Age-related loss of lean body mass (LBM), particularly skeletal muscle LBM, and its relationship to mortality is well cited in epidemiological studies( Reference Bunout, de la Maza and Barrera 1 , Reference Muscaritoli, Anker and Argilés 2 ). Skeletal muscle LBM has been shown to decline with increasing age, and its reversal made more difficult, because of development of ‘anabolic resistance’, which may be related to factors such as dietary protein inadequacy( Reference Houston, Nicklas and Ding 3 , Reference Beasely, LaCroix and Neuhouser 4 ), physical inactivity( Reference Hughes, Roubenoff and Wood 5 ), ageing vasculature( Reference Dillon, Casperson and Durham 6 , Reference Timmerman, Lee and Fujita 7 ) and chronic low-grade inflammation( Reference Beyer, Mets and Bautmans 8 ).
Previous prospective cohort studies have shown that higher total protein intake is positively associated with measures of LBM retention( Reference Houston, Nicklas and Ding 3 , Reference Stookey, Adair and Popkin 9 ), whereas additional cross-sectional studies have reported similar( Reference Morris and Jacques 10 ) as well as contrasting( Reference Mitchell, Haan and Steinberg 11 , Reference Baumgartner, Waters and Gallagher 12 ) results. The most recent Nordic Nutritional Recommendations, which summarise recent key findings( Reference Volpi, Campbell and Dwyer 13 , Reference Bauer, Biolo and Cederholm 14 ), indicate that the optimal daily protein requirements are suggested as 1·1–1·3 g/kg body weight (BW) in elderly populations (i.e. older than 65 years)( 15 ). A number of smaller well-designed controlled experiments have further elucidated that increasing amounts of complete protein sources containing 2 g or more leucine effectively stimulate muscle protein synthesis (MPS) in elderly men( Reference Yang, Breen and Burd 16 ), with larger doses being related to a greater response( Reference Breen and Phillips 17 , Reference Churchward-Venne, Burd and Phillips 18 ). Investigations have shown that leucine alone can signal MPS, and leucine peak, in part, can direct the amplitude of the resulting anabolic response from protein ingestion. However, in the long-term, leucine alone has been shown to have no effect on actual muscle hypertrophy over time( Reference Breen and Phillips 17 , Reference Norton, Layman and Bunpo 19 ).
Longer-term smaller intervention studies among patients and healthy elderly (>6 months) that have added a combination of leucine and the other branched-chain amino acids to normal intake have shown mixed results, with some reporting an increase in LBM( Reference Aquilani, Opasich and Gualco 20 , Reference Fuller, Baier and Flakoll 21 ), whereas another found no significant change in LBM compared with control( Reference Verhoeven, Vanschoonbeek and Verdijk 22 ). Therefore, accretion of skeletal LBM is most likely to be reliant upon the provision of adequate leucine in combination with a complete protein source.
It has been well established that physical activity in conjunction with the ingestion of a complete and adequate protein source yields a greater hypertrophic effect than either alone( Reference Yang, Breen and Burd 16 , Reference Churchward-Venne, Burd and Phillips 18 ).
Therefore, a recent position study has indicated that foods containing 2·3–2·8 g of leucine should be recommended at each meal for those over 65 years of age. In addition, a recommendation that adequate protein intake should be combined with regular aerobic and resistance training is also suggested( Reference Bauer, Biolo and Cederholm 14 ).
Although there are cross-sectional( Reference Morris and Jacques 10 , Reference Aubertin-Leheudre and Adlercreutz 23 ) and prospective data( Reference Houston, Nicklas and Ding 3 ) describing associations between total protein intake, LBM and LBM change, none of these studies have reported the effect on LBM in relation to dietary intake of leucine. Therefore, the purposes of this study were to investigate the relationship between dietary intake of leucine and its relationship with LBM and LBM change over 6 years and how this may differ for those younger or older than 65 years of age.
Methods
Subjects
The present study was part of the Danish MONICA (Multinational MONItoring of trends and determinants in CArdiovascular disease) project, an international study conducted under the auspices of the WHO, to monitor CVD trends and determinants. In 1982, a sample of 4807 subjects was randomly selected from the Central Person Register of all citizens born in 1922, 1932, 1942 and 1952 and living in eleven municipalities in the Copenhagen County( Reference Hartz, Rupley and Rimm 24 ). Of these, 226 were of non-Danish origin and were excluded. Among the 4581 invited, 3608 participated in a subsequent health examination in 1982( Reference Jensen and Jorgensen 25 ). Those who participated in the baseline study (MONICA1) were invited to a follow-up examination in 1987–1988 (GEN-MONICA), of which 2987 (83 %) attended. After 11 years, 4130 of the 1982 sample were still alive and lived in Denmark. These participants were invited to the second follow-up (MONICA10) examination from 1993 to 1994, where 2554 (65 %) attended. The non-respondents have been described in detail elsewhere( Reference Heitmann 26 ).
Among survivors of the participants of MONICA1, one in six individuals was invited for a diet history interview at GEN-MONICA and MONICA10 surveys conducted 6 and 11 years later (n 552). Body composition measures were collected only at the GEN-MONICA and MONICA10 surveys, and total information on both dietary and body composition data for both GEN-MONICA and MONICA10 was available for 368 participants. Of those, leucine intake at MONICA1 was also available for 233 participants. For the purposes of this study, data from only GEN-MONICA and MONICA10 will be used. These two surveys will be referred to as ‘baseline’ and ‘follow-up’, respectively.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki (1989) of the World Medical Association, and the Ethics Committee of the Medicine Faculty of the Copenhagen County approved all the procedures involving human subjects. All subjects were informed of the aims and procedures of the study, and they gave their written consent.
Anthropometry
BW was measured to the nearest 0·1 kg using a lever balance, with subjects dressed in light clothing or underwear. Height was measured without shoes to the nearest 0·5 cm. A bioelectrical impedance (BIA)-103 body-composition analyzer (RJL Systems) was used to measure electrical impedance, following the manufacturer’s instructions. Measurements were taken with a tetra polar electrode arrangement while the subjects were lying relaxed on a couch. The electrodes were placed on the dorsal surfaces of the right hand and foot at the distal metacarpals and metatarsals, respectively, and between the distal prominence of the radius and the ulna at the wrist and the medial and lateral malleoli at the ankle. A specific equation for estimating body fat (BF) from impedance, with measurements of total body water and K as a reference, has been developed specifically for this population and reported previously( Reference Yang, Breen and Burd 16 ):
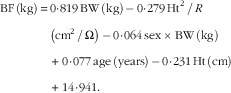 $$\eqalignno{ {\rm BF}\,\left( {{\rm kg}} \right)\,{\equals}\, & 0\!\cdot\!819\,{\rm BW}\,\left( {{\rm kg}} \right)\,{\minus}\,0\!\cdot\!279\,{\rm Ht}^{{\rm 2}} {\rm \,/\,}R\, \cr & \left( {{\rm cm}^{{\rm 2}} {\rm \,/\,}\Omega } \right)\,{\minus}\,0\!\cdot\!064\,{\rm sex}\,{\rm {\times}}\,{\rm BW}\,\left( {{\rm kg}} \right) \cr & {\plus}\,0\!\cdot\!077\,{\rm age}\,\left( {{\rm years}} \right)\,{\minus}\,0\!\cdot\!231\,{\rm Ht}\,\left( {{\rm cm}} \right) \cr & {\plus}\,14\!\cdot\!941. $$
$$\eqalignno{ {\rm BF}\,\left( {{\rm kg}} \right)\,{\equals}\, & 0\!\cdot\!819\,{\rm BW}\,\left( {{\rm kg}} \right)\,{\minus}\,0\!\cdot\!279\,{\rm Ht}^{{\rm 2}} {\rm \,/\,}R\, \cr & \left( {{\rm cm}^{{\rm 2}} {\rm \,/\,}\Omega } \right)\,{\minus}\,0\!\cdot\!064\,{\rm sex}\,{\rm {\times}}\,{\rm BW}\,\left( {{\rm kg}} \right) \cr & {\plus}\,0\!\cdot\!077\,{\rm age}\,\left( {{\rm years}} \right)\,{\minus}\,0\!\cdot\!231\,{\rm Ht}\,\left( {{\rm cm}} \right) \cr & {\plus}\,14\!\cdot\!941. $$
where BF is the body fat, Ht the height and BW the body weight. Sex was coded as 1 for men and 0 for women. LBM (kg) was calculated by subtracting BF (kg) from total BW (kg).
LBM and BF measures were available at baseline and follow-up surveys, but unavailable at MONICA1. Thus, our analysis focused on change in LBM between these two latter time points.
Dietary assessment
At baseline and follow-up, the same dietitian interviewed participants to assess dietary intake based on information from the previous month, and average daily nutrient and energy intakes were calculated from this information. Meal patterns, dishes and foods were explored by interview using a detailed pre-coded interview form. Quantities were explored using food models, photo series, cups and measures. This instrument has been validated in another study( Reference Heitmann 27 ). Nutrient calculations have been carried out with the DANKOST programme, which is derived from the Danish food composition tables. This database included 835 food items at baseline in 1988, and it was extended and comprised 1250 food items in 1995. In 1987–1988, participants gave information on diet intake based on 498 different foods, and at follow-up in 1993–1994 1177 were recorded. Food contents of leucine and all the other amino acids were supplied from the food database, which can be found at www.foodcomp.dk.
Physical activity
Leisure-time activity was based on participants marking one out of four alternatives: (i) almost completely inactive, reading, television viewing, cinema-going; (ii) some physical activity (at least 4 h/week); (iii) regular activity; and (iv) regular, hard physical training for competition. These variables were then reduced by restricting sedentary activity to (i) only and combining (ii)+(iii)+(iv) as the physically active group.
Demographical variables
Participants recorded present and previous smoking habits and were classified as either current smokers or non-current smokers. Women recorded whether they had entered menopause. Information on previous or prevalent disease, including CVD and cancer diagnoses, was obtained by following-up each individual via the unique personal number in the centralised hospital discharge registry, which is complete with information for all individuals hospitalised in Denmark since 1973.
Statistical analysis
Differences in demographical data between older (>65 years of age) and younger (<65 years of age) participants at baseline were assessed using Mann–Whitney U and χ 2 tests for continuous and categorical variables, respectively.
Multivariable linear regressions and ANCOVA were used to assess the relationship between leucine at baseline in 1987–1988 and LBM change until 1993–1994, when leucine was expressed as a continuous variable and categorical (quartiles) variable, respectively. The covariates included in the primary model were LBM (kg), BW (kg) and physical activity at baseline, sex and age. Physical activity was reduced to a binary variable by dividing the groups as (i) and (ii)+(iii)+(iv). Interaction product and terms were used in the linear regression for sensitivity analyses. Sex, physical activity, and presence of CVD were assessed for their interaction on the relationship between leucine and LBM. For interactions that approached significance (P<0·1), stratified analyses were carried out to determine their influence on the overall model.
Results
For the 368 subjects who had diet information and complete data on changes in body composition between assessments at baseline in 1987–1988 and follow-up in 1993–1994, the mean age was 50·4 (sd 10·8) years, 51·1 % were female and all were Caucasian (Table 1). The mean daily leucine intake was significantly greater for those aged 35–55 years compared with those aged >65 years. In addition, those aged >65 years had significantly lower LBM, total energy and protein intakes, higher BF percentage and BMI, and were less likely to have never smoked. For the whole population, there was a mean LBM loss, which was significantly greater for those aged >65 years compared with those aged <65 years.
Table 1 Baseline demographics (Numbers and percentages; mean values and standard deviations; mean values with their standard errors)

LBM, lean body mass; BF%, body fat percentage.
* P value comparing those over 65 years of age with those younger than 65 years.
† χ 2 Test, all others Mann–Whitney U tests with the grouping >65 or <65 years of age; sedentary: almost completely inactive: reading, television viewing, cinema-going; physically active: some physical activity (at least 4 h/week) or more.
Dietary leucine intake and subsequent lean body mass change over 6 years
After adjustment for sex, age, total energy intake, physical activity and LBM at baseline, no significant associations were found between baseline leucine intake and the subsequent 6-year LBM change for all individuals (β=0·000, P=0·99). For those aged >65 years at baseline, a linear relationship was found for leucine intake as a continuous variable (β=0·434, P=0·028). Fig. 1 shows results of the adjusted ANCOVA for LBM changes by quartiles of leucine intake, which indicated that only those in highest quartile of leucine intake (7·1 (sd 0·6) g/d) maintained LBM (adjusted mean change in LBM: 0·24 kg; 95 % CI −0·67, 1·14). The lower three quartiles experienced loss of LBM over time (Q1: −1·24 kg; 95 % CI −1·9, −0·5; Q2: −0·99 kg; 95 % CI −0·88, 0·5; Q3: −0·2 kg; 95 % CI −0·88, 0·5). For those aged <65 years at baseline, no association was found between leucine intake as a continuous variable and change in LBM (β=−0·082, P=0·39). In addition, no differences were seen between any of the quartile groups (Q1: 0·01 kg; 95 % CI −0·5, 0·5; Q2: −0·06 kg; 95 % CI −0·5, 0·4; Q3: 0·4 kg; 95 % CI 0·0, 0·8; Q4: −0·1 kg; 95 % CI −0·3, +0·1. Table 2 shows total protein and leucine intakes across the four quartiles of intake and the corresponding change in LBM for younger and older individuals. Compared with those older than 65 years, relative total protein and leucine intakes were higher in all four corresponding quartiles for those younger than 65 years.

Fig. 1 Six-year lean body mass (LBM) change for quartiles of leucine intake. *P=<0·05. ![]() Relates to quartile values for those >65 years of age (for trend as a continuous variable: β=0·43, P=0·028);
Relates to quartile values for those >65 years of age (for trend as a continuous variable: β=0·43, P=0·028); ![]() relates to those <65 years of age (for trend: β=−0·08, P=0·393). Adjusted model for ANCOVA includes LBM at baseline, total energy intake, age, sex and physical activity.
relates to those <65 years of age (for trend: β=−0·08, P=0·393). Adjusted model for ANCOVA includes LBM at baseline, total energy intake, age, sex and physical activity.
Table 2 Quartiles of total leucine and corresponding relative total protein intakes for those younger and older than 65 years of age (Mean values and standard deviations)
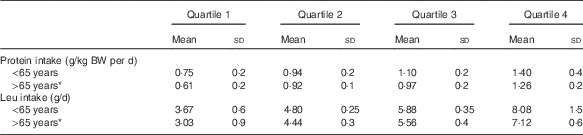
BW, body weight.
* Compared with those <65 years, those aged >65 years had significantly lower intakes of protein and leucine for all quartiles (P<0·05).
Sensitivity analyses were performed that included interactions with history of CVD diagnosis at baseline and/or follow-up, sex or physical activity. For those older than 65 years, a significant interaction was found for ‘any diagnosis of CVD’ (i.e. diagnosed with CVD at baseline or follow-up)×leucine (β: −1·031, P=0·008), whereas no interaction was seen for younger individuals (β: 0·199, P=0·25). After restricting the analysis to only older individuals free of CVD, a significant association for leucine and change in LBM (β=0·481, P=0·04; n 65) was seen. However, for those over 65 years and with ‘any diagnosis of CVD’, no relationship was found between leucine and LBM change (β=−0·171, P=0·76; n 14). Post hoc analyses revealed that those free of CVD (n 65) lost less LBM than those with any diagnosis of CVD (n 14) over 6 years (mean change: −0·35 (sd 1·4) v. −1·3 (sd 1·8) kg, respectively; t=2·2, P=0·03).
For those younger than 65 years, a significant interaction was found by level of physical activity (P=0·03). However, when analyses were restricted to those who were sedentary (P=0·28; n 69) or physically active (P=0·065; n 217), no significant associations were found between leucine and LBM change. No statistically significant interaction for leucine×physical activity was seen in older individuals. A trend for sex×leucine interaction was noted in older individuals (β: 0·72, P=0·11). However, no significant trends were noted when analyses were restricted to males (β: 0·445, P=0·10) or females only (β: 0·385, P=0·14).
Discussion
In a healthy population subset of adult Danes, higher dietary protein and leucine intakes were associated with greater LBM retention in those aged 65 years, whereas no association was found for those younger than 65 years. For those aged >65 years, LBM was maintained over 6 years in individuals who consumed a mean of 7·1 g of leucine/d, with a corresponding mean total protein intake of 1·25 g/kg BW per d. Therefore, a long-term relationship between leucine intake in the presence of adequate total protein consumption and LBM retention was shown. This association remained significant after adjustment for initial LBM, total energy intake, age, sex, physical activity, smoking status and presence of CVD.
The presence of a diagnosis of CVD at any time during the 6- year follow-up period was associated with greater LBM loss, and similarly in CVD-free individuals, leucine and total protein intakes had a more marked influence over LBM. Individuals with a diagnosis of CVD may have been exposed to greater levels of inflammation that may have increased anabolic resistance in them( Reference Beyer, Mets and Bautmans 8 ), which in turn may have dampened the effect of anabolic stimuli including the muscle protein synthetic effects of protein intake. Alternatively, as previous case–control studies in Nordic countries have indicated that those with CVD reported higher-quality diets than healthy controls at the time after diagnosis( Reference Ma, Olendzki and Pagoto 28 ), it is plausible to suggest that those diagnosed with CVD may have altered their dietary intake rendering their baseline data on diet intake unreliable, which may explain why the relationship between leucine and LBM change was strengthened when including only those without CVD.
Previous observational research findings from the Health, Ageing and Body Composition (Health ABC) study have indicated that individuals in the lowest quintile of protein intake (0·7 g/kg BW) experienced a 40 % greater loss of LBM over 3 years compared with the highest quintile (1–1·2 g/kg BW)( Reference Houston, Nicklas and Ding 3 ). Similar observations were made in a Chinese sample assessing LBM change by mid-arm circumference( Reference Stookey, Adair and Popkin 9 ). In addition, protein intakes from animal meats, which typically contain the highest concentrations of leucine( 29 ), have been associated with greater LBM retention compared with vegetable-based protein( Reference Houston, Nicklas and Ding 3 , Reference Aubertin-Leheudre and Adlercreutz 23 ). Laboratory data in younger and older individuals have indicated that after a meal containing 2 g of leucine in the form of a complete whey protein isolate( Reference Yang, Breen and Burd 16 ), or after 170 g of meat( Reference Robinson, Burd and Breen 30 ), MPS is increased, resulting in net LBM gain. More in-depth investigations have shown that for those over 65 years, a positive dose–response relationship between protein ingestion and MPS exists for up to 40 g of protein (containing 4 g of leucine). However, younger individuals experienced no further gains after 20 g of leucine (containing 2 g leucine) at one meal( Reference Breen and Phillips 31 ). To frame these findings correctly, it is important to note that free leucine added to an adequate and complete protein source does not provide further anabolic advantages( Reference Koopman, Verdijk and Beelen 32 ). Thus, leucine is an essential factor and a useful surrogate marker for determining effect on MPS, yet total essential amino acid intake will ultimately determine the anabolic effect of protein ingestion.
Combining both bodies of literature, a recently published position stand concluded that protein sources containing 2·3–2·8 g of leucine are recommended at each main meal (total intake of 6·6–8·4 g/d) to optimise LBM outcomes in older populations (>65 years)( Reference Bauer, Biolo and Cederholm 14 ). Our study is the first to confirm both observational and experimental data with regard to LBM and leucine intake. Furthermore, our results confirmed that protein consumed through foods without protein supplementation seems sufficient to maintain LBM for a longer term in older populations.
In the present study, there was no association between protein intake and LBM change for those younger than 65 years. From laboratory data, it is known that older individuals need more protein than younger individuals in order to elicit equivalent responses in MPS( Reference Yang, Breen and Burd 16 ). Therefore, declines in anabolic response( Reference Taaffe 33 , Reference Haran, Rivas and Fielding 34 ) in older individuals may partly explain why higher protein intake may be required for clinically relevant LBM preservation, than for those younger than 65 years. Similarly, markers of chronic inflammation are known to contribute to age-related sarcopenia( Reference Beyer, Mets and Bautmans 8 ) and are predictive of CVD( Reference Danesh, Whincup and Walker 35 ). No statistically significant interactions were seen overall for sex×leucine; however, a trend for interaction was noted for older but not younger individuals. Previous literature suggests that women may have a higher rate of basal MPS( Reference Henderson, Nair and Dhatariya 36 , Reference Smith, Atherton and Villareal 37 ); however, one study indicated a similar MPS response to feeding in older men and women (65–80 years)( Reference Smith, Atherton and Villareal 37 ). Our findings agree with one previous study that reported no differences between sexes in middle-aged men and women in either basal or fed states( Reference Smith, Atherton and Reeds 38 ). However, a greater sample size with more detailed physical activity data may elucidate sex differences in future studies. No interaction was noted for physical activity×leucine. The current literature indicates that resistance training and leucine intake combined produce greater MPS responses and LBM gains compared with either intervention alone( Reference Churchward-Venne, Burd and Phillips 18 ). In addition, a cross-sectional study reported greater skeletal muscle LBM for those having higher protein intakes in conjunction with both vigorous aerobic training or strength training( Reference Morris and Jacques 10 ). Our physical activity classification of ‘sedentary’ or ‘non-sedentary’ did not delineate aerobic and resistance exercise, thus potentially explaining the lack of effect seen in the previous literature.
A few limitations should be noted. It is possible that assessment of dietary intake of leucine at baseline may have been inadequate to capture important dietary exposures over the subsequent 6 years; however, the power was adequate to detect a linear relationship. Furthermore, we showed previously that fat- and/or carbohydrate-rich foods are most under-reported, and protein intake is also under-reported in general and particularly among the more obese( Reference Heitmann and Frederiksen 39 ). Therefore, both random reporting error, which would tend to attenuate, and non-random reporting error, which may have inflated the observed association, are possible in the present study( Reference Heitmann and Frederiksen 39 ). The net effect of such opposite acting errors is unknown. However, LBM retention in older individuals occurred at similar amounts protein and leucine previously reported to elicit net LBM growth in both laboratory and epidemiological studies( Reference Bauer, Biolo and Cederholm 14 ). In addition, the reliability of our results related to measurement of LBM was strengthened by using a population-specific algorithm generated from the measurement of total body water and total K as reference( Reference Heitmann 40 ). Our entire sample included Danes of Caucasian descent; thus, our results are most likely valid for Caucasian populations and will require confirmation in other races. In contrast, the use of BIA as the method of body composition assessment is likely to be less accurate than a more current gold standard measure using dual-energy X-ray absorptiometry or computer tomography scan( Reference Brodie, Moscrip and Hutcheon 41 ). However, the use of a population-specific BIA algorithm used for this trial specifically is a factor that may have improved the precision of these measures. Finally, resistance training has been the most common type of exercise stimulus for measurement of anabolic response, and data for resistance training were not available.
Conclusion
For Danes aged 65 years, greater dietary intake of leucine is associated with greater retention of LBM over 6 years. These results confirm previous observational and laboratory findings that to avoid loss of LBM in older populations a mean intake of 1·25 g of protein/kg BW per d containing 7·1 g/d of leucine (approximately 2·3–2·4 g/main meal) may be required. These findings should be confirmed in a larger and more diverse population.
Acknowledgements
The authors thank the participants in the Danish cohort of the WHO MONICA trial.
Funding for travel to perform the research was provided by the University of Queensland (UQ) through the Graduate Student International Travel Award (GSITA). Research and statistical analysis support was provided by the Institute of Preventive Medicine, Bispebjerg and Frederiksberg Hospitals. This research was supported by the UQ GSITA and by a Danish MRC grant from the FREJA programme (Female Researchers in Joint Action).
The authors’ responsibilities were as follows – C. K. M.: analysis, drafting of the manuscript; M. Z. A., S. C., J. B. and B. L. H.: critical revision of the manuscript; K. R.: data extraction, translation and design of data analysis; C. K. M. and B. L. H.: study design; B. L. H.: collection of data; C. K. M., M. Z. A. and B. L. H.: interpretation of data. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








