Introduction
Major depressive disorder (MDD) is a prevalent disease (Kessler et al. Reference Kessler, Berglund, Demler, Jin and Walters2005; De Graaf et al. Reference de Graaf, ten Have, van Gool and van Dorsselaer2012), characterized by a persistent depressed mood and/or marked loss of pleasure (American Psychiatric Association, 2013). MDD is a highly recurrent disorder and risk of recurrence is related to the number of previous episodes (Solomon et al. Reference Solomon, Keller, Leon, Mueller, Lavori and Shea2000; Kessing et al. Reference Kessing, Hansen, Andersen and Angst2004). This observation stimulated the idea that depressive episodes leave more or less permanent residual impairments or scars that confer risk of subsequent episodes; the ‘scar hypothesis’ (Rohde et al. Reference Rohde, Lewinsohn and Seeley1990; Burcusa & Iacono, Reference Burcusa and Iacono2007). Indeed, many studies comparing remitted depressed patients with healthy controls have shown that the former differ in certain ways from the latter (Just et al. Reference Just, Abramson and Alloy2001; Bhagwagar & Cowen, Reference Bhagwagar and Cowen2008; Paykel, Reference Paykel2008). Such studies, however, cannot rule out the possibility that post-morbid impairments already existed before the onset of the episode, predisposing people to depression. Thus, individuals with a history of depression may already have had certain characteristics that made them vulnerable to recurrent depression beforehand. This is called the ‘vulnerability hypothesis’ (Just et al. Reference Just, Abramson and Alloy2001; Christensen & Kessing, Reference Christensen and Kessing2006; Sowislo & Orth, Reference Sowislo and Orth2013) or ‘trait marker hypothesis’ (Rohde et al. Reference Rohde, Lewinsohn and Seeley1990).
To distinguish between pre-existing vulnerabilities and scars, prospective studies with pre- and post-morbid data of first-onset depressed subjects are required (i.e. subjects with a first-lifetime occurrence of an MDD episode). The few available prospective studies, most often done in population-based samples, have addressed scarring in psychosocial functioning (Burcusa & Iacono, Reference Burcusa and Iacono2007, review), cognitive functioning (Burcusa & Iacono, Reference Burcusa and Iacono2007; Allott et al. Reference Allott, Fisher, Amminger, Goodall and Hetrick2016, reviews), self-esteem (Sowislo & Orth, Reference Sowislo and Orth2013, review), personality (Christensen & Kessing, Reference Christensen and Kessing2006, review), and after stressful life events (Burcusa & Iacono, Reference Burcusa and Iacono2007, review). These reviews have shown that there is more evidence for the vulnerability hypothesis than for the scar hypothesis. There is slight evidence in favor of scarring in neuroticism (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1993; Burcusa & Iacono, Reference Burcusa and Iacono2007) and some evidence that sensitization to stressful life events occurs (Monroe & Harkness, Reference Monroe and Harkness2005; Burcusa & Iacono, Reference Burcusa and Iacono2007), but evidence for psychosocial or cognitive scars is virtually absent.
A limitation of the few longitudinal population studies done thus far is that in most studies the number of people who developed a depressive episode during the study follow-up was very small, leaving limited power to detect vulnerability or scarring effects. Further, it may be that scarring only occurs when depressive episodes are severe, long-lasting, or recurrent. Ormel et al. (Reference Ormel, Oldehinkel, Nolen and Vollebergh2004a) indeed observed scarring effects in psychosocial disability only in individuals with severe recurrent episodes, although they could not replicate this regarding scars in neuroticism, depressive coping, and self-esteem (Ormel et al. Reference Ormel, Oldehinkel and Vollebergh2004b), and did not control for possible confounders. Most longitudinal studies on scarring, however, did not separately investigate severe or recurrent episodes. Further, most studies focus on mental scars; there are hardly any studies that investigated physical functioning pre- and post-morbidly.
We investigated vulnerability and scar effects in mental and physical functioning among adults with a major depressive disorder in a large sample representative of the general Dutch population. We used three waves from the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2), a prospective epidemiological study with a 6-years follow-up. We specifically focus on the questions: (1) whether levels of premorbid functioning of individuals who develop a first-onset depressive episode differ from those of healthy controls (vulnerability effect); (2) whether residual impairments in functioning remain after remission of the depressive episode (scar effect); (3) whether vulnerability or scar effects are different for mental compared with physical functioning; and (4) whether vulnerability or scar effects are different for recurrent v. first-onset episodes, and for episodes of different severity. We hypothesized that vulnerability but not scar effects will be found in general, but that scarring will be detected in the subgroup with recurrent or severe depression. Further, we examined whether the results may be confounded by comorbid disorders or by prodromal or residual symptom severity.
Methods
Participants
We used data from the first three waves of NEMESIS-2, a prospective epidemiological cohort study in the Dutch adult general population (de Graaf et al. Reference de Graaf, ten Have and van Dorsselaer2010). The inclusion criterion for this study was age between 18 and 64 at the time of the baseline interview. Insufficient fluency in Dutch was an exclusion criterion. Participants were randomly selected by means of a multistage, stratified sampling procedure, with one respondent being randomly sampled from randomly selected households sampled from randomly selected municipalities. In the baseline wave (T0), between November 2007 and July 2009, 6646 individuals were assessed by means of face-to-face interviews (response rate 65.1%). This sample was reasonably representative of the Dutch general population, though younger individuals were somewhat underrepresented (de Graaf et al. Reference de Graaf, ten Have and van Dorsselaer2010). During the second wave (T1), 3 years after T0, 5303 participants were interviewed again (response rate 80.4%). In the third wave (T2), 3 years after T1, 4618 participants were re-interviewed (response rate 87.8%). NEMESIS-2 was approved by an independent Medical Ethics Committee and all participants provided written informed consent. For more details about the study design, see de Graaf et al. (Reference de Graaf, ten Have and van Dorsselaer2010).
Measures
The Medical Outcomes Study Short Form Health Survey (SF-36, Ware & Sherbourne, Reference Ware and Sherbourne1992) was used to assess mental and physical functioning at each wave. The SF-36 is a self-report questionnaire consisting of 36 items and eight subscales which assess the level of functioning during the previous 4 weeks (McHorney et al. Reference McHorney, Ware, Rachel Lu and Sherbourne1994). The scale is composed of 36 items, rated on Likert-type scales. Item responses are converted to a scale ranging from 0 to 100, with higher scores indicating better functioning. The scores are averaged to create the subscale scores. The SF-36 has been widely used and its psychometric properties are good (McHorney et al. Reference McHorney, Ware and Raczek1993, Reference McHorney, Ware, Rachel Lu and Sherbourne1994; Aaronson et al. Reference Aaronson, Muller, Cohen, Essink-Bot, Fekkes and Sanderman1998). We combined four of the SF-36 subscales into a mental health component scale, namely mental health, role limitations due to emotional problems, social functioning, and vitality (Cronbach's α = 0.78 at T0). The other four SF-36 subscales were combined into a physical health component scale, namely general health perceptions, physical functioning, role limitations due to physical health problems, and bodily pain (Cronbach's α = 0.79 at T0). Earlier principal component analyses on the SF-36 have provided support for the validity of these two component scales (McHorney et al. Reference McHorney, Ware and Raczek1993; Sanson-Fisher & Perkins, Reference Sanson-Fisher and Perkins1998). Scores on these scales range from 0 (poor) to 100 (good).
DSM-IV diagnoses were established with the Composite International Diagnostic Interview (CIDI) version 3.0, a fully structured lay-administered diagnostic interview (Kessler & Üstün, Reference Kessler and Üstün2004). At all three waves, the disorders assessed were mood disorders (major depressive disorder, dysthymia, bipolar disorder), anxiety disorders (generalized anxiety disorder, panic disorder with or without agoraphobia, agoraphobia without panic disorder, specific phobia, and social phobia), and substance use disorders (alcohol/drug abuse and dependence). The CIDI 3.0 assesses these disorders with generally good validity in comparison with blinded clinical reappraisal interviews (Haro et al. Reference Haro, Arbabzadeh-Bouchez, Brugha, de Girolamo, Guyer and Jin2006). At all waves, 12-month diagnoses were assessed. Lifetime diagnoses were assessed at T0. At T1 and T2, an adapted CIDI version was used with as timeframe the period between T0–T1 and T1–T2, respectively.
Healthy controls (n = 2826) were defined as participants who did not have any lifetime diagnosis of a mood, anxiety, or substance use disorder at T0, and who did not develop any within the T0–T1 interval (Table 1). MDD (n = 295) individuals were defined as participants who did not have a 12-month diagnosis of MDD at T0 and who developed an MDD within the T0–T1 interval. The distinction between a first-onset (n = 181) and recurrent MDD (n = 114) was determined according to the absence or presence of a CIDI lifetime diagnosis of MDD at T0. Severity of the MDD was assessed at T1 in those who had a CIDI 12-month diagnosis of MDD and was based on criteria used in previous studies (Demyttenaere et al. Reference Demyttenaere, Bruffaerts, Posada-Villa, Gasquet, Kovess and Lepine2004; Ten Have et al. Reference Ten Have, Nuyen, Beekman and de Graaf2013). Disorders were classified as severe if participants reported severe impairments in at least two areas of role functioning on the Sheehan Disability Scales (SDS, Leon et al. Reference Leon, Olfson, Portera, Farber and Sheehan1997); as moderate in case of moderate role impairment in any domain of the SDS; and the remaining were classified as mild. The SDS is a self-report measure of disability in four role domains (home, work, social, close relationships) and is incorporated in the CIDI 3.0. The scale has shown good reliability and validity (Leon et al. Reference Leon, Olfson, Portera, Farber and Sheehan1997). It consists of four questions assessing, on a scale from 0 to 10, the extent to which a particular disorder interfered with activities in one of the four role domains during the month in the past year when the disorder was most severe. Note that this measure of severity is based on the impact the disorder has on a person's functioning rather than on symptom severity.
Table 1. Baseline characteristics of participants who developed a 12-month major depressive disorder between T0 and T1 (first-onset and recurrent MDD) and healthy controls (HC)
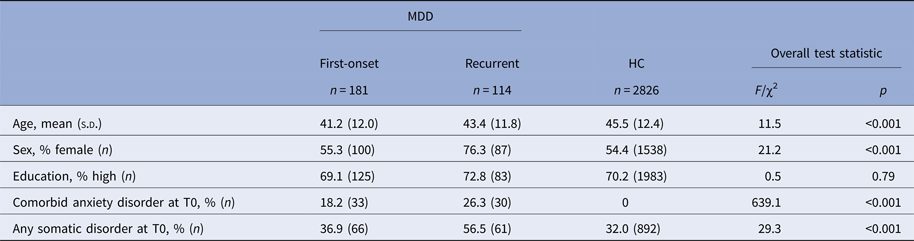
F, F-value from analysis of variance test; χ2, Chi-square value from Chi-square test.
Post-hoc tests show that the difference in sex and somatic comorbidity is only significant for the recurrent subgroup versus HC comparison.
Covariates
Covariates used in the analyses consisted of demographic and clinical variables, and were chosen based on their presumed association with both predictor and outcome. Demographic variables were assessed at baseline and included age, sex, and education. Education was defined as either low (primary, basic vocational, and low secondary education) or high (high secondary education, higher professional education, and university). Clinical variables included the presence of a comorbid anxiety disorder and the presence of a somatic disorder. The presence of a comorbid anxiety disorder (yes/no) was defined as any 12-month anxiety disorder according to the CIDI. This was assessed at all three waves. The presence of somatic disorders was also assessed at all three waves and ‘any somatic disorder’ was considered present if one or more of 17 chronic somatic disorders from a standard checklist was reported and had been treated or monitored by a medical doctor in the previous 12 months. These disorders included the most common chronic somatic disorders such as hypertension, chronic backache, rheumatoid disease, asthma, and diabetes. Comparisons between self-reports of chronic somatic disorders and medical records show moderate to good concordance (Baker et al. Reference Baker, Stabile and Deri2004; NCHS, 2004). Subthreshold residual symptoms were assessed at T2 with the K10, a self-report screening questionnaire with strong psychometric properties assessing psychological distress in the depression-anxiety spectrum in the past 4 weeks with 10 items on a five-point scale (Kessler et al. Reference Kessler, Andrews, Colpe, Hiripi, Mroczek and Normand2002; Donker et al. Reference Donker, Comijs, Cuijpers, Terluin, Nolen and Zitman2010). The K10 has proven to be both reliable and valid (Kessler et al. Reference Kessler, Andrews, Colpe, Hiripi, Mroczek and Normand2002; Donker et al. Reference Donker, Comijs, Cuijpers, Terluin, Nolen and Zitman2010).
Statistical analysis
Vulnerability effect
To examine the presence of a vulnerability effect, we compared the level of functioning at baseline (T0) between the healthy controls (n = 2826) and participants who did not have a lifetime diagnosis of MDD at T0 and who developed a first-onset MDD between T0 and T1 (n = 181). Differences in demographic characteristics between the healthy and MDD group were examined using Chi-square tests for categorical variables and one-way ANOVA for continuous variables. First, mental functioning and physical functioning at T0 (thus prior to the development of the depressive episode) were compared between the first-onset MDD and the healthy group using linear regression analyses. The analyses were subsequently adjusted for age, sex, and education. The analyses on mental functioning were additionally adjusted for 12-month comorbid anxiety disorders at baseline, and the analyses on physical functioning were additionally adjusted for 12-month somatic disorders at baseline. We also examined whether the recurrent MDD subgroup (n = 114) differed from the HC group, and whether the first-onset and the recurrent MDD subgroups differed from each other. We further examined whether vulnerability effects were different for those who developed a mild, v. a moderate, v. a severe first-onset depressive episode. Because severity could only be assessed in those who had a 12-month diagnosis at T1 (n = 118, of the total of 181 in the first-onset MDD group), we restricted the analyses on severity subtype to these participants. Multiple tests were conducted, with a maximum of nine tests for each outcome measure. To ensure that the cumulative type I error remained below 0.05, an effect was considered significant if the p value was below 0.0056. A power analysis showed that with this adjusted alpha level we still had a power of 0.99 to detect a difference with a moderate effect size (d = 0.50) between the first-onset MDD group and the HC group.
Scar effect
To assess the presence of a scar effect, we compared the level of functioning prior to the onset of the depressive episode with the level of functioning following remission of the depressive episode. We did so in participants without a 12-month MDD at T0, who had a 12-month MDD at T1, and who had no 12-month MDD at T2 (i.e. were remitted at T2). This sample consisted of 108 participants. We used the 12-month diagnosis at T1 (and not the 3 years interval diagnosis at T1) to be able to assess the severity of the disorder. To account for the repeated measurements in the data, linear mixed models were used with mental and physical functioning as dependent variables and dummy variables for time T1 and time T2 as independent variables (time T0 being the reference category). The restricted maximum likelihood was used as the estimation method and the Akaike Information Criterion was used for model selection. First, we examined a model with the dummy variables for time T1 and time T2 only. For mental functioning this resulted in a model with a random intercept, a random slope for time T2, and an independent covariance structure for the random effects and the residuals. For physical functioning, the same model without random slopes was optimal.
Next, we adjusted the models for age, sex, and education, and additionally for comorbid anxiety disorders (mental functioning model) and somatic disorders (physical functioning model). The latter variables were time-varying. When a scar effect was present after remission, lower functioning scores would be found at time T2 compared with time T0. A state effect would be visible in worse functioning scores at time T1 compared with T0. Finally, we examined the presence of a scar effect in specific subgroups (first-onset v. recurrent episode; mild v. moderate v. severe episode), by adding the subgroup variable and the interaction between this variable and the time variables to the adjusted model (two separate models). In view of the smaller sample size in the scar analyses and the lower total number of tests, we adopted a somewhat less conservative p value adjustment here (Leon, Reference Leon2014), using an α-level of 0.0167. A power analysis showed that with this adjusted alpha level we still had a power of 0.92 to detect a difference with a moderate effect size in functioning before v. after the depressive episode in the sample of 108 MDD patients.
Prodromal and residual symptoms
Vulnerability effects may be biased by prodromal symptoms, because premorbid assessments done just before the onset of the depressive episode may reflect prodromal symptomatology (Ormel et al. Reference Ormel, Oldehinkel, Nolen and Vollebergh2004a). Scar effects, as estimated in the pre-post comparison, may be biased by both prodromal and residual symptoms; if the post-morbid assessment takes place just after remission of the depressive episode, scarring effects may be overestimated due to the presence of residual symptoms. If the premorbid assessment takes place just before the onset of the depressive episode, scarring effects may be obscured due to the presence of prodromal symptoms. To examine whether residual symptoms may have biased the results on scarring, we checked whether the type and severity subgroups differed with respect to their K10 scores at T2 using one-way ANOVA. Because the K10 was only assessed at T2, not at T0, we examined whether prodromal symptoms may have biased the results in an indirect way, by comparing MDD participants with a depression onset within 1 year after T0 v. those with a later onset in the T0–T1 period on mental and physical functioning.
Results
Baseline demographic and clinical characteristics of the 181 first-onset and 114 recurrent MDD participants are presented in Table 1 and compared with those of the 2826 healthy individuals. Individuals in both MDD subgroups were on average younger and more often suffered from a comorbid anxiety disorder at baseline than healthy individuals. Individuals in the recurrent subgroup were also more often female and more often suffered from a somatic disorder at baseline than healthy individuals.
Vulnerability effect
The regression models showed that mental functioning at T0, before the depressive episode developed, was significantly lower in the first-onset MDD group compared to the healthy group (B = −7.8, s.e. = 0.8, p < 0.001). Adjusting the analyses for age, sex, education, and anxiety comorbidity did not substantially change these results (see Table 2). Physical functioning was also significantly lower at baseline in the first-onset MDD group than in the healthy group in unadjusted (B = −8.8, s.e. = 1.2, p < 0.001) and adjusted analyses (Table 2). Thus, in participants who developed a first depressive episode after T0, both mental and physical functioning were already impaired prior to the onset of the depressive episode.
Table 2. Evaluating vulnerability effects; mental and physical functioning at T0 of participants who developed a 12-month major depressive disorder between T0 and T1 (first-onset and recurrent MDD) and healthy controls
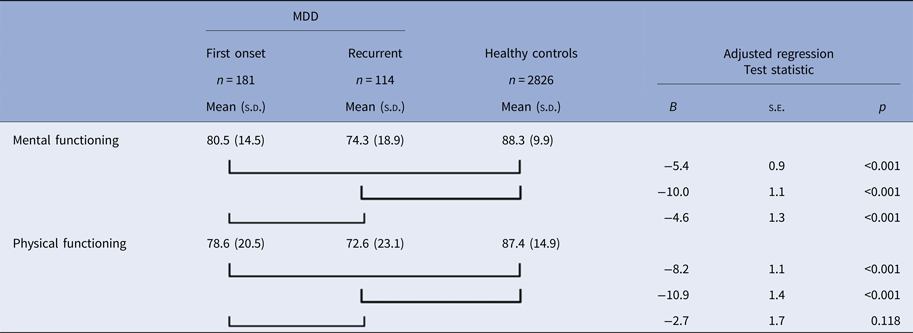
Regression analyses on mental functioning adjusted for age, sex, education, and any anxiety disorder; regression analyses on physical functioning adjusted for age, sex, education, and somatic disorders.
Mental functioning at T0 was significantly lower also in the recurrent MDD subgroup (n = 114) compared with the healthy group (Table 2). The same was true for physical functioning. Further, the recurrent subgroup showed lower mental functioning than the first-onset subgroup (Table 2). The difference in physical functioning between the recurrent and first-onset subgroups was not significant.
Next, we examined whether the severity of the depressive episode influenced the results. Of the 118 first-onset participants in which episode severity was assessed, 12 had a mild, 39 a moderate, and 67 a severe depressive episode. Mental functioning was significantly lower at T0 in the severe subgroup compared with the healthy group (Table 3). The mild and moderate subgroup did not differ significantly from the healthy group. The severe subgroup also had lower mental functioning at T0 than the moderate subgroup, while the difference between the severe and mild subgroup showed a trend (p = 0.010). The moderate subgroup did not differ significantly from the mild subgroup. Similar results were found for physical functioning, which was also significantly lower at T0 only in the severe subgroup compared with the healthy group (Table 3). The severe subgroup also had lower physical functioning than the moderate subgroup, while the difference between the severe and mild subgroup showed a trend (p = 0.011). The moderate subgroup did not differ significantly from the mild subgroup. Thus, those who developed the most severe depressive episode between T0 and T1 also had the worst level of mental and physical functioning prior to episode onset compared with those with less severe or no episodes.
Table 3. Evaluating vulnerability effects in different severity groups; mental and physical functioning at T0 of participants who developed a first-onset 12-month major depressive disorder between T0 and T1 (mild, moderate, and severe MDD) and healthy controls
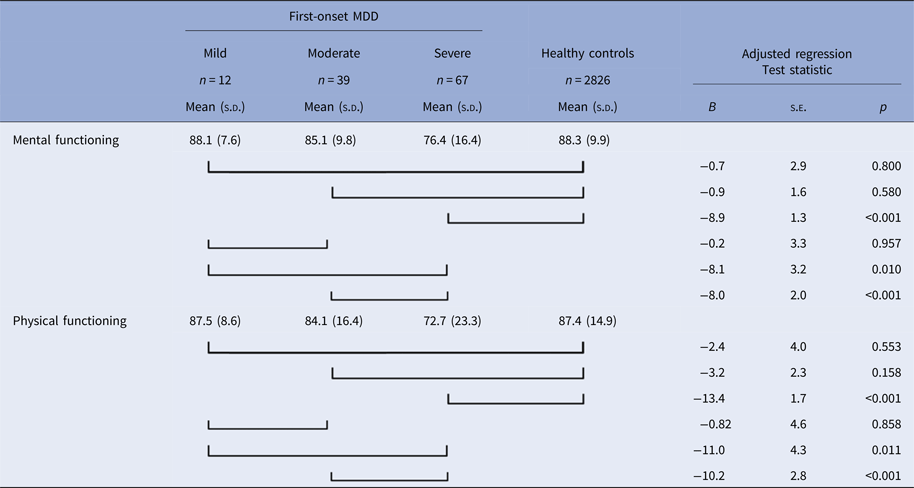
Regression analyses on mental functioning adjusted for age, sex, education, and any anxiety disorder; regression analyses on physical functioning adjusted for age, sex, education, and somatic disorders.
Severity refers to role functioning impairment due to the depressive episode, as assessed by the Sheehan Disability Scales.
To test whether prodromal symptoms may have biased the results, we examined whether first-onset participants whose depressive episode started within 1 year after T0 (n = 87) differed from participants with a later onset (n = 94) on baseline mental and physical functioning. This was not the case (adjusted analyses; mental functioning, B = −0.3, s.e. = 2.1, p = 0.88; physical functioning, B = −2.3, s.e. = 2.7, p = 0.40). Thus, the observed vulnerability effects in mental and physical functioning were not likely to be reflections of prodromal symptoms.
Scar effect
The characteristics of the 108 MDD participants who had developed a 12-month diagnosis of depression at T1 and were remitted at T2 are described in Table 4. Mean mental functioning in this group was lower at T1 than at T0, prior to the development of the depressive episode, and returned to premorbid levels following remission at T2 (see Table 5). The linear mixed models showed a state effect but no scar effect with regard to mental functioning, both in unadjusted (time T1, B = −15.1, s.e. 2.4, p < 0.001; time T2, B = −2.3, s.e. 1.8, p = 0.18) and adjusted analyses (Table 5). The random slope for time T2 was significant (s.d. = 17.3, 95% CI 13.7–21.7), which implies that there was large heterogeneity in the scarring effect. Physical functioning was higher at T0 than at T1 and T2. In the unadjusted model for physical functioning, both the state effect (time T1, B = −5.7, s.e. = 2.1, p = 0.006) and the scar effect (time T2, B = −5.1, s.e. = 2.1, p = 0.014) showed significance. In the adjusted analyses, neither the state nor the scar effect was significant (Table 5).
Table 4. Characteristics of participants who developed a 12-month major depressive disorder between T0 and T1 and were remitted at T2, as used to examine a scar effect
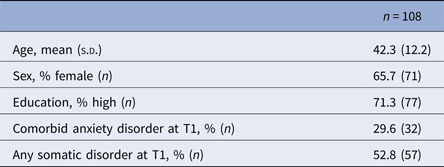
Table 5. Evaluating scar effects; mental and physical functioning of participants who developed a 12-month major depressive disorder between T0 and T1 and were remitted at T2
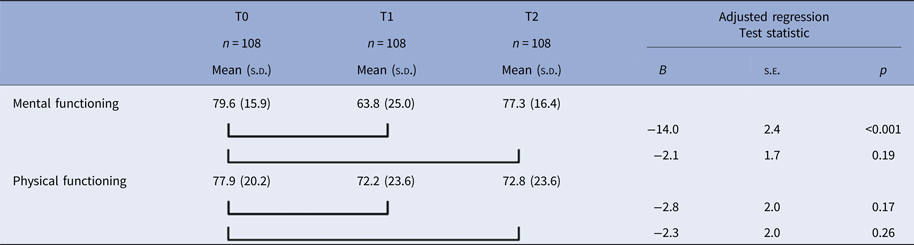
Regression analyses on mental functioning adjusted for age, sex, education, and any anxiety disorder; regression analyses on physical functioning adjusted for age, sex, education, and any somatic disorder.
Subsequently, we examined whether any scar effects were different for specific subgroups, according to the type of disorder (first-onset, n = 70 v. recurrent, n = 38) and disorder severity (mild, n = 10 v. moderate, n = 41 v. severe, n = 57). In both the mental functioning and the physical functioning model, neither of the interaction terms were significant, indicating that scar effects were not more pronounced in individuals with recurrent of more severe episodes (results not shown).
Next, we examined whether scarring effects were different in participants with an onset within one year after T0 (n = 35) and participants with a later onset (n = 73). Also in these models, no significant interaction effects were observed. Finally, we examined whether residual symptoms levels (K10 scores) were different for those with a first-onset v. a recurrent episode, which was not the case [F (1106) = 0.37, p = 0.54]. Neither were K10 scores different for subgroups with different disorder severity [F (2105) = 0.43, p = 0.52]. So, the results were not likely influenced by prodromal or residual symptoms.
Discussion
The present three-wave population-based study was aimed to distinguish between pre-existing vulnerability and scar effects in mental and physical functioning after the experience of an episode of major depressive disorder. We did so by comparing individuals who developed a first-onset depressive episode during follow-up with healthy controls (vulnerability effect), and with themselves after remission of the depressive episode (scar effect). The results provide more support for the vulnerability than the scar hypothesis: both mental and physical functioning were already impaired prior to the onset of the depressive episode, and no firm evidence of scarring after remission was found. Subgroup analyses showed that the vulnerability effects mainly applied to persons with a severe depressive episode (‘severe’ in terms of role functioning impairment).
The results are congruent with those of previous studies done on this topic, the majority of which showed vulnerability but not scar effects on diverse outcomes (for reviews, see Christensen & Kessing, Reference Christensen and Kessing2006; Burcusa & Iacono, Reference Burcusa and Iacono2007; Sowislo & Orth, Reference Sowislo and Orth2013; Allott et al. Reference Allott, Fisher, Amminger, Goodall and Hetrick2016). Notably, studies that did find effects congruent with the scar hypothesis most often had a design in which individuals with remitted depression were compared with healthy controls, a design in which vulnerability and scar effects are confounded (Just et al. Reference Just, Abramson and Alloy2001). Possibly, much of the impairments observed in remitted patients can be accounted for by pre-existing characteristics that predispose these people to depression (vulnerability) or are due to common causes. Similarly, the often-made assertion that risk of recurrence increases with every further episode (Kessing et al. Reference Kessing, Hansen, Andersen and Angst2004), which has often been considered as support for the scar hypothesis, may actually be explained by pre-existing between-subjects differences: people with more previous episodes than other people may have a higher risk of recurrence just because they were vulnerable to recurrent episodes right from the start. Also in our study persons with recurrent MDD showed lower mental functioning before the onset of the episode than healthy persons, and their functioning was also lower than persons with first-onset depression. This may thus reflect a pre-existing differential vulnerability. Alternatively, the latter difference could be a reflection of scarring that occurred earlier in life. However, our subsequent analyses did not show evidence of scarring.
Although we did not find firm evidence for scarring in our study (only a scarring effect in physical functioning in unadjusted analyses), there was substantial heterogeneity in the degree to which mental functioning was still lowered after remission of the depressive episode. This heterogeneity could not be explained by type, severity, or timing of the depressive episode. There are not many studies who examined such subgroups. Ormel et al. (Reference Ormel, Oldehinkel, Nolen and Vollebergh2004a) did so, and found some scarring in psychosocial functioning in individuals with severe recurrent episodes, in unadjusted analyses. In our unadjusted analyses, we found significant scarring in physical functioning, but this effect disappeared after adjustment, which suggests confounding by the presence of somatic disorders. An alternative explanation for this finding might be that the presence of MDD increases the likelihood of developing a somatic disorder. The high percentage of people with recurrent MDD having comorbid somatic disorders (Tables 1 and 4) is remarkable in this respect. If this alternative explanation would be true, we might have over-adjusted by using somatic disorders as a confounder, since this somatic disorder may be considered a scar. These reflections point to the more general problem that it is always hard to decide which variables should be used as potential confounders. In a recent study on scarring after remission of an anxiety disorder (Schopman et al. Reference Schopman, ten Have, van Dorsselaer, de Graaf and Batelaan2017), scarring was found in mental but not physical functioning in the subgroup with recurrent anxiety, also after adjustment. Power issues are also prevailing here, because subgroups were often very small, also in our study. Further, it might be that any scarring already occurred before T0 in those with previous episodes, limiting further scarring. Thus, although it may still be too early to discard the idea that scarring occurs altogether – maybe scarring does occur, but in a subtle way (Wichers et al. Reference Wichers, Geschwind, van Os and Peeters2010), in subgroups we did not investigate, or in outcome measures we did not investigate (Burcusa & Iacono, Reference Burcusa and Iacono2007; Bhagwagar & Cowen, Reference Bhagwagar and Cowen2008) – our study suggests there is no robust scarring effect.
In contrast to the scarring effects, the vulnerability effects observed in the present study were robust and substantial. Effect sizes, calculated by dividing the adjusted estimated mean difference between the groups by the pooled standard deviation (Cohen, Reference Cohen1988, p.44), were 0.43 for mental functioning and 0.46 for physical functioning (first-onset MDD group), and especially large in the severe subgroup (0.66 for mental functioning; 0.69 for physical functioning). These effect sizes are slightly larger than those found for the average prospective association between neuroticism and depression, one of the most notorious risk factors for depression (Jeronimus et al. Reference Jeronimus, Kotov, Riese and Ormel2016). Functional limitations following remission from depression thus presumably reflect pre-existing vulnerabilities rather than scars. This may have clinical implications, as people with impaired functioning in either mental, social, or physical areas are at increased risk of (recurrence of) depression (Lewinsohn et al. Reference Lewinsohn, Rohde and Seeley1998; Oldehinkel et al. Reference Oldehinkel, Bouhuys, Brilman and Ormel2001; Solomon et al. Reference Solomon, Leon, Endicott, Mueller, Coryell and Shea2004; Conradi et al. Reference Conradi, de Jonge and Ormel2008), and these impairments are not merely reducible to subthreshold symptoms (Spijker et al. Reference Spijker, de Graaf, Oldehinkel, Nolen and Ormel2007). Treatment and prevention strategies may be thus improved by targeting patients’ level of functioning. Solely focusing on symptom reduction may be ‘trying to dry out a flooded room without turning off the taps’, if the underlying vulnerability is not addressed.
A vulnerability perspective of depression may also have implications for how we conceive this disorder. Depression is, even though operationalized as episodic, seldom an isolated event, but rather an expression of an underlying vulnerability. Many persons suffering from a current depressive episode have had other mental or somatic disorders in their past, which may be indicative of this vulnerability. Previous work on lifetime comorbidity of mental disorders has shown that disorders tend to cluster in large domains of psychopathology, such as internalizing and externalizing disorders (Kessler et al. Reference Kessler, Cox, Green, Ormel, Mclaughlin and Merikangas2011a, Reference Kessler, Ormel, Petukhova, McLaughlin, Green and Russob). Depression has a median age of onset around 30 (Bromet et al. Reference Bromet, Andrade, Hwang, Sampson, Alonso and de Girolamo2011), while some of the other disorders in the internalizing domain, such as specific phobia (age 8) or social phobia (age 14), have a considerably earlier onset (Lijster et al. Reference Lijster, Dierckx, Utens, Verhulst, Zieldorff and Dieleman2017; Wardenaar et al. Reference Wardenaar, Lim, Al-Hamzawi, Alonso, Andrade and Benjet2017). Perhaps such disorders with an early age of onset can be seen as markers of an underlying internalizing vulnerability that eventually leads to disorders such as depression and generalized anxiety disorder. Viewing psychopathology from a lifespan perspective opens the way of preventing depression in such targeted subpopulations. It should also be noted that although disorders are typically defined as discrete, the underlying vulnerability may well be continuous (Ten Have et al. Reference Ten Have, Lamers, Wardenaar, Beekman, de Jonge and van Dorsselaer2016).
Limitations of the present study include the self-report nature of our measures and the fact that they were assessed retrospectively, which might have induced some mood-related reporting bias. However, functioning was assessed prior to onset and 1 year after remission of the depression, presumably reducing this bias to acceptable levels. Further, we examined whether the timing of the onset (as a proxy for prodromal symptom severity) and residual symptom severity influenced the results, which was not the case. Another limitation may be that we used the Sheehan disability scale as a measure of depression severity, which may potentially overlap with our outcome measures. However, the SDS assesses disability due to the presence of the disorder, whereas the SF-36 assesses mental and physical functioning in general. A further limitation is that we did not assess prodromal symptom severity directly, but instead used the timing of the onset, because the K10 was not assessed at T0.
To conclude, this study suggests that limitations in mental and physical functioning following remission of depression reflect a pre-existing vulnerability rather than a scar. Depressive disorders might be conceived as life-stage-dependent manifestations of an underlying internalizing vulnerability dimension. A lifespan perspective of psychopathology opens the way of prevention strategies targeting this vulnerability early in life.
Acknowledgements
NEMESIS-2 is conducted by the Netherlands Institute of Mental Health and Addiction (Trimbos Institute) in Utrecht. Financial support has been received from the Ministry of Health, Welfare and Sport, with supplementary support from the Netherlands Organization for Health Research and Development (ZonMw) and the Genetic Risk and Outcome of Psychosis (GROUP) investigators. All financial support was without any role or interference in the design and conduct of the study or interpretation of the data.
Declaration of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







