acetate, propionate and butyrate), propionate+butyrate and butyrate alone in HT29 cells
(Mean values and standard deviations)
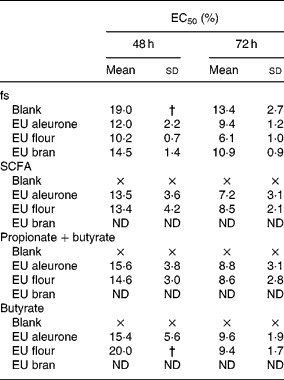
EU, European.
× Indicates that EC50 was not achieved.
* EC50 is defined as effective concentration at which cell number decreased to 50 % of viable cells.
† Standard deviation was not determinable because EC50 was not achieved in all three experiments.
Furthermore, the effects of DCA, a potential tumour-promoting fermentation metabolite, alone or in combination with SCFA, on cell growth of HT29 cells were analysed. Treatment with DCA ( < 7 μm) alone did not modulate cell growth. Additionally, cytotoxic effects of SCFA were not enforced by the addition of the secondary bile acid DCA (data not shown). In general, the pH of the cell culture medium was not affected by the highest concentration of the fs used (20 %).
Modulation of cell growth by fermentation supernatants (series II) and corresponding SCFA
The fermentation series II was used to analyse the effect of different varieties of aleurone (EU and US) on cell growth. After treatment with the fs aleurone (EU and US), the number of HT29 cells was efficiently reduced in a time- and dose-dependent manner. The calculated EC50 ranged from 11·7 to 19·1 % after 48 h (Table 3). No EC50 was, however, detectable after 24 h. The fermented aleurone affected the cell growth more effectively than fs blank independently of the variety after 48 and 72 h of treatment. Again, the growth of cells was efficiently retarded in HT29 cells by the SCFA mixtures (acetate, propionate and butyrate), as well as mixtures of butyrate and propionate or butyrate alone of both fs aleurone. The synthetic mixtures, however, were found to be slightly less effective than the respective fs in inhibiting growth of the cells. Higher concentrations of butyrate in fs US aleurone (24 mm) compared with fs EU aleurone (20 mm) had no increased influence on cell growth. Up to 65 %, butyrate was responsible for the cytotoxic effect of fs. Acetate and propionate had no clear additional effect on cell growth. Opposed to this, the cell-modulating effect of fs blank could not be explained by the contained SCFA (Table 3). Comparable to fermentation series I, fs blank had a significantly increased inhibitory effect on cell growth than the equivalent synthetic mixtures.
Table 3 EC50 (%)* of fermentation supernatants (fs; series II) and corresponding synthetic mixtures SCFA (acetate, propionate and butyrate), propionate+butyrate and butyrate alone in HT29 cells
(Mean values and standard deviations)
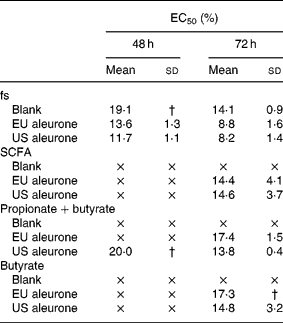
EU, European, US, American.
× EC50 was not achieved.
* EC50 is defined as effective concentration at which cell number decreased to 50 % of viable cells.
† Standard deviation was not determinable because EC50 was not achieved in all three experiments.
Modulation of cell growth by aqueous extracts of wheat samples
To determine the biological activity of the water-soluble compounds of the wheat samples (aleurone, flour and bran), the aqueous extracts were analysed for their influence on cell growth. Treatment of HT29 cells with the aqueous extracts of the wheat samples inhibited cell growth in a dose-dependent manner. After 24 h, the calculated EC50 was not detectable for any extract, whereas after 48 h, the EC50 was determinable only for EU aleurone (22·7 g/l) and both varieties of bran (EU: 31·2 g/l; US: 39·3 g/l). EU aleurone was most effective compared with other fractions of the same variety or to US aleurone.
Genotoxic potential of fermentation supernatants of different wheat samples
Fs of both fermentation series were used to analyse the genotoxic potential of concentrations that were used in further experiments (5 and 10 %). Treatment of HT29 cells with fs of different wheat samples (5 and 10 %) for 1 or 24 h did not induce DNA damage as reflected by tail intensities below 5 % (DNA in tail: 1 h, 1·92 (sd 0·12) % to 4·16 (sd 2·32) %; 24 h, 1·79 (sd 0·40) % to 3·57 (sd 1·87) %).
Modulation of cell cycle by fermentation supernatants of aleurone and corresponding SCFA mixtures
The fs of different aleurone varieties were used to analyse their antiproliferative effect on HT29 cells, a relevant marker of secondary chemoprevention. In HT29 cells treated with fs aleurone (10 %) for 48 h, an increased amount of cells in the G0/G1 phase was observed indicating a cell cycle arrest. This increase was accompanied by a decrease of cells in S and G2/M phases (Fig. 1). In comparison, the fs blank (10 %), lower concentrations of the fs aleurone (5 %) or a shorter incubation time (24 h) did not influence the cell cycle progression. Mixtures with analogous SCFA and butyrate concentrations caused a cell cycle arrest in G0/G1 phase in a similar manner like fs.

Fig. 1 Effects of medium control (□), blank (![]() ), European aleurone (■) and American aleurone (■) on cell cycle phases (G0/G1; S; G2/M) in HT29 cells after treatment with fermentation supernatants (10 %) (a), the corresponding SCFA mixtures (acetate, propionate and butyrate) (b) and butyrate alone (c) for 48 h. Cell cycle was assessed by intercalation of 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) into DNA and quantification by flow cytometry. Values are means with their standard deviations depicted by vertical bars (n 3). Student's t test was used to calculate the difference from the respective medium control (* P < 0·05) and from blank († P < 0·05).
), European aleurone (■) and American aleurone (■) on cell cycle phases (G0/G1; S; G2/M) in HT29 cells after treatment with fermentation supernatants (10 %) (a), the corresponding SCFA mixtures (acetate, propionate and butyrate) (b) and butyrate alone (c) for 48 h. Cell cycle was assessed by intercalation of 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) into DNA and quantification by flow cytometry. Values are means with their standard deviations depicted by vertical bars (n 3). Student's t test was used to calculate the difference from the respective medium control (* P < 0·05) and from blank († P < 0·05).
Induction of apoptosis by fermentation supernatants of both aleurone varieties
Fs of both aleurone varieties were used to analyse the effect on apoptosis as another important marker of secondary chemoprevention. The basal levels of early apoptotic cells detected in HT29 cells were 3·36 (sd 0·89) % after 24 h and 4·02 (sd 1·03) % after 48 h, respectively. The fs (10 %) of different aleurone varieties (EU and US) induced a significant increase in apoptosis in HT29 cells (Fig. 2). The fs blank was able to induce apoptosis after 48 h, but levels of apoptotic cells were significantly lower after treatment with the fs blank than with both fs aleurone. Synthetic mixtures of SCFA (acetate, propionate and butyrate) or butyrate alone had no influence on apoptosis and were significantly different from the fs. A higher butyrate concentration of 10 mm (positive control) significantly shifted the rate of early apoptotic cells to 10·82 (sd 2·46) % after 24 h and 19·07 % (sd 5·78) % after 48 h, respectively.

Fig. 2 Effects of fermentation supernatants (fs, 10 %), the corresponding SCFA mixtures (acetate, propionate and butyrate) and butyrate alone on early apoptosis in HT29 cells after treatment with blank (a), European aleurone (b) and American aleurone (c) for 24 h (□) and 48 h (![]() ). Apoptosis was assessed by binding of annexin V and exclusion of 7-amino-actinomycin D quantified by flow cytometry. Values are means with their standard deviations depicted by vertical bars (n 3). Student's t test was used to calculate the difference from the respective medium control (* P < 0·05), from blank († P < 0·05) and from each fs (‡ P < 0·05).
). Apoptosis was assessed by binding of annexin V and exclusion of 7-amino-actinomycin D quantified by flow cytometry. Values are means with their standard deviations depicted by vertical bars (n 3). Student's t test was used to calculate the difference from the respective medium control (* P < 0·05), from blank († P < 0·05) and from each fs (‡ P < 0·05).
To confirm the results obtained using annexin V/7-amino-actinomycin D and to get more detailed information about the underlying molecular mechanisms, the influence of fs on caspase activity was measured. Fermented aleurone of both varieties (10 %) significantly induced caspase-3 activity compared with the medium control, which confirms the results obtained by flow cytometry (Fig. 3). In contrast, the fs blank did not induce the activity of caspase-3. Initiator caspases-8 and -9 were not modulated by all fs (data not shown). Therefore, no information was obtained on which pathway underlies fs-dependent induction of apoptosis.

Fig. 3 Effects of fermentation supernatants (fs, 10 %) on caspase-3 activation in HT29 cells after treatment with blank (a), European aleurone (b) and American aleurone (c) for 24 h (□) and 48 h (■). Caspase-3 activity as an indicator for apoptotic effects was assessed in whole-cell lysates of HT29 cells in the absence and presence of caspase-3 inhibitor Ac-DEVD-CHO. Fold changes of results out of three separate experiments were calculated on the basis of the respective medium control that was set to 1·0. Values are means with their standard deviations depicted by vertical bars (n 3). Student's t test was used to calculate the difference from the respective medium control (* P < 0·05) and from blank († P < 0·05).
Discussion
It is a matter of debate whether dietary fibre actually does play an important role in human cancer prevention. While recent large trials document a reduction in the risk of getting CRC, other well-conducted epidemiological studies do not support the conclusion that fibre intake and colon cancer are inversely associated(Reference Young, Hu and Le Leu4). Reasons for this controversy are multifaceted and possibly result from limited expressiveness of epidemiological studies(Reference Schatzkin and Kipnis23). Furthermore, lack of protective effects of dietary fibre may depend on less intake ( < 30 g dietary fibre), the quality of fibre (different fermentability by colonic microflora lead to different SCFA patterns) as well as the presence or absence of phytoprotectants in the foodstuffs(Reference Hamer, Jonkers and Venema3, Reference Young, Hu and Le Leu4, Reference Beyer-Sehlmeyer, Glei and Hartmann6, Reference Munjal, Glei and Pool-Zobel7). However, the protective characteristics, which have been proven for wheat grain components in numerous studies(Reference Lupton and Turner13), could be assigned neither to a single compound nor to a certain group of substances so far. There are some indications that dietary fibres of wheat bran inhibit colon tumours in animal experiments more consistently than other dietary fibres. Fermentation of dietary fibre by intestinal bacterial flora may lead to enhanced levels of potential chemoprotective metabolites such as SCFA indicated by a lower pH in the colon(Reference Young, Hu and Le Leu4). Here, fast metabolism of arabinoxylans of wheat compounds, particularly of the aleurone layer by luminal microflora, resulted in high concentrations of SCFA and thus in a lowered pH. Survey data from various populations showed that faecal SCFA are in the range from acetate >propionate ≥ butyrate in a ratio of 60:20:20(Reference Hamer, Jonkers and Venema3, Reference Wong, de Souza and Kendall24). Depending on the diet, total SCFA concentrations in the proximal colon are 70–140 mm falling to 20–70 mm in the distal colon(Reference Wong, de Souza and Kendall24). Thus, the ratio of fs blank resulting from fermentation I and II of 59:18:23 and 54:23:23, respectively, fits very well to physiological conditions. In addition, the ratio of SCFA shifted from acetate to butyrate during bacterial degradation of fermentable material, primarily of arabinoxylans, contained in the investigated wheat samples. Moreover, a lowered luminal pH caused by increased SCFA concentrations may affect intestinal microflora by conveying growth of acidophilic bacteria species such as lactobacilli and bifidobacteria(Reference Blaut25).
In general, Western style diets, characterised by high concentrations of fat and animal protein, as well as low amounts of fibre, fruits and vegetables, are connected with a high release of bile acids, which seems to be a risk factor for the development of colon cancer(2). Especially, secondary bile acids, produced by bacterial conversion of primary bile acids, showed tumour-promoting properties by acting directly on the mucosa or by promoting the effects of carcinogenic substances present in the lumen(Reference Zampa, Silvi and Fabiani26). The present results demonstrated that fermentation of wheat sources extensively lowered the concentration of the secondary bile acid DCA. Next to a reduced solubility and conversion of bile acids, a lower pH also can reduce the activity of the enzyme 7-α-dehydroxylase, which is responsible for the conversion of primary to secondary bile acids(Reference Owen and Hill27). An increase in number and activity of acidophilic bacteria strains could lead to an enhanced binding of bile acids by these bacteria(Reference Zampa, Silvi and Fabiani26). Furthermore, direct binding of bile acids by dietary fibre could also lead to reduced concentrations in the gut lumen(Reference Das, Arber and Jankowski28). Since bile acids are presumed to promote colon cancer growth and progression(Reference Pai, Tarnawski and Tran29) and are known to interfere with antiproliferative properties of butyrate(Reference Zampa, Silvi and Fabiani26, Reference McMillan, Butcher and Wallis30), this observed decline in bile acids in the presence of dietary fibre can be considered to be favourable in terms of colon cancer prevention.
Ammonia, which is a product from proteolytic bacterial fermentation in the distal colon(Reference Hamer, Jonkers and Venema3), could also promote colon cancer, since ammonia exhibits a number of effects on the colonic mucosa, including increased mucosal cell turnover and the incidence of colon carcinomas induced by N-methyl-N′-nitro-N-nitrosoguanidine in rats(Reference Hughes, Magee and Bingham31). Dietary fibre sources are thought to reduce ammonia concentrations in the intestinal lumen. This is attributed to their role as bacterial substrates resulting either in an increased utilisation of ammonia for bacterial cell growth or a reduced deamination of nitrogenous compounds(Reference Cummings, Hill and Bone32) or indirectly by lowering the pH and so affecting enzyme activity e.g. of amino acid decarboxylase(Reference Cummings and Englyst33). In the present study, the ammonia concentration in all fs showed a direct correlation to the protein content of the test substances. Therefore, fermented aleurone exhibited the highest amounts of ammonia. Cummings et al. (Reference Cummings, Hill and Bone32) showed that fibre sources with relatively high-protein contents diminish the ammonia-lowering effect of fibre. So, non-cereal dietary fibre sources that contain less protein may be more effective at lowering faecal ammonia levels than fibres from wheat grains.
Secondary chemoprevention is defined as the use of natural, synthetic or biological agents to reverse or suppress the promotion and progression of neoplastic cells to cancer(Reference Bonovas, Tsantes and Drosos34). Investigations on effects of the fs on the growth of transformed cells as a useful indicator of secondary chemoprotection showed that the growth of HT29 cells was efficiently retarded by all fs in a time- and dose-dependent manner, with increased effectiveness of the fermented wheat samples compared to the faeces control. No important differences were seen between different wheat fractions and aleurone varieties. Hence, fermentable dietary fibre, concentrated in the aleurone layer, seems to be responsible for the growth-inhibitory effects of whole-wheat grains. In this study, SCFA were the major biologically active and growth-inhibitory components in the fs of wheat samples, especially aleurone. However, the fermentation samples were more effective in inhibiting the cell growth than the synthetic SCFA mixtures. These findings are in line with previous experiments in which several fs of various fibre sources were analysed for their effects on cell growth and survival(Reference Beyer-Sehlmeyer, Glei and Hartmann6). Even though it has been hypothesised that butyrate is the primarily SCFA, which inhibits colon tumour cell proliferation(Reference Ruemmele, Dionne and Qureshi35, Reference Coradini, Pellizzaro and Marimpietri36), results of Beyer-Sehlmeyer et al. (Reference Beyer-Sehlmeyer, Glei and Hartmann6) and Gamet et al. (Reference Gamet, Daviaud and Denis-Pouxviel37) indicate that propionate also exhibits appreciable antiproliferative properties. The present study showed that the cell growth-inhibiting effects of SCFA in the fermented wheat sources are caused almost solely by butyrate. Propionate concentrations comparable with concentrations found in fs aleurone (2 mm) exhibited an antiproliferative effect only after 8 d of treatment(Reference Siavoshian, Blottiere and Le38). Coradini et al. (Reference Coradini, Pellizzaro and Marimpietri36) have demonstrated that 4 mm butyrate for 72 h reduces the growth of HT29 cells by 75 %. A comparable growth reduction (60–70 %) was found in the present work in cells treated with butyrate concentrations contained in 20 % fs of wheat sources (3·7–5·9 mm). In comparison, the fs reduced cell growth by 95 %. Thus, the growth-inhibiting effect of the fs in the present study is probably not only due to the butyrate content. The additional activity of the fermentation samples reflects the growth-inhibitory properties of other metabolites, which have not yet been identified, or synergistic effects of these metabolites with SCFA. Furthermore, fs blank, containing less amounts of SCFA than the fermented wheat samples, also efficiently reduced cell growth. Glei et al. (Reference Glei, Hofmann and Kuster17) suggested that secondary bile acids possibly cause the effects of fs blank on cell number. But, the DCA concentrations found in fs blank (7 μm in 20 % fs) as well as the concentrations of ammonia (4 mm in 20 %) are too low to affect cells (data not shown). Thus, so far, unidentified metabolites probably resulting from the faecal inoculum must be partially responsible for the cell-modulating effects of fs blank. Growth inhibition by unfermented extracts of wheat sources is possibly caused by the presence of free phenolic acids, which have been shown to act antiproliferative in colon tumour cells(Reference Ferguson, Zhu and Harris39).
Additionally, the impact of the fs on cell cycle modulation was investigated to enhance our knowledge about mechanisms, which could be responsible for the effects on cell growth. Butyrate has been shown to inhibit cell proliferation by arresting cell cycle in the G0/G1 phase by a mechanism of action still not completely understood(Reference Blottiere, Buecher and Galmiche40). The present results indicated that fs aleurone independently of the variety inhibited HT29 cell growth after 48 h related to blocking cells in the G0/G1 phase of the cell cycle. This impact is attributed to butyrate's effect, since butyrate mimicking the concentration of fs aleurone (2–2·4 mm) caused a cell cycle arrest in the G0/G1 phase in a similar manner. Fs blank, in which only low concentrations of butyrate were found (0·5 mm), did not modulate cell cycle progression. G0/G1 arrest induced by butyrate is related to a downregulation of genes involved in cell proliferation(Reference Iacomino, Tecce and Grimaldi41). Results of Coradini et al. (Reference Coradini, Pellizzaro and Marimpietri36) indicated that butyrate (>1 mm) reduced cyclin D1 and maintained the synthesis of p21waf1/cip1, probably in a p53-independent way. Overexpression of p21waf1/cip1 selectively inhibits the G1/S cdk-cyclin complexes; thus, it is inversely associated with proliferation and directly affects terminal differentiation(Reference Williams, Coxhead and Mathers5).
In addition to growth inhibition, induction of apoptosis is another relevant marker for secondary chemoprevention of CRC. Although the molecular basis for the proapoptotic effects of butyrate has not been fully worked out, there are indications that extrinsic as well as intrinsic pathways of programmed cell death are involved(Reference Williams, Coxhead and Mathers5, Reference Kim, Park and Lee42). In contrast, effects of fs or SCFA mixtures have not been well investigated. Using annexin V staining, the externalisation of phosphatidylserine as an early event in apoptosis could be detected after treatment of HT29 cells with the fs. Fs aleurone (10 %) independently of variety (EU and US) was able to induce apoptosis in a time-dependent manner and was more effective than the fs blank, although fs blank also had proapoptotic effects in HT29 cells. Beyond this, the proapoptotic effects of the fs aleurone resulted in an enhanced activity of caspase-3. Caspase-3 is a member of a cysteine protease family that is involved in the execution of the apoptotic programme. Since fs aleurone did not induce activities of caspase-8 or -9, no information is given if the extrinsic or intrinsic pathway plays a role in activation of apoptosis. Therefore, experimental conditions like the time frame or less sensitivity of the method could be the reason for the lack of induction of the initiator caspases. Furthermore, SCFA (acetate, propionate and butyrate) or butyrate mimicking the concentration in the fs did not enhance the level of apoptotic cells. A study by Medina et al. (Reference Medina, Edmonds and Young43) has shown that butyrate (4 mm) induces activation of caspase-3 in CRC cells. Kim et al. (Reference Kim, Park and Lee42) suggested that butyrate sensitises human cancer cells to TRAIL-mediated apoptosis by upregulation of expression of death receptor DR5 that is mediated by activation of Sp1-transcriptional factor. In HT29 cells, 4 mm butyrate induced a 2·4-fold increase in DR5 expression, whereas lower concentrations (2 mm), found in our fs, did not modulate expression levels. In addition, only higher butyrate concentrations (>5 mm) enhanced histone acetylation resulting in reactivation or silencing of genes critical for apoptosis(Reference Kiefer, Beyer-Sehlmeyer and Pool-Zobel22). Therefore, further studies are needed to identify which molecular cascades are involved in the proapoptotic activity of fs and which metabolites are responsible for its effect.
In conclusion, the present results provide evidence on the biological effects of fermentation samples from different wheat sources, in particular aleurone. Gut flora-mediated fermentation of wheat aleurone results in reduced levels of tumour-promoting DCA but higher levels of potentially chemopreventive SCFA, especially butyrate. The aleurone layer was identified as the most beneficial subfraction, since its fermentation metabolites were able to induce apoptosis and to block cell cycle – two essential markers of secondary chemoprevention. Partly, chemoprotective activities of fermented aleurone are caused by butyrate. Thus, a fibre-rich diet (e.g. with aleurone-enriched products) could be an effective strategy, firstly to enhance gut health and secondly to inhibit growth of transformed colon cells.
Acknowledgements
We would like to thank the Federal Ministry of Education and Research, Germany (BMBF 0313829A) for funding. The authors have no conflicts of interest. A. B., K. S., D. S. and M. G. designed the research and wrote the paper. A. B. and K. S. performed the in vitro fermentation and experiments on cell growth. A. B. performed the experiments on quantification of cell cycle parameters and apoptosis. K. S., G. B-.W. and U. O. performed the analysis of SCFA and bile acids and wrote the part describing the corresponding methods. J. H. and M. L. performed the analysis of dietary fibre sources that were provided by N. W. We thank Walter von Reding, Bühler AG, Switzerland, for preparation of the aleurone fraction from wheat sources.










