Introduction
When outbreaks of the spruce budworm (Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) came to the attention of Canadian foresters in the mid-20th century, entomologists already recognised the distinct forms of budworms living on different conifer tree species in different forest regions of North America. They also knew that spruce budworm outbreaks had damaged Canadian forests several times in the past (Craighead Reference Craighead1924; Mathers Reference Mathers1932; Turner Reference Turner1952; Blais Reference Blais1954). What was new by mid-century was that both forests and the industry they supported were changing rapidly and disturbances such as the spruce budworm were seen as a significant threat. Most jurisdictions in Canada had legislation that enabled active forest management and by the 1950s aerial application of pesticides to protect forests from spruce budworm became routine (Webb et al. Reference Webb, Blais and Nash1961).
Since then, there has rarely been a year in living memory without significant forest damage caused by a budworm outbreak somewhere in Canada (Fig. 1). The basic research that accompanied the forest management response to these outbreaks has resulted in a forest-insect system for which knowledge and alternative interpretations are uncommonly rich. Much of this knowledge has been gained by scientists working in Canada. The concentration of expertise in a few government agencies and universities working on a mostly publically owned natural resource resulted in continuous effort that sustained both extensive annual surveys as well as investigation of the fundamental biology and ecology of these insects.
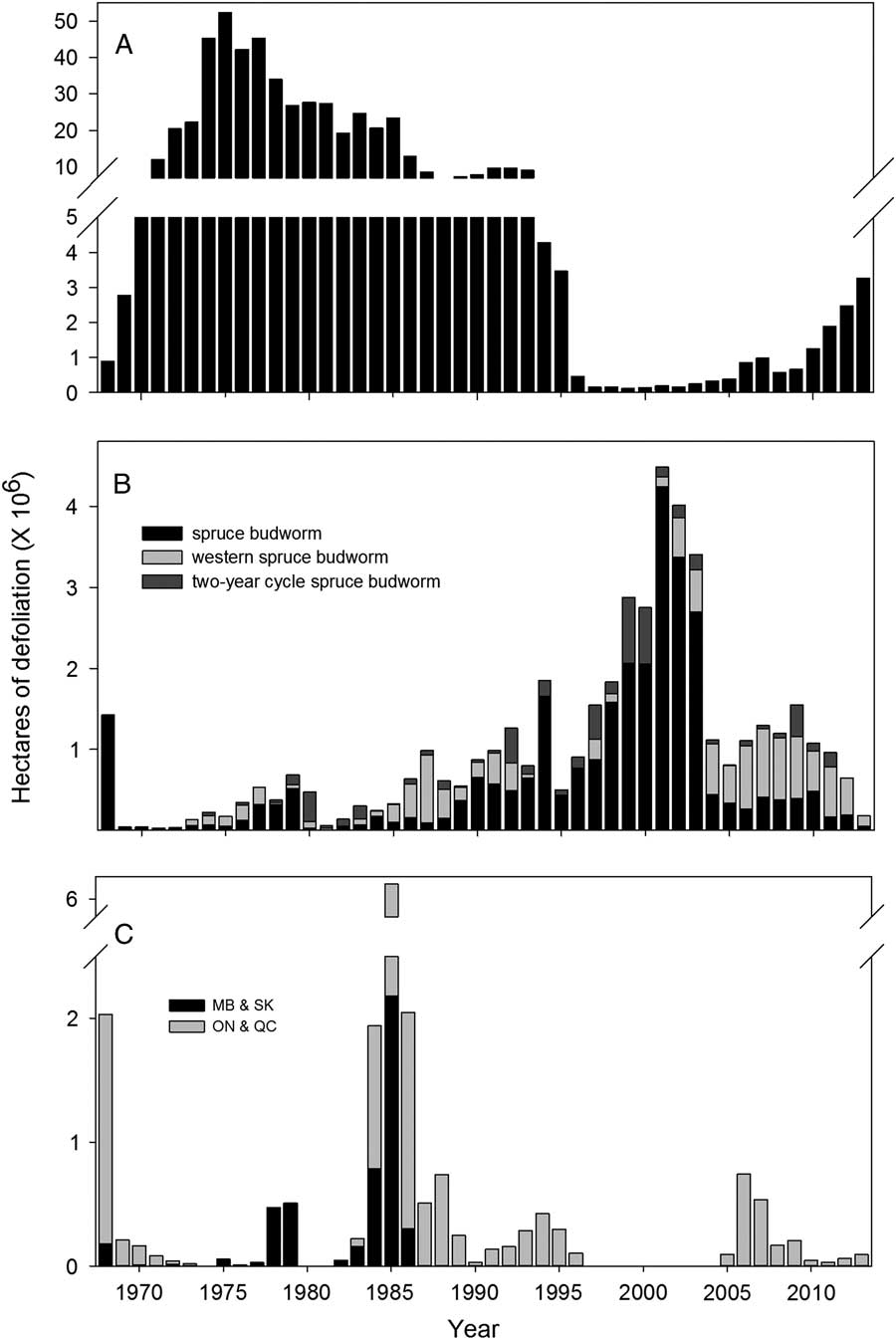
Fig. 1 Area of defoliation (millions of ha) by (A) spruce budworm in eastern Canada (Newfoundland and Labrador, New Brunswick, Nova Scotia, Prince Edward Island, Québec, Ontario), (B) spruce budworm, two-year cycle spruce budworm, and western spruce budworm in western Canada (Manitoba, Saskatchewan, Alberta, British Columbia, Yukon, Northwest Territories), and (C) jack pine budworm in Manitoba (MB), Saskatchewan (SK), Ontario (ON), and Québec (QC). (Source: National Forestry Database. http://nfdp.ccfm.org/insects/quick_facts_e.php accessed 23 September 2014).
This paper compares the ecology of the major conifer-feeding species in the genus Choristoneura Lederer (Lepidoptera: Tortricidae) in Canada. These closely-related species share phylogenetic constraints which characterise their common evolutionary histories, and related adaptive syndromes which mitigate the fitness consequences of these constraints (Price Reference Price1994). The ecological relationships that emerge from these adaptations determine macro-ecological phenomena such as eruptive population cycles. I begin with a review of budworm systematics, life history, and seasonality followed by the evidence on historic outbreaks. These are the foundational elements and resulting patterns of population ecology. Next, I examine trophic interactions with a focus on new knowledge compiled in the past 30 years, coincidentally the duration of a typical research career. Trophic interactions are interpreted as ecological drivers that transcribe the consequences of adaptation in a variable environment to patterns of abundance in space and time.
The primary literature on the spruce budworm, C. fumiferana, alone is formidable. Including publications on other conifer-feeding budworm species makes parsimonious selection of citations a challenge. In keeping with the theme of this special issue, I have favoured publications by Canadian scientists where appropriate although given the volume of these the choices were not reduced by much. Excellent reviews became available following the last outbreak (Harvey Reference Harvey1985; Volney Reference Volney1989; Sanders Reference Sanders1991) so I also have tried to restrict myself to post-1980 publications except what I consider to be original, seminal contributions that provide the enduring ideas and evidence that have influenced my view of budworm ecology.
Conifer-feeding spruce budworms
Systematics
So who are these budworms? Biosystematic analysis supports eight species in the spruce budworm complex of conifer-feeding Choristoneura in North America including six species in Canada (Lumley and Sperling Reference Lumley and Sperling2010). Many more biotypes are recognised (Volney and Fleming Reference Volney and Fleming2007) and so more cryptic species may be revealed eventually (Lumley and Sperling Reference Lumley and Sperling2011). Populations of four species: (eastern) spruce budworm; jack pine budworm, C. pinus pinus Freeman; western spruce budworm, C. occidentalis Freeman; and two-year cycle spruce budworm, C. biennis Freeman; have erupted extensively at least once during the last 50 years, a period representing barely one generation of tree and forest (Fig. 1). Collectively these species occupy most of Canada’s major conifer forest ecozones (Fig. 2). The distribution and status in Canada of the coastal spruce budworm, C. orae Freeman, and a western pine-feeding budworm, C. lambertiana (Busck), are poorly known and not considered further.
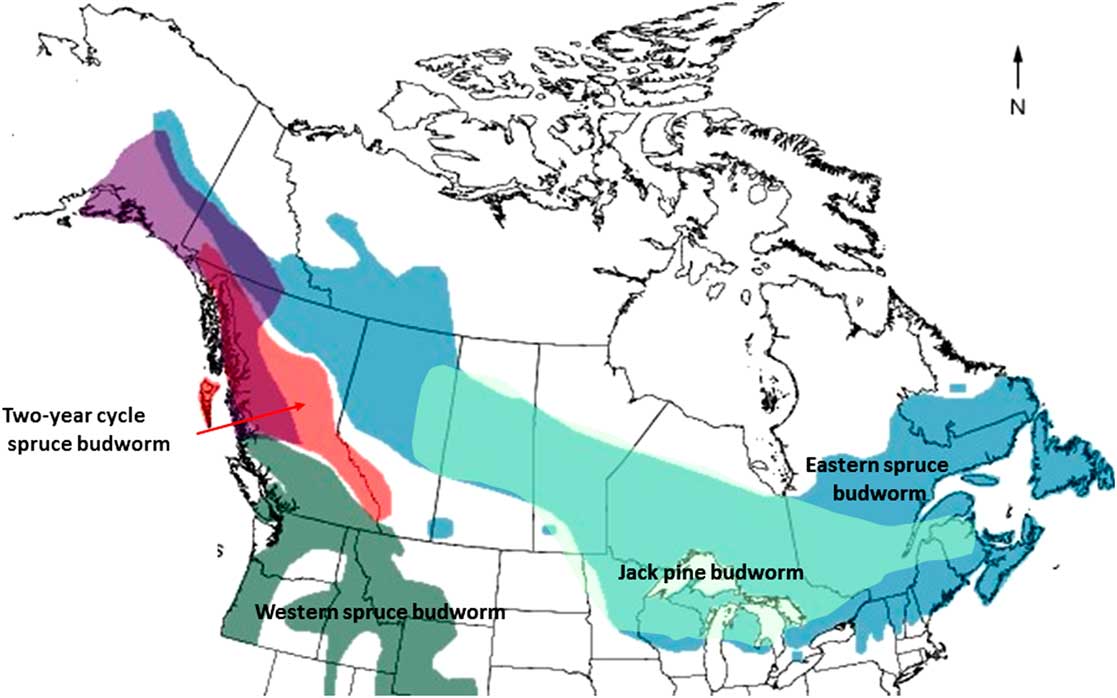
Fig. 2 Geographic distribution of major conifer-feeding budworms in the genus Choristoneura in North America (after Harvey Reference Harvey1985, courtesy of R Alfaro).
The near identical life histories of these budworm species suggest close phylogenetic relationships and recent divergence. Until the 1950s, forest entomologists applied the species name “fumiferana” to all budworms even though they recognised different biogeographic entities associated with different host-tree species. The outbreaks that occurred in the mid-20th century prompted taxonomic revision of the group drawn from detailed morphological studies of adults (Freeman Reference Freeman1947) and larvae (MacKay Reference MacKay1962). The many polymorphisms within species and overlap of characters among species, however, obscured clear separation of species based on conventional morphology (Harvey Reference Harvey1985, Reference Harvey1996). Further, successful hybridisation between most species combinations and their progeny demonstrated high genetic compatibility within the genus (Volney Reference Volney1989; Harvey Reference Harvey1997; Nealis Reference Nealis2005). Nonetheless, Freeman (Reference Freeman1967) recognised the distinct species mentioned above among the different forms while the search continued for diagnostic morphological characters (Dang Reference Dang1985) and biochemical attributes such as pheromones (Silk and Kuenen Reference Silk and Kuenen1988), isozymes (Harvey Reference Harvey1985), and mitochondrial DNA (Sperling and Hickey Reference Sperling and Hickey1995).
Despite this dedicated search for clear distinctions, effective separation of species requires information on morphology, geography, ecology, and genetics (Lumley and Sperling Reference Lumley and Sperling2010). As noted by Stehr (Reference Stehr1967), the simplest, most reliable method for distinguishing budworm species in Canada remains the geographic location and host-tree associations on which the population in question is found. This practical method of identification may become less reliable in western Canada as changing climate and forest composition create new areas of sympatry and potential hybridisation (Volney and Fleming Reference Volney and Fleming2007). Nonetheless, this biogeographical pattern and ecological relationship with the host-tree forms the reference point for this comparative review of their ecology. For brevity, I refer to “budworms” when describing attributes common to all species and either the scientific or accepted common name when making species distinctions.
Life history
Conifer-feeding budworms are relatively specialised, feeding on one or at most a few genera of tree species within a single plant family (Pinaceae). Each life stage is intimately associated with their preferred host species (Table 1). Adult moths mate soon after eclosion in mid-summer to late-summer and lay eggs on needles in imbricated masses ranging from 20 or fewer eggs per mass in the spruce budworm (Nealis and Régnière Reference Nealis and Régnière2004a) to 60 eggs per mass in the jack pine budworm (Fowler and Simmons Reference Fowler and Simmons1987) with the other species intermediate. Foliage architecture affects female oviposition behaviour (Grant Reference Grant2006) and so the number of eggs per mass characteristic of a budworm species may be related to needle length of its preferred host (balsam fir and spruces have the shortest, and jack pine the longest, needle lengths in the spectrum). Lifetime fecundity for female spruce budworms can vary nearly three-fold (80–220 eggs) depending on the quality and quantity of food (Nealis and Régnière Reference Nealis and Régnière2004a). A similar range in lifetime fecundity has been estimated for western spruce budworm (personal observation) and available estimates of mean fecundity for jack pine budworm (≈130 eggs) are comparable (Nealis Reference Nealis1995).
Table 1 Host-trees and mean (range) outbreak periodicity and duration for major conifer-feeding budworms in North America.
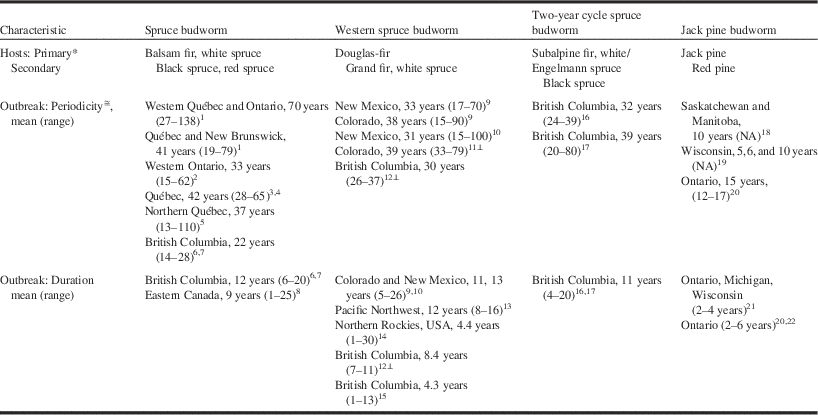
1Blais (Reference Blais1983), 2Blais (Reference Blais1985), 3Boulanger and Arseneault (Reference Boulanger and Arseneault2004), 4Boulanger et al. (Reference Boulanger, Arseneault, Morin, Jardon, Bertrand and Dagneau2012), 5Morin et al. (Reference Morin, Paprise and Bergeron1993), 6Shore and Alfaro (Reference Shore and Alfaro1986), 7Burleigh et al. (Reference Burleigh, Alfaro, Borden and Taylor2002), 8Gray (Reference Gray2013), 9Swetnam and Lynch (Reference Swetnam and Lynch1989), 10Swetnam and Lynch (Reference Swetnam and Lynch1993), 11Ryerson et al. (Reference Ryerson, Swetnam and Lynch2003), 12Alfaro et al. (Reference Alfaro, Berg and Axelson2014), 13Flower et al. (Reference Flower, Gavin, Heyerdahl, Parsons and Cohn2014), 14Johnson and Denton (Reference Johnson and Denton1975), 15Parfett et al. (Reference Parfett, Clarke and Van Sickle1994), 16Zhang and Alfaro (Reference Zhang and Alfaro2002), 17Zhang and Alfaro (Reference Zhang and Alfaro2003), 18Volney (Reference Volney1988), 19Volney and McCullough (Reference Volney and McCullough1994), 20Scarr et al. (Reference Scarr, Ryall and Hodge2012), 21McCullough (Reference McCullough2000), 22Nealis et al. (Reference Nealis, Magnussen and Hopkin2003).
* Tree species on which extensive and significant damage are observed in Canada.
≅ Interval between initial years of observed damage in regional outbreaks.
⊥ Calculated from reported means.
Hatch occurs in one to two weeks. Neonate larvae do not feed but disperse immediately in search of sheltered niches on the host tree where they spin an individual hibernaculum, moult, and pass late summer, autumn, and winter as a second-stage larva. Diapause terminates gradually in late winter after which budworms remain dormant until warmer spring conditions prevail (Régnière Reference Régnière1990; Nealis and Régnière Reference Nealis and Régnière2014). Emergence of second-instar budworm from their hibernacula in the spring occurs in advance of bud-flush, at least in univoltine individuals, so that budworms must first mine previous-years’ needles (McGugan Reference McGugan1954; Shepherd Reference Shepherd1992) and forage continuously within and between the crowns of trees to find current-year pollen cones and swelling buds (Sanders Reference Sanders1991). Many small budworms are lost during this dispersal stage (Miller Reference Miller1958; Nealis and Lomic Reference Nealis and Lomic1994; Nealis and Régnière Reference Nealis and Régnière2009). I return to this key point in later sections.
As bud development proceeds, budworms enter the buds, construct feeding shelters and pass through four or five more instars (McGugan Reference McGugan1954; Nealis Reference Nealis1987; Nealis and Régnière Reference Nealis and Régnière2014). As in all parts of the life cycle following egg hatch, budworms prefer a solitary habit during the feeding period and are reluctant to move from their feeding shelters as long as current-year foliage is available. Back-feeding on previous-years’ foliage may occur in extremely high population densities, especially when the hosts are true fir (Abies Miller; Pinaceae), but it is distinctly less common when the hosts are spruce (Picea Dietrich; Pinaceae), Douglas-fir (Pseudotsuga Carrière; Pinaceae), or especially pine (Pinus Linneaus; Pinaceae) in which older foliage is exceptionally tough. In all cases, survival and fecundity are significantly reduced when budworm are forced to feed on previous years’ foliage (Blais Reference Blais1952; Dodds et al. Reference Dodds, Clancy, Leyva, Greenberg and Price1996). In fact, the seasonal window of high nutritional quality of the host-plant for budworms is quite brief. There is a race to begin the feeding season because the quality of current-year foliage declines rapidly so that late-feeding budworms are also subject to reduced survival and fecundity (Lawrence et al. Reference Lawrence, Mattson and Haack1997; Nealis Reference Nealis2012). Pupation occurs near the final feeding site and adults emerge approximately one week later.
The only variation in this life cycle is for two-year cycle spruce budworm which feed for a brief period in the spring of their first year, then settle into a new hibernaculum as a third instar, moult to a fourth instar, and enter a second diapause where they remain throughout the first summer, autumn, and second winter. They emerge in the spring of the second year to complete their life cycle (Mathers Reference Mathers1932; Shepherd Reference Shepherd1961; Shepherd et al. Reference Shepherd, Gray and Harvey1995). A small proportion of univoltine budworm populations can be biennial (Harvey Reference Harvey1967), especially in western spruce budworm (Nealis Reference Nealis2005). The hybrid progeny of two-year cycle spruce budworm parents and univoltine parents of western spruce budworm can be either univoltine or biennial depending on photoperiod, indicating both heritable and environmental influences (Nealis Reference Nealis2005).
Adult budworms employ chemically similar, although species-specific, pheromones for mating (Silk and Kuenen Reference Silk and Kuenen1988), which, combined with differences in moth phenology, serve to reduce potential hybridisation in sympatric species (Sanders Reference Sanders1991; Shepherd et al. Reference Shepherd, Gray and Harvey1995; Lumley and Sperling Reference Lumley and Sperling2011; Nealis and Régnière Reference Nealis and Régnière2014). Female fecundity is inversely related to local defoliation (Nealis and Régnière Reference Nealis and Régnière2004a) and seasonal foliage quality (Lawrence et al. Reference Lawrence, Mattson and Haack1997; Nealis Reference Nealis2012). Our knowledge of adult behaviour is restricted to C. fumiferana but at a coarse level probably applies to all budworms considered here. Female moths oviposit at least half of their egg complement in the natal site irrespective of host condition provided that some needles are available. Moths are capable of long-distance, nocturnal flights with the direction and distance influenced by ambient meteorological conditions (Greenbank et al. Reference Greenbank, Schaefer and Rainey1980; Sturtevant et al. Reference Sturtevant, Cooke, MacLean and Kneeshaw2015).
Behaviour of populations
Spatial and temporal patterns
Historical evidence for budworm outbreaks has been reconstructed by study of growth rings in trees (e.g., Blais Reference Blais1954, Reference Blais1965a; Burleigh et al. Reference Burleigh, Alfaro, Borden and Taylor2002; Zhang and Alfaro Reference Zhang and Alfaro2002; Alfaro et al. Reference Alfaro, Berg and Axelson2014), lumber in old buildings (Krause Reference Krause1997; Boulanger et al. Reference Boulanger, Arseneault, Morin, Jardon, Bertrand and Dagneau2012), insect remains in sediments (Simard et al. Reference Simard, Morin and Lavoie2006), and defoliation surveys (Kettela Reference Kettela1983; Volney Reference Volney1988; Parfett et al. Reference Parfett, Clarke and Van Sickle1994; Simpson and Coy Reference Simpson and Coy1999). These reveal that the spruce budworm has been resident in northern forest ecosystems of Canada for at least 8500 years and outbreaks have been recurrent for at least the past four centuries. There is also evidence of outbreaks of western and two-year cycle spruce budworms for several centuries (Zhang and Alfaro Reference Zhang and Alfaro2002; Alfaro et al. Reference Alfaro, Berg and Axelson2014). Interpreting these patterns assembled from a combination of direct and indirect evidence gathered at different scales and variable sampling intensities is hazardous in detail (Régnière and Lysyk Reference Régnière and Lysyk1995) but nonetheless represents our best evidence of historical population patterns.
Blais (Reference Blais1954, Reference Blais1965a, Reference Blais1983, Reference Blais1985) used tree rings as indicators of spruce budworm outbreaks across the southern boreal forest of eastern Canada and concluded that outbreaks have been regional in scale but not clearly periodic as the intervals between outbreaks for a given location were highly variable (Blais Reference Blais1968). Royama (Reference Royama1984) was convinced of a regular periodicity of ~35 years in the historical record and argued the unusually long intervals between outbreaks observed by Blais were the result of an undetected outbreak in the late 19th century for which there is now some evidence (Morin et al. Reference Morin, Paprise and Bergeron1993). But even without this speculative correction, most of the historical data compiled subsequent to Blais’ research report a regular outbreak interval of 33–42 years albeit with considerable range in all datasets. More limited data from the extreme western range of C. fumiferana indicate a shorter interval of 22 years (Table 1). Overall the historical record for spruce budworm suggests a low frequency periodicity synchronised over a wide geographic range.
Evidence of outbreaks in western spruce budworm has been reconstructed from tree rings in New Mexico, United States of America (Swetnam and Lynch 1993), Colorado, United States of America (Swetnam and Lynch Reference Swetnam and Lynch1989; Ryerson et al. Reference Ryerson, Swetnam and Lynch2003), Pacific Northwest, United States of America (Flower et al. Reference Flower, Gavin, Heyerdahl, Parsons and Cohn2014), and British Columbia, Canada (Campbell et al. Reference Campbell, Smith and Arsenault2006; Alfaro et al. Reference Alfaro, Berg and Axelson2014). Here, too, there is evidence of regional-level outbreaks that share large-scale patterns for several centuries. As in spruce budworm, western spruce budworm outbreaks occur at 30-year to 40-year intervals with considerable regional range (Table 1). A series of data for two-year cycle spruce budworm from northern British Columbia suggests an outbreak interval of 32 years (Zhang and Alfaro Reference Zhang and Alfaro2002, Reference Zhang and Alfaro2003), a curiosity since this interval represents only half as many generations as the comparable interval in the one-year cycle budworms.
The duration of these low frequency outbreaks of conifer-feeding budworms is difficult to estimate from historical records because it varies spatially with geographic conditions (Kemp et al. Reference Kemp, Everson and Wellington1985; Shepherd Reference Shepherd1985), the methods and assumptions of the measurements (Alfaro et al. Reference Alfaro, Van Sickle, Thomson and Wegwitz1982), and the scale of observation (Gray Reference Gray2013). Durations estimated from dendrochronologies are usually longer than those estimated from direct observation of defoliation for the same species in the same area. Mean outbreak duration for C. fumiferana is approximately nine years in eastern and 12 years in western Canada, but varies from a few to as many as 25 years in all regions (Table 1; Candau et al. Reference Candau, Fleming and Hopkin1998; Gray et al. Reference Gray, Régnière and Boulet2000). The duration of western spruce budworm outbreaks is similar to spruce budworm outbreaks (Swetnam and Lynch Reference Swetnam and Lynch1989, Reference Swetnam and Lynch1993; Flower et al. Reference Flower, Gavin, Heyerdahl, Parsons and Cohn2014) although apparently is shorter in the northern portions of the range where outbreaks usually last less than 10 years and often no more than four years (Table 1; Johnson and Denton Reference Johnson and Denton1975; Maclauchlan et al. Reference Maclauchlan, Brooks and Hodge2006; Alfaro et al. Reference Alfaro, Berg and Axelson2014).
An informative variation to these low-frequency, persistent outbreak patterns is the jack pine budworm. The historical record on this species is restricted to survey data from the 20th century but even with this direct evidence, patterns are irregular and currently changing. Volney (Reference Volney1988) proposed a 10-year periodicity for jack pine budworm outbreaks in the Prairie Provinces of Canada but this was confounded with the periodicity of forest fires, which influence this system significantly. Regardless, this apparent historical pattern has changed because outbreaks of jack pine budworm have been essentially absent from the prairie region since Volney’s analysis and have become recurrent, if not yet periodic, more than 1000 km east in Ontario, Canada (Scarr et al. Reference Scarr, Ryall and Hodge2012; Hughes et al. Reference Hughes, Fortin, Nealis and Régnière2014). Surveys from Wisconsin, United States of America, revealed highly variable periodicities averaging from five to 12 years depending on specific site conditions (Volney and McCullough Reference Volney and McCullough1994). An associated characteristic of jack pine budworm outbreaks is their short duration, lasting only two to six years at the regional level (McCullough Reference McCullough2000; Scarr et al. Reference Scarr, Ryall and Hodge2012) and even fewer for individual trees within infested stands (Nealis et al. Reference Nealis, Magnussen and Hopkin2003).
Despite high variability in the range of outbreak periodicity and duration at the local level, budworm outbreaks appear synchronised over large areas via several potential mechanisms including correlated, large-scale weather-related perturbations and dispersal of moths (Cooke et al. Reference Cooke, Nealis and Régnière2007). Once again, deviations from this generalisation are informative. Synchrony of spruce budworm in the contiguous, balsam-fir rich forests of eastern Canada is clear (Royama Reference Royama1984) but less so in the more mixed, climatically diverse forests of central Canada (Blais Reference Blais1985; Candau et al. Reference Candau, Fleming and Hopkin1998), and even less so in the sinuous, river-valley spruce forests of northwestern Canada (Burleigh et al. Reference Burleigh, Alfaro, Borden and Taylor2002). During the last outbreak, observed defoliation in Canada began and ended in regions east of −80° longitude 10 years before these same events further west (Fig. 1A, B; Régnière and Nealis Reference Régnière and Nealis2007). Synchrony in outbreaks of western spruce budworm is limited to smaller geographic areas (Alfaro et al. Reference Alfaro, Berg and Axelson2014) with occasional overlap among adjacent areas otherwise distinct (Swetnam and Lynch Reference Swetnam and Lynch1989, Reference Swetnam and Lynch1993; Ryerson et al. Reference Ryerson, Swetnam and Lynch2003). This different pattern may be related to western spruce budworm’s broad latitudinal distribution over mountainous terrain, which would constrain moth dispersal and result in more localised weather effects than would be the case for the spruce budworm. The long duration and greater amplitude of outbreaks in both spruce budworm and western spruce budworm makes apparent synchrony over large areas in these species likely. In jack pine budworm with short-duration outbreaks, large-scale synchrony is less apparent with the extensive outbreak of the 1980s being the notable exception (Fig. 1C).
Most studies of budworm outbreak patterns cited here emphasise mean values, which reinforce the perception of the regularity of cycles with characteristic durations. However, there is also variation in outbreak behaviour that contains relevant information for understanding dynamics. For example, catastrophic forest change resulting from high mortality of dominant trees is not universal in any budworm system and actually atypical in most. Today’s northern forests are shaped by the legacies of past disturbance caused by budworms. Severe outbreaks may be ecologically transient at one scale but transformative at another. Since variation in the frequency, severity, and duration of outbreaks determines their ecological and socio-economic impacts at all spatial and temporal scales, understanding how and why outbreaks vary in these parameters helps prepare for future disturbances and identification of ecosystem-level indicators of climate or land-use changes in prevailing forest disturbance regimes.
Population dynamics
Widespread, severe damage to trees in mature stands was the most evident, empirical information available to early investigators of population dynamics. A heuristic explanation emerged in which outbreaks occurred in homogeneous, mature stands dominated by preferred host species. The abundant food source permitted rapid population growth under favourable environmental conditions. Persistent defoliation reduced foliage and killed the most vulnerable trees. The resulting reduction in foliage, in turn, forced decline in spruce budworm populations (Blais Reference Blais1983). Outbreak cycles were thus related to the average period between destruction and regeneration of vulnerable stands within a resilient forest system. This was the basis for synthetic models of spruce budworm dynamics (Ludwig et al. Reference Ludwig, Jones and Holling1978; Jones Reference Jones1979) and conceptual explanations of associated forest dynamics (Baskerville Reference Baskerville1975).
This resource-driven perspective appealed to forest managers because of its cause-and-effect relationship to stand attributes. It led to clear management decisions as intervention was required if severe impacts were to be avoided in highly vulnerable stands (MacLean Reference MacLean1996). This perspective also explained apparent, increased severity and extent of outbreaks in the 20th century as selective harvesting, regeneration, and fire-suppression increased the abundance of the most susceptible host species for spruce budworm (balsam fir) (Blais Reference Blais1983), western spruce budworm (Douglas-fir) (Anderson et al. Reference Anderson, Carlson and Wakimoto1987; Swetnam and Lynch Reference Swetnam and Lynch1993), and jack pine budworm (jack pine) (Volney Reference Volney1988).
There were, however, problems with this explanation. First, not all outbreaks result in catastrophic reduction in basal area of susceptible tree species for spruce budworm (Morris Reference Morris1963a; Alfaro et al. Reference Alfaro, Taylor, Brown and Clowater2001) and especially for jack pine budworm (Gross Reference Gross1992) and western spruce budworm (Alfaro et al. Reference Alfaro, Van Sickle, Thomson and Wegwitz1982; Van Sickle Reference Van Sickle1987). Second, even in the case where forest stands were severely impacted, the length of the outbreak cycle, 30–40 years, was not long enough to replace a northern conifer forest. Other significant ecological factors must be modifying this periodicity.
The Green River Project in New Brunswick, Canada, introduced a quantitative life system approach to spruce budworm population dynamics (Morris Reference Morris1963a). The data were used in several interpretations of population ecology (Morris Reference Morris1963b; Jones Reference Jones1979; Royama Reference Royama1984) but all featured eruptive, periodic outbreak dynamics driven by lagged, density-related processes associated with trophic interactions modulated by weather and confounded by moth dispersal. Two paradigms emerged; one in which spruce budworm populations rapidly switch between multiple equilibria as a function of resource availability (Ludwig et al. Reference Ludwig, Jones and Holling1978) and an alternative view in which populations oscillate gradually with a unique conditional equilibrium that varies according to the current state of different forcing agents such as natural enemies (Royama Reference Royama1992). The ensuing debate has drawn useful attention to how the relative strength and direction of trophic interactions influence dynamics (Cooke et al. Reference Cooke, Nealis and Régnière2007; Sturtevant et al. Reference Sturtevant, Cooke, MacLean and Kneeshaw2015). I return to this in detail after first considering the pervasive effect of weather and the obfuscating effect of moth dispersal.
Influence of climate and weather
Climate often defines the primary environmental range of insects via the cumulative and cross-scale effects of local weather on populations (Fig. 3). A simple, explanatory relationship between weather and budworm outbreaks, however, remains elusive. The influence of weather on population dynamics of budworms is more complex than direct, modulating rather than driving rates of change and absolute levels of population densities at multiple scales through its effect on ecological relationships that determine fecundity and survival. Weather is an environmental context for understanding variability in the intrinsic drivers of population change.
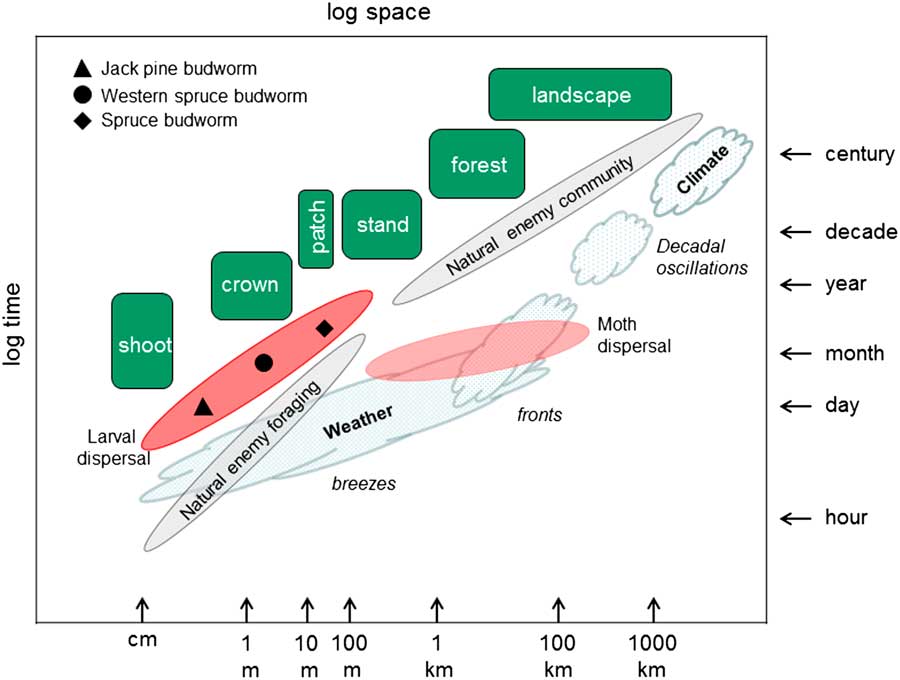
Fig. 3 Time and space scales (log) of northern conifer forests and their relationship to ecological processes that influence the population dynamics of budworms. Dispersal of budworm larvae and moths provide linkage between macroscale atmospheric processes and microscale landscape processes. The results of microscale processes such as larval dispersal vary among jack pine budworm, spruce budworm, and western spruce budworm systems whereas natural enemy foraging and community composition are similar over all scales (adapted from Peterson et al. Reference Peterson, Allen and Holling1998).
Wellington et al. (Reference Wellington, Fettes, Turner and Belyea1950) analysed synoptic weather patterns in northern Ontario and concluded that spruce budworm outbreaks were preceded by years with relatively warm, dry summers and fewer storms. Supporting evidence was found for spruce budworm elsewhere by Greenbank (Reference Greenbank.1956) and Pilon and Blais (Reference Pilon and Blais1961), and for western spruce budworm by Thomson et al. (Reference Thomson, Shepherd, Harris and Silversides1984). All suggested these conditions favour foraging by larvae and moth dispersal. Statistical approaches identified specific meteorological variables correlated to outbreak behaviour of jack pine budworm (Clancy et al. Reference Clancy, Giese and Benjamin1980), western spruce budworm (Kemp et al. Reference Kemp, Everson and Wellington1985) and spruce budworm (Candau and Fleming Reference Candau and Fleming2005; Gray Reference Gray2013) but explanatory ecological processes were difficult to interpret or returned contrary results (Swetnam and Lynch Reference Swetnam and Lynch1993; Flower et al. Reference Flower, Gavin, Heyerdahl, Parsons and Cohn2014). This ambiguity is not surprising given the formidable number of weather variables that can enter an analysis and risk spurious inferences (Royama Reference Royama1984) or the differences in data sources and analytical techniques among studies (Gray Reference Gray2013). These empirical studies have most value in synthesis of large datasets and identification of likely and unlikely hypotheses. For example, most historical analyses conclude the importance of weather is contingent on location-specific forest composition, in particular, abundance of susceptible hosts (Shepherd Reference Shepherd1959; Blais Reference Blais1968; Candau and Fleming Reference Candau and Fleming2005; Gray Reference Gray2013) but becomes a more dominant influence at the margins of budworms’ ranges (Blais Reference Blais1968; Volney and Fleming Reference Volney and Fleming2007; Régnière et al. Reference Régnière, St-Amant and Duval2012b).
Understanding population dynamics within a species’ range requires consideration of the effects of weather on contemporaneous trophic interactions (Volney and Fleming Reference Volney and Fleming2007; Gray Reference Gray2008). Process-oriented phenology models re-scale seasonal events in an insect’s life history from calendar to physiological time. Phenology models for budworms vary from simple heat unit accumulations (Shepherd Reference Shepherd1961; Lysyk and Nealis Reference Lysyk and Nealis1988) to nonlinear functions incorporating individual variation for all life stages (Lysyk Reference Lysyk1989a; Régnière et al. Reference Régnière, St-Amant and Duval2012b; Nealis and Régnière Reference Nealis and Régnière2014). They have been used to investigate ecological relationships between budworms and their tree hosts (Thomson et al. Reference Thomson, Shepherd, Harris and Silversides1984; Lysyk Reference Lysyk1989b; Volney and Cerezke Reference Volney and Cerezke1992; Thomson and Benton Reference Thomson and Benton2007; Nealis Reference Nealis2012) and natural enemies (Nealis Reference Nealis1988; Quayle et al. Reference Quayle, Régnière, Cappuccino and Dupont2003) as well as to predict shifts in geographic range with climate change (Régnière et al. Reference Régnière, St-Amant and Duval2012b).
Dispersal of moths and recruitment of eggs
Both male and female budworm moths disperse. The direct contribution of female moths actively transporting eggs has received most attention (Greenbank et al. Reference Greenbank, Schaefer and Rainey1980; Royama Reference Royama1984) but dispersing male moths may contribute to increase in populations by enhancing mating success at low population densities (Régnière et al. Reference Régnière, Delisle, Pureswaran and Trudel2012a). Moth dispersal appears to be more weather than population related (Greenbank et al. Reference Greenbank, Schaefer and Rainey1980; Anderson and Sturtevant Reference Anderson and Sturtevant2011) and there is credible anecdotal evidence that immigration has been the source of sudden increases in local population densities (Wellington et al. Reference Wellington, Fettes, Turner and Belyea1950; Greenbank Reference Greenbank1957; Dobesberger et al. Reference Dobesberger, Lim and Raske1983). Whether dispersal leads to persistent new outbreaks or populations return to their former, low-density state in these situations depends on local conditions. Nonetheless, the likelihood of moth immigration in the first place is more dependent on location of the receiving area relative to a source with respect to direction of prevailing wind during the adult period, than on the ecological suitability of the forest receiving budworms (Greenbank Reference Greenbank1957; Anderson and Sturtevant Reference Anderson and Sturtevant2011). Moths have limited, if any, choice as to where they land. So the extent to which large-scale patterns are the result of moth dispersal remains controversial in theory (Royama et al. Reference Royama, MacKinnon, Kettela, Carter and Hartling2005) and problematic in practise (Sturtevant et al. Reference Sturtevant, Achtemeier, Charney, Anderson, Cooke and Townsend2013) because the net effect of observed dispersal activity in a given location is not obvious. What is clear, however, is that the capacity and inclination for moths to migrate long distances is a phylogenetic trait common to all Choristoneura which adds significant potential for stochastic influence on local rates of change and results in a meta-population structure of sustained and synchronised outbreaks over a regional scale for all species (Fig. 3; Cooke et al. Reference Cooke, Nealis and Régnière2007).
Trophic relationships
Top-down: the influence of natural enemies
Surveys of natural enemies such as parasitoids, predators, and pathogens are available from throughout the ranges of most budworms. Parasitoids have been particularly well studied and there are recent identification keys and tables summarising associations with the different budworm species (Huber et al. Reference Huber, Eveleigh, Pollock and McCarthy1996; O’Hara Reference O’Hara2005; Bennett Reference Bennett2008; Fernández-Triana and Huber Reference Fernández-Triana and Huber2010). The number of parasitoid species found reflects the search effort and so the greatest number of the more than 200 species recorded from Choristoneura in North America is associated with C. fumiferana and the lowest number for the four species considered here is from the lesser known C. biennis (Fernández-Triana and Huber Reference Fernández-Triana and Huber2010). The impressive diversity of parasitoids associated with spruce budworm from one intensively sampled area has been analysed as a food web comprising at least five trophic levels from the balsam fir producer through the budworm herbivore to tertiary hyperparasitoids (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007).
Despite the complexity of natural enemy assemblages, some useful qualitative generalisations are possible. First, while there is a rich, potential diversity of parasitoids attacking conifer-feeding Choristoneura, there are only a few species that are relatively abundant and these same species are abundant throughout the continental range of Choristoneura (Fig. 3). Second, the most common parasitoid species parasitise all of the budworm species considered here (Huber et al. Reference Huber, Eveleigh, Pollock and McCarthy1996; O’Hara Reference O’Hara2005; Bennett Reference Bennett2008; Fernández-Triana and Huber Reference Fernández-Triana and Huber2010) and several are true generalists, attacking other Lepidoptera hosts. The generalist characteristic of the parasitoid fauna attacking budworms has led to the notion that most parasitoids in budworm outbreaks are opportunistic (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007) and have a limited ability to respond numerically to increases in budworm abundance because of their reliance on alternative hosts to satisfy their seasonal life history requirements (Miller Reference Miller1963; Blais Reference Blais1965b; Sanders Reference Sanders1991). The few parasitoid species with seasonal life histories that do match budworms’, such as Apanteles fumiferanae Viereck (Hymenoptera: Braconidae) and Glypta fumiferanae Viereck (Hymenoptera: Ichneumonidae), are ubiquitous and common but it is debatable whether their rates of attack track, rather than drive, rates of change in their host populations (Miller Reference Miller1963; Royama Reference Royama1984; Nealis Reference Nealis1988). Another potential limit to strong numerical responses of primary parasitoids is the diverse set of secondary parasitoids and hyper-parasitoids that attack these parasitoids during the outbreak period (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007).
A less obvious faunal pattern for parasitoids of budworms is the association of particular species with the density, or outbreak phase, of the budworm population. The relative diversity of the parasitoid fauna changes as outbreaks progress (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007) and apparent parasitism by a few species such as Meteorus trachynotus Viereck (Hymenoptera: Braconidae), increases during collapse of budworm populations (Miller Reference Miller1963; Blais Reference Blais1965b; Nealis Reference Nealis1991). Endemic spruce budworm populations are attacked by very few of the parasitoid species common at higher densities but, interestingly, are attacked by a completely distinct set of species rarely encountered in outbreak populations (Miller and Renault Reference Miller and Renault1976; Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007).
Similarly, the importance of predators varies over the outbreak cycle. Several species of specialist migratory birds and small mammals have negligible impacts on outbreak populations (Morris et al. Reference Morris, Cheshire, Miller and Mott1958; Mattson et al. Reference Mattson, Knight, Allen and Foltz1968) despite initial numerical responses as budworm populations increase (Holmes et al. Reference Holmes, Sanders, Fillman and Welsh2009). The impact of bird predation is inversely related to budworm densities (Campbell et al. Reference Campbell, Torgersen and Srivastava1983). The prevailing hypothesis is that bird predation has an important, perhaps regulatory, influence on low-density budworm populations although critical data remain unavailable (Sanders Reference Sanders1991; Venier and Holmes Reference Venier and Holmes2010). A qualitative analysis of bird predation in budworm dynamics argued that the role of predators gains more insight when viewed as functional (e.g., body size), as opposed to taxonomic, groups (Holling Reference Holling1988). As is becoming apparent with parasitoids, guilds of bird predators have distinct impacts at different budworm densities with distinct consequences on population behaviour.
Pathogens also have been well studied in budworm populations (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007) but their role in the ecology of budworms is equivocal. Royama (Reference Royama1984) evoked a potential suite of diseases as the “fifth agent” contributing to population collapse observed in the Green River Project but it is not clear whether these were actual pathogens or the result of insufficient information in the original study. As with parasitoids, the same pathogens or their homologues have similar roles in the different budworm systems. Common pathogens such as Nosema Nägeli (Microsporida: Nosematidae) become more prevalent over the course of an outbreak (Régnière Reference Régnière1984; van Frankenhuyzen et al. Reference van Frankenhuyzen, Ryall, Liu, Meating, Bolan and Scarr2011; Eveleigh et al. Reference Eveleigh, Lucarotti, McCarthy and Morin2012) but their effects on individuals are generally sub-lethal and their impact on rates of population change enigmatic (Wilson Reference Wilson1987; Eveleigh et al. Reference Eveleigh, Lucarotti, McCarthy and Morin2012). Fungal epizootics are infrequent and localised (Perry and Régnière Reference Perry and Régnière1986). Detailed molecular characterisation of several viruses isolated from spruce budworm and western spruce budworm show them to be essentially variants of otherwise similar genomes (Arif et al. Reference Arif, Guangyu and Jamieson1986; Graham et al. Reference Graham, Morin, Lapointe, Nealis and Lucarotti2008; Thumbi et al. Reference Thumbi, Béliveau, Cusson, Lapointe and Lucarotti2013); more evidence of the close phylogenetic lineage of these budworms. Baculoviruses are widely distributed in budworm populations but their prevalence is very low and epizootics are rare to absent in C. fumiferana (Cunningham and Kaupp Reference Cunningham and Kaupp1995). They appear more frequent in C. occidentalis populations but only at exceptionally high densities where their impacts are transient (Nealis et al. Reference Nealis, Turnquist, Morin, Graham and Lucarotti2015).
The association of generalist natural enemies common to all budworms over their continental range suggests that natural enemies in the budworm system respond to, rather than determine, the population density of budworms; the “bird-feeder” effect whereby high-density budworm populations attract or enable a greater number of widespread generalist natural enemies to exploit dense populations (Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007). The third pattern of a few species of natural enemies commonly associated with collapsing or endemic populations, however, suggests that the critical relationship between natural enemies and budworms emerges near transition points in the outbreak cycle where budworm densities tend to increase or decrease relatively rapidly through several orders of magnitude as a result of significant trends in generation survival and recruitment (Fig. 4).
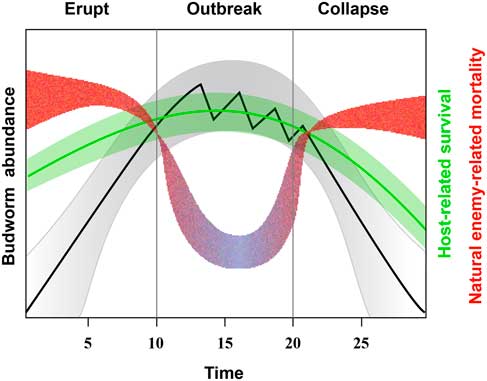
Fig. 4 Idealised budworm population cycle showing change in budworm density and associated change in trophic interactions in different phases. Budworm populations (grey) increase with an increase in resource availability (green) although this increase is dampened by strong, persistent top-down effects by specialised natural enemies (red). Outbreaks occur when host-related survival increases budworm density and specialised natural enemies effective at low population densities are replaced by generalists that inflict relatively low levels of generation mortality (purple). Collapses occur when persistent damage by budworm feeding reduces host-related survival in combination with increases in mortality by a small subset of natural enemies.
The difficulties of inferring this transient but critical role of natural enemies from population measurements has been long recognised but proposed solutions based on direct sampling seem formidable (Royama Reference Royama2001). A qualitative working hypothesis using comparative analysis and the context of trophic interactions (Cooke et al. Reference Cooke, Nealis and Régnière2007) offers a robust alternative that makes use of the weight of evidence provided by uncommonly rich historical knowledge available for budworms. I return to this point in the final section.
Bottom up: influence of the resource
Blais (Reference Blais1985) stressed the central importance of balsam fir in the location and severity of outbreaks of C. fumiferana. Certainly most analysis of historical records find a clear relationship between growth reduction or defoliation, proxies for spruce budworm population density, and some measure of balsam fir abundance (e.g., Gray Reference Gray2013). Similar associations between outbreaks and abundance of preferred host trees are found in the other Choristoneura species (Campbell Reference Campbell1993; Shepherd et al. Reference Shepherd, Gray and Harvey1995; McCullough Reference McCullough2000; Maclauchlan et al. Reference Maclauchlan, Brooks and Hodge2006). While it may be an obvious prerequisite for outbreaks to occur where host-trees are abundant, the extent to which the influence of the preferred host pervades every aspect of the life history and ecology of conifer-feeding budworms, with significant effects on outbreak characteristics, may be less clear. Viewed as a cross-scale factor, the ecological relationship between budworms and their hosts tells us much about the similarities and differences in their dynamics.
At the coarsest scales, basal area of susceptible host species is repeatedly confirmed as the dominant indicator of likely infestation and significant damage (Turner Reference Turner1952; MacLean Reference MacLean1980; Bouchard et al. Reference Bouchard, Kneeshaw and Bergeron2005; Gray Reference Gray2013). Thus, stand composition and age (size) of host trees emerge as significant factors over other site-related indicators (Bergeron et al. Reference Bergeron, Leduc, Morin and Joyal1995; MacLean and MacKinnon Reference MacLean and MacKinnon1997) although at slightly larger scales, abiotic environmental gradients may become evident (Magnussen et al. Reference Magnussen, Boudewyn and Alfaro2004; Bouchard and Auger Reference Bouchard and Auger2014). The presence of non-host trees reduces impacts whether they are mixed in the stand (Su et al. Reference Su, MacLean and Needham1996) or the stand is embedded in a landscape-scale matrix of host and non-host stands (Campbell et al. Reference Campbell, MacLean and Bergeron2008; Bouchard and Auger Reference Bouchard and Auger2014). But is it simply food supply? Certainly, foliage depletion can reduce survival and fecundity by depriving larvae of food but these are transient, first-order effects not associated with population collapse (Morris Reference Morris1963c; Royama Reference Royama1984; Nealis and Régnière Reference Nealis and Régnière2004a; Régnière and Nealis Reference Régnière and Nealis2007).
Qualitative changes in the nutritional adequacy of host foliage or active host responses to defoliation have been proposed as correlates of population decline, especially in western spruce budworm and jack pine budworm (Campbell Reference Campbell1993); i.e., host quality does matter. A defining phylogenetic constraint for all of these budworms is their requirement to feed on rapidly growing, current-year shoots when nitrogen availability is greatest (White Reference White1993). Outside this relatively narrow seasonal window of nutritional adequacy, current-year foliage is more difficult to exploit. In early spring, buds are hard, enclosed by scales, and difficult to penetrate (Shepherd Reference Shepherd1992; Nealis and Lomic Reference Nealis and Lomic1994; Nealis and Nault Reference Nealis and Nault2005). In mid-summer, development of foliage is characterised by decreasing fitness of budworms associated with increased lignification of current-year foliage (Lawrence et al. Reference Lawrence, Mattson and Haack1997; Nealis Reference Nealis2012). However, these are normal, seasonal changes in the physical characteristics of the foliage, whereas most of the studies of host quality examine plant-chemical and physiological relationships to identify either constitutive or induced mechanisms of resistance (Clancy Reference Clancy2002). For good experimental reasons, this work often uses artificial diet containing extracted, controlled nutritional supplements (e.g., Albert and Bauce Reference Albert and Bauce1994; Delvas et al. Reference Delvas, Bauce, Labbé, Ollevier and Bélanger2011) and so remove the seasonal physical characteristics of intact foliage. Overall results show that aside from the dominant importance of nitrogen, variability in host-plant chemistry has minor negative effects on the performance of budworms and there is little evidence of induced plant responses with significant negative feedbacks to population rates of change (Mattson et al. Reference Mattson, Haack, Lawrence and Slocum1991). A simpler, more direct ecological relationship between budworms and their hosts lies in the distribution, abundance, and physical characteristics of the host resource and its effect on recruitment of eggs at the coarse scale and the survival of small budworm larvae at finer scales.
Summer dispersal of neonates and overwinter survival
All budworms disperse at least twice before feeding; first following hatch in summer to find hibernation sites and then the following spring to forage for food. Their likelihood of surviving these events is their risk of dispersal (Nealis Reference Nealis2003). This risk varies with dispersal distance, weather conditions under which dispersal occurs, and the success of these small larvae, first in finding suitable hibernation sites and then adequate feeding sites. In between these dispersal events, budworms must survive several months of variable weather without feeding or imbibing water. The details of this sequence are important to understand for the comparative analysis.
Moths lay eggs on foliage at the periphery of the tree crown. When they hatch, larvae react positively to light except at warmer temperatures (>29 °C) at which they become photonegative (Wellington and Henson Reference Wellington and Henson1947). If walking toward the light, many reach the branch tips and drop, suspended on a silken thread. The risk of this dispersal event, however, is relatively low because summer weather in northern forests tends to be strongly convective and downdrafts force dislodged budworms downwards where they are likely to be intercepted by the lower branches of the conical-shaped crowns of their host tree; even more so for larvae dispersing during warmer summer days when they are photonegative. So, dispersal of first-instar budworms is mostly within the crown of the tree (Régnière and Fletcher Reference Régnière and Fletcher1983).
When budworms encounter rough surfaces, the photic stimulus is replaced by a tactile stimulus that results in settling and constructing a sheltered hibernaculum (Wellington and Henson Reference Wellington and Henson1947). The net effect is that neonates tend to establish their overwinter sites more interior and lower in the crown than where eggs were laid (Régnière et al. Reference Régnière, Lysyk and Auger1989). The final distribution of overwintering budworms, however, varies significantly among species because of the interaction between their common behaviour to settle in sheltered locations and the specific characteristics of their associated host trees in providing such locations. Balsam fir has woody bracts on its branch tips, which are preferred overwintering sites for spruce budworm (Blais Reference Blais1952; Greenbank Reference Greenbank1963). Spruces have pronounced leaf-pegs on branch internodes (Farrar Reference Farrar1995), which also provide sheltered niches. The result is that most C. fumiferana find suitable overwintering sites along the foliated branches close to where eggs were laid and do not need to disperse a great distance to find overwinter sites on the tree bole (Miller Reference Miller1958). This is also likely true for two-year cycle spruce budworm in the spruce-fir forests of western Canada. Jack pine, by comparison, produces no such woody structures and its slender peripheral shoots have smooth bark. Pronounced bark flakes and grooves providing sheltered niches only become common on the basal branch surfaces and tree bole of jack pine, which is where most jack pine budworm overwinter (Nealis and Lysyk Reference Nealis and Lysyk1988). Douglas-fir also has fewer suitable overwintering sites on its branch tips compared to the deeply furrowed surfaces near the branch base and especially on the tree bole. Similarly, most western spruce budworm overwinter on the bole than elsewhere (McKnight Reference McKnight1969; Nealis and Régnière Reference Nealis and Régnière2009). Thus while all budworms have a common need to find suitable locations to overwinter, their ultimate choice is determined by the specific surface features of their host-trees.
Wellington and Henson (Reference Wellington and Henson1947) stressed the importance of a sheltered niche in protecting spruce budworm in hibernacula from desiccation during the warm days of late summer and autumn. Direct field measurements found a positive correlation between survival of spruce budworm and their propensity to choose hibernation sites on complex branch surfaces (e.g., bracts, lichen mats) compared to smooth-barked shoots (Régnière and Duval Reference Régnière and Duval1998). Exposure to relatively warm temperatures in the laboratory during the pre-diapause period increased mortality by desiccation (Bauce and Han Reference Bauce and Han2001) and accelerated loss of lipid and glycogen reserves so budworms also were more vulnerable to mortality later during the post-diapause period (Han and Bauce Reference Han and Bauce1998). This negative effect of warm summer temperatures on subsequent overwinter survival is apparent in field populations but the effect can be reduced in shelters that buffer weather conditions (personal observation). So, the behaviour of first-instar budworm in finding protected overwinter niches reduces the negative effects of exposure to inclement conditions on their survival. But this adaptive syndrome results in a trade-off between improved overwinter survival and increased risk of dispersal the following spring.
Spring dispersal and survival during foraging
Emerging, second-instar budworms are strongly photopositive throughout the foraging period (Wellington and Henson Reference Wellington and Henson1947). This is what brings them to the branch tips in search of feeding sites. Weather during the spring dispersal period is typically cooler and more turbulent than during first-instar dispersal the previous summer resulting in extensive inter-tree dispersal of larvae as they balloon on silk threads between tree crowns (Beckwith and Burnell Reference Beckwith and Burnell1982; Régnière and Fletcher Reference Régnière and Fletcher1983). As mentioned, all univoltine budworms considered here emerge in the spring in advance of bud-flush so it is normal that they use temporary alternatives including pollen cones and previous-year needles (Sanders Reference Sanders1991). The availability of these critical, alternative resources varies among host species and over time affects patterns of survival of their budworm guests and, in turn, has consequences for respective dynamics.
Coniferous trees periodically produce pollen cones early in the season before vegetative buds flush. These are favoured by foraging budworms as they bridge the period between spring emergence and bud-flush (Blais Reference Blais1952; Greenbank Reference Greenbank1963; Nealis and Lomic Reference Nealis and Lomic1994). Pollen cone production is most frequent in mature trees growing in open situations and within a species, production of pollen cones tends to be synchronised regionally (Blais Reference Blais1952; Greenbank Reference Greenbank1963; Hughes et al. Reference Hughes, Fortin, Nealis and Régnière2014). The relative importance of pollen cones to spring emerging budworms depends on alternative resources of the host trees, essentially previous years’ needles that can be mined or tied together with silk to form a protected feeding site. Spruce budworm and western spruce budworm readily mine older needles in the absence of pollen cones (McGugan Reference McGugan1954; Shepherd Reference Shepherd1992; Trier and Mattson Reference Trier and Mattson1997; Nealis and Nault Reference Nealis and Nault2005). Jack pine budworm, however, is unable to mine the needles of jack pine and so pollen cones are critical during its spring dispersal period (Nealis and Lomic Reference Nealis and Lomic1994). Fortunately for jack pine budworm, jack pine has a high propensity to produce pollen cones (Nealis et al. Reference Nealis, Magnussen and Hopkin2003; Hughes et al. Reference Hughes, Fortin, Nealis and Régnière2014). So an adaptive syndrome for all budworm species that mitigates the problems of precocious emergence is the temporary use of previous years’ needles and pollen cones when available.
What happens to these early-season feeding sites during outbreaks emphasises the differences among host tree species and consequent dynamics of their associated budworms. When defoliated, conifers reduce pollen cone production abruptly (Blais Reference Blais1952; Nealis et al. Reference Nealis, Magnussen and Hopkin2003; Hughes et al. Reference Hughes, Fortin, Nealis and Régnière2014). This has an immediate impact on jack pine budworm that now must forage more extensively for scarce resources critical to its survival (Nealis and Lomic Reference Nealis and Lomic1994). By comparison, the immediate impact of fewer pollen cones on spruce budworm and western spruce budworm is less dire because these species can mine needles and their hosts compensate for defoliation by producing even more vegetative buds (Shepherd Reference Shepherd1992; Nealis and Régnière Reference Nealis and Régnière2004b). Nonetheless, severe defoliation by western spruce budworm reduces needle density of the host, which similarly increases foraging dispersal in subsequent generations (Nealis and Régnière Reference Nealis and Régnière2009). There is no evidence of such tree-level effects between spruce budworm and its spruce-fir hosts.
Continuous foraging during the spring emergence period may increase the likelihood that budworms will find fresh buds (McGugan Reference McGugan1954; Shepherd Reference Shepherd1992; Nealis and Lomic Reference Nealis and Lomic1994) but at the cost of an increased risk of dispersal at the stand level that also differs among systems (Régnière and Nealis Reference Régnière and Nealis2008). Spruce budworm and two-year cycle spruce budworm occupy boreal and cordilleran spruce-fir forests where their shade-tolerant hosts characteristically develop full crowns forming dense stands with a high degree of canopy closure at maturity. By comparison, western spruce budworm and jack pine budworm use hosts that are less shade tolerant and have more open crowns and sparser canopy densities in the dry sites they occupy (Farrar Reference Farrar1995). These features mean that between-crown dispersal of budworm characteristic of the spring emergence period is more hazardous to western spruce budworm and jack pine budworm than to spruce budworm as it is more likely to result in larvae landing off the host. Field measurements show a high rate of loss of early-stage larvae in jack pine budworm and western spruce budworm (Nealis and Lomic Reference Nealis and Lomic1994; Nealis and Régnière Reference Nealis and Régnière2009). The comparable situation only occurs in spruce-fir forests once the stand has become thinned by prolonged defoliation and significant gaps develop that change the characteristics of the stand to one that resembles the more open condition of Douglas-fir and jack pine stands (Régnière and Nealis Reference Régnière and Nealis2008).
To summarise, all early-stage budworms share the same adaptive syndrome; find a sheltered niche to overwinter and emerge early in the spring to forage for the earliest available resources. But the population outcome can be different among budworm species because of the interaction of these dispersal events with the distinct characteristics of their respective host trees. Budworms exploiting jack pine and Douglas-fir overwinter on rough surfaces in the interior of the crown and so must travel further to hibernate in the summer and again in the spring to forage for resources than budworms exploiting spruce and fir. Differences in risk of dispersal are accentuated at the stand level as jack pine and Douglas-fir trees have sparser crowns and density of trees per ha is typically lower than in spruce-fir stands. These normal differences among tree and stand characteristics become magnified as delayed negative feedbacks from defoliation first reduce pollen cone and needle density at the tree level and eventually create resource gaps at the stand level as trees die (Fig. 3).
Are these relative differences in the ecological relationships between budworms and their respective hosts significant to population dynamics? Both theory and empirical evidence would suggest so. In theory, lagged, reciprocal, density-dependent responses between a consumer and its resource can generate outbreak cycles (Royama Reference Royama1992). The empirical evidence of large-scale outbreak dynamics of these species shows the same graded response in their defining parameters as the specific budworm-host ecological relationships summarised here. Spruce budworm, with its low frequency, long duration, and extensively synchronised cycles correlates to the slow negative feedbacks of the host to the effects of budworm density (Régnière and Nealis Reference Régnière and Nealis2008). At the other extreme, the short-lived, relatively high-frequency, vaguely periodic and more spatially fragmented nature of jack pine budworm outbreaks correlates to the near immediate reduction of critical pollen cones with subsequent impact on jack pine budworm survival following the first episode of significant defoliation (Nealis and Lomic Reference Nealis and Lomic1994; Nealis et al. Reference Nealis, Magnussen and Hopkin2003). Outbreaks of western spruce budworm are certainly more regular and long-lived than those of jack pine budworm but there are regional differences that make generalisations regarding extensively synchronised and persistent outbreaks less convincing than those of spruce budworm in eastern Canada. The final section examines how these bottom-up ecological relationships interact with top-down factors to shape the eruptive behaviour of all budworms.
Integrating trophic interactions
Cooke et al. (Reference Cooke, Nealis and Régnière2007) present a graphical model in which spruce budworm population cycles are induced by delayed, density-related negative feedbacks among three trophic levels. This tri-trophic concept is dissected here to reveal marked changes in the interaction between bottom-up and top-down factors as the population cycle proceeds from endemic to outbreak to collapse (Fig. 4).
Within suitable climatic zones, budworm survival is favoured in forests with high foliage densities characterised by large, overlapping tree crowns with abundant current-year buds augmented by pollen cones. Forest growth is a relatively slow-moving variable (Fig. 3) so the change toward a condition that favours budworm survival takes many budworm generations. But in the absence of other disturbances, the trend toward improved resource conditions will proceed. Sustained increase in budworm populations commensurate with resource improvement, however, depends on more than foliage and good weather as low density budworm populations are subject to very high rates of mortality from natural enemies (Sanders Reference Sanders1991; Eveleigh et al. Reference Eveleigh, McCann, McCarthy, Pollock, Lucarotti and Morin2007) as well as by reduced mating success (Régnière et al. Reference Régnière, Delisle, Pureswaran and Trudel2012a) that curb sustained increases in density.
Over the immense biogeographic range characterised by extensive northern conifer stands, stochastic elements will result in areas where the coincidence of abundant foliage and limited parasitoid activity permit some increase in budworm densities over a few generations. With higher local densities, there will be more dispersing moths, homogenising the local increase in rate of recruitment over a larger area. Because of the persistent impact of natural enemies, however, even favourable bottom-up conditions do not necessarily propel budworm densities to outbreak. Favourable local conditions must be sustained for several generations for an outbreak to develop locally, at least in C. fumiferana (Royama Reference Royama1984). But relaxation of top-down mortality dominating at low densities will happen somewhere in the extensive geographic range as the bottom-up conditions improve for budworm and the importance of natural enemies effective in endemic populations diminish. This change in the tri-trophic interaction reinforces further increase in survival, recruitment, and population density to outbreak levels (Fig. 4).
Conifer hosts are tolerant of feeding budworms as these herbivores are essentially grazers of non-vital tissues. Occasional, severe defoliation causes first-order reductions in population densities through a combination of starvation, reduced fecundity, and net emigration so defoliation the next year is seldom as severe. An important change occurs, however, in the tri-trophic interaction as budworm populations reach outbreak levels. The natural enemies associated with endemic populations are replaced functionally at outbreak levels by a different guild of common, generalist species that collectively impose a fairly consistent but relatively low level of mortality during the outbreak. This absence of strong, variable top-down effects that were present during the increase phase permit rapid recovery of budworm populations from short-term, negative bottom-up effects such as severe defoliation so that outbreaks are sustained, sometimes for more than a decade (Fig. 4).
However, as cumulative damage from budworm becomes significant, the bottom-up interaction changes systematically with significant impacts on budworm survival (Fig. 5). First, defoliation reduces pollen cone production in all conifers. This has an immediate, negative impact on small jack pine budworms which are reliant on this resource (Nealis and Lomic Reference Nealis and Lomic1994) but less so for spruce budworm and western spruce budworm which may still mine previous years’ needles in the absence of pollen cones. Nonetheless, dispersal losses will increase. Eventually the negative feedback from damage will become more significant for these species as needles become sparse and needle-mining more difficult, particularly for western spruce budworm (Nealis and Régnière Reference Nealis and Régnière2009). It takes even longer for these negative feedbacks to affect spruce budworm where host foliage remains plentiful and palatable for several years and resource-related mortality does not become apparent until the vulnerable conifer component of the stand dies and becomes replaced by gaps and non-host species (Régnière and Nealis Reference Régnière and Nealis2008). Thus delayed negative feedback from the host occurs in all species; it is just a question of how long it takes to become significant for population rates of change (Fig. 5).
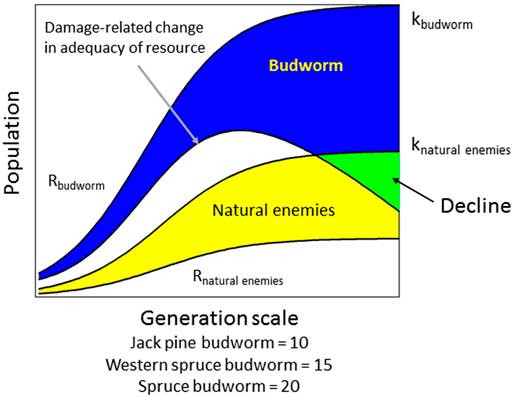
Fig. 5 Trophic interactions and decline of budworm populations. As budworm populations (Rbudworm) approach the capacity of the host resource (Kbudworm), negative feedbacks result in greater variation and declining trend in population density. At the same time, natural enemy capacity (Knatural enemies) increases relative to budworm densities creating a greater probability of population decline. Note the generation scale for development of this tri-trophic interaction varies by species although the absolute values are an approximation (modified from graphic by J. Régnière).
Once bottom-up feedbacks change the resource environment, the rate of change in budworm densities becomes neutral and then negative (Nealis and Régnière Reference Nealis and Régnière2004a). Population density decreases unless subsidised by immigration. Apparent, or relative, rates of parasitism increase even without a substantive increase in parasitoid density simply because budworm densities are lower and parasitoid densities are not closely dependent on local budworm populations. So, the collapse of budworm populations initiated by negative, bottom-up feedbacks becomes more likely as the great diversity of predators, parasitoids, and diseases further depress survival and recruitment (Fig. 5). When these top-down losses do not occur, budworm outbreaks persist, causing significant tree mortality and driving the forest to a major episode of change (Ostaff and MacLean Reference Ostaff and MacLean1989; Nealis and Régnière Reference Nealis and Régnière2004b). The frequency and extent of such severe events varies by species. In the jack pine budworm and western spruce budworm where the shift from a positive to a negative bottom-up feedback is relatively fast and direct, stand destruction is less common than death of smaller trees and thinned crowns with the exception of stands with a substantial Abies component (Wulf and Cates Reference Wulf and Cates1987). In spruce-fir forests, where bottom-up feedbacks are weakest and have a significant effect on budworm populations only in the extreme, significant damage to forests is more frequent, especially in balsam-fir dominated stands (MacLean Reference MacLean1980).
Summary
Trophic interactions are fundamental ecological relationships that integrate several small-scale biotic interactions such as top-down or bottom-up perturbations and shed light on population patterns. To explore this for conifer-feeding budworms, factors that influence populations are stratified by trophic level and their interactions examined as populations proceed through different phases of change. This perspective gains further generality by comparing the ecology of closely related budworm species to identify shared phylogenetic constraints and adaptive syndromes that shape their common life history strategies. When these common life histories play out in different forest environments, emergent patterns reveal how ecological relationships translate the adaptations of individuals to population patterns in variable environments.
A defining phylogenetic constraint for Choristoneura budworms is their specialised feeding on current-year buds of a small group of related host plants. The constraint is temporal as fresh buds provide adequate nutrition only for a short period of time relative to the time required for larval feeding and maturation. The adaptive syndrome mitigating this constraint is that budworms overwinter as larvae and emerge early in the spring ready to feed immediately on the earliest available buds. This precocious emergence, however, comes with a cost as budworms typically emerge before current-year foliage is suitable and so must forage extensively for several days and even weeks until buds are available. The risk of dispersal associated with this foraging varies within and among species such that different survival patterns emerge. At the finest spatial scale, early-instar budworms expand the narrow seasonal window of plant adequacy by exploiting previous years’ needles and pollen cones as temporary resources. The production of pollen cones itself is periodic and regionally synchronised in northern conifer species and so there are periods when this alternative resource is especially abundant over large areas. Budworm species feeding on true fir and, to some extent, spruce and Douglas-fir can also use previous years’ foliage but old foliage of pines is unpalatable so jack pine budworm is more dependent on pollen cones to bridge the period between emergence and bud flush. As budworm damage increases, pollen cone production is reduced with immediate, dire consequences for jack pine budworm but less so for non-pine feeding budworms. The increasing negative, bottom-up feedbacks from feeding damage ultimately occurs in all budworm systems but at different temporal rates and spatial scales (Cooke et al. Reference Cooke, Nealis and Régnière2007). Whereas the bottom-up feedback affects survival at the branch and tree levels within a few years for jack pine budworm (Nealis and Lomic Reference Nealis and Lomic1994) and a little longer in western spruce budworm (Nealis and Régnière Reference Nealis and Régnière2009), it does not become significant for survival of spruce budworm until the change in resources occurs at the stand level (Régnière and Nealis Reference Régnière and Nealis2008) (Figs. 3, 5).
Delayed, density-dependent feedbacks from bottom-up sources might be sufficient to explain the basic oscillation in budworm populations via changes in generation survival but more can be explained by including the interaction with top-down sources of mortality. Endemic budworm population levels can be determined, in part, by resource availability but also maintained by intense pressure from specialised natural enemies with strong functional responses. Budworm populations may be kept so low by natural enemies that local mating success is compromised, further impeding population increase (Régnière et al. Reference Régnière, Delisle, Pureswaran and Trudel2012a). Improved suitability of forest condition for budworms as conifer stands develop post-disturbance has two components: (1) the relatively rapid increase in foliage, augmented by periodic pollen cone production and (2) the more gradual creation of tree-scale and stand-scale structure which acts to reduce losses of dispersing small larvae and perhaps buffers microclimates.
Under the conditions set by the resource, budworm populations will become sufficiently abundant to saturate the functional response of natural enemies so that the generation rate of survival favours continued increase in budworm density. Moth dispersal accelerates the inclusion of more and more stands in the outbreak so that the dynamics of any one stand becomes connected to that of the landscape, reducing the dominant influence of local conditions. Knowing the density and spatial scale at which the dynamics transition from local to area-wide outbreak phases is a critical contemporary question in budworm population dynamics.
Periods of more rapid change in local budworm density are associated with major, relatively fast shifts in the nature and impacts of natural enemies; first the apparent disappearance of specialist natural enemy species as budworm populations increase beyond the endemic phase and again at the end of the outbreak when generalist natural enemies increase their impact as forest condition is degraded and negative feedbacks reduce local densities (Figs. 4, 5).
This review has focussed on trophic interactions and the characteristics of outbreak patterns in several related budworm species based on the past three decades of laboratory and stand-level studies where processes and population structure and density can be measured and experiments conducted. Population models produced during this same period were built entirely on evidence compiled during the 30 years previous to most of the studies reviewed here. Those models identified phases in the outbreak cycle where trophic interactions had characteristic and significant effects on population rates of change but lacked sufficient information to analyse these interactions over widely variable environments and timescales. For example, the changing interactions of bottom-up and top-down effects conditioned by budworm density and recruitment discussed here may create a boom-and-bust system with multiple equilibria (Ludwig et al. Reference Ludwig, Jones and Holling1978) but there is insufficient evidence that rates of change in population densities actually accelerate at these critical points. A unique conditional equilibrium that varies with the local state of trophic interactions would also generate richly complex dynamics (Royama Reference Royama1992) and may be a more powerful, if less prescriptive, model of the system.
The influences of weather and dispersal have been mentioned only insofar as they modulate these trophic interactions. But these stochastic modulations can be significant: think of large-scale immigration of moths to a susceptible forest or a sequence of weather patterns that promotes production of pollen cones or increases overwinter survival over a larger geographic area with susceptible hosts (Fig. 3). Certainly any future model must deal explicitly with moth dispersal as it obscures detection of alternative tri-trophic possibilities by accelerating rates of increase extrinsically; an irruptive process. Recent progress promises new insights from different perspectives (Régnière et al. Reference Régnière, Delisle, Pureswaran and Trudel2012a; Sturtevant et al. Reference Sturtevant, Cooke, MacLean and Kneeshaw2015).
A new cycle of population models is surely due. Population models that include processes informed by the last generation of research will reduce the inherent uncertainty of the nonlinear effects of weather and dispersal of moths, which historically have been assumed, ignored, or dismissed. In turn, a greater understanding of weather and dispersal will shed light on how changes in climate and forest landscape will interact with our understanding of trophic relationships to produce the disturbance patterns that will continue to shape the future of northern forest ecosystems.
Acknowledgements
Tom Royama’s critical approach to data and insistence on rigorous methods launched the latest generation of research with a clear goal and high standard of scientific conduct. Jacques Régnière has been my long-time research partner, challenging my half-baked ideas while always adding value. Barry Cooke has been a generous listening post and broadens my thinking with his insight. I am grateful to him and Brian Van Hezewijk for improving an earlier draft of this paper.










