1. Introduction
With the realization of gallium nitride laser diodes Reference Nakamura, Senoh, Nagahama, Iwasa, Yamada, ToshioMatsushita, Kiyoku and Sugimoto[1], GaN has attracted much attention and it is obvious that GaN will be an important semiconductor in optoelectronic applications. Although all the major semiconductor devices have now been realized with GaN, the material is far from being mastered. State-of-the art aluminum nitride layers grown by MBE or MOCVD on SiC or sapphire substrates typically show a broad band centered around 3 eV in luminescence measurements as the dominant feature. This band has been assigned to oxygen accommodating defects with donor-acceptor pair transitions Reference Pasternak, Pocesova and Roskovcova[2] and to transitions within the optical bandtail states Reference Harris, Youngman, Partridge, Li, Chen and Barfknecht[3]. M. D. Bremser et al. Reference Bremser, Perry, Zheleva, Edwards, Nam, Parikh, Aspnes and Davis[4] observe: (a) an increase of the violet luminescence (VL) with the density of defects (b) a sublinear shift of the VL of AlN to the yellow luminescence of GaN as a function of the decrease of the bandgap of AlxGa1−xN. In this paper we discuss the temperature dependent time-resolved spectroscopy of the VL band and compare it to the generically similar yellow luminescence band of GaN.
2. Results and Discussion
The AlN samples investigated here were grown on sapphire and on SiC Substrates by molecular beam epitaxy (MBE). The time-resolved measurement of this luminescence has been done with a correlation method which has the advantage of a greater signal to noise ratio. It is approximately one order of magnitude higher than with the conventional single shot mode method (Figure 1). The exciting beam (electron or photon beam) is modulated with a pseudorandom binary sequence which has a two level autocorrelation function. The crosscorrelation function of the system response and the binary sequence is, with a multiplicative and an additive constant, the pulse response of the system. The violet photoluminescence of AlN on sapphire excited by a HeCd-Laser at 325 nm is very weak because it is a sub-bandgap excitation. Therefore only below band edge states could absorb the laser and only a few percent can be absorbed by AlN. Nevertheless it was possible to excite a measurable violet photoluminescence which is red-shifted by about 30 nm compared with the violet cathodoluminescence (Figure 2).
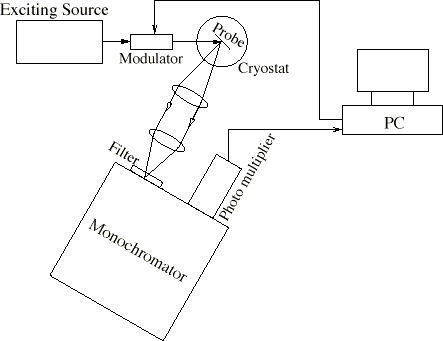
Figure 1. Breadboard construction with the correlation method

Figure 2. Cathodoluminescence spectrum of AlN on sapphire (sample LS005). The inset shows a fit (black dashed) of the luminescence intensity near the band edge (red). The fit consists of peaks from LO phonons and bound excitons which indicates donors and acceptors from unintentionally doping.
The result is an algebraic decay of the luminescence with I~t−1.9±0.3 at 5 Kelvin at medium and long times ( Figure 3). While the time-resolved cathodoluminescence varies only a little from sample to sample at the same temperature ( Figure 3), the exponent does vary between 1.2 and 1.6 at 5 K. For clarity Figure 3 shows the photoluminescence measurement together with two cathodoluminescence measurements with exponents 1.2 and 1.4. The photoluminescence (PL) decays significantly faster than the cathodoluminescence with an exponent about 1.9. The reason for the faster decay of the PL is the sub-bandgap excitation which leaves no additional energy and therefore no time for relaxing into the states which are involved into the radiative recombination.
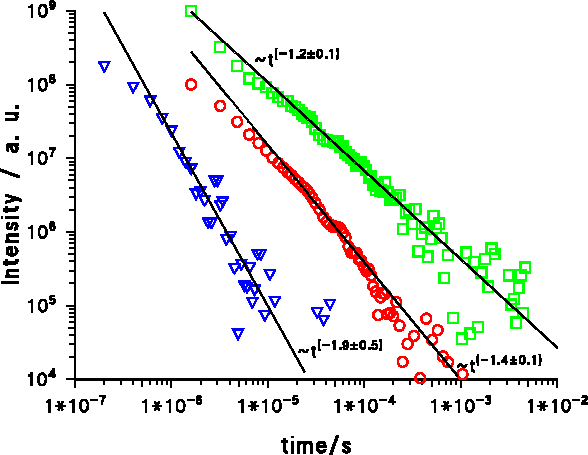
Figure 3. Decay of the violet luminescence of AlN at 5 K: Triangles: Photoluminescence of AlN on sapphire (sample LS005: 400 nm MBE-ALN on Al2O3) at 400 nm, Circles: Cathodoluminescence of AlN on sapphire (sample LK48: 600nm MOCVD-ALN on Al2O3) at 423 nm Squares: Cathodoluminescence of AlN on SiC (sample LS12: 575 nm MBE-ALN on SiC) at 355 nm. The measurements where done at the maximum intensity wavelength.
At 77 K the decay is slightly faster and it can be seen that the decay of the violet band at long wavelengths is slightly slower than the decay at short wave lengths (Figure 4). The slower decay at long wavelengths is common for donnor-acceptor bands and is not temperature dependent.
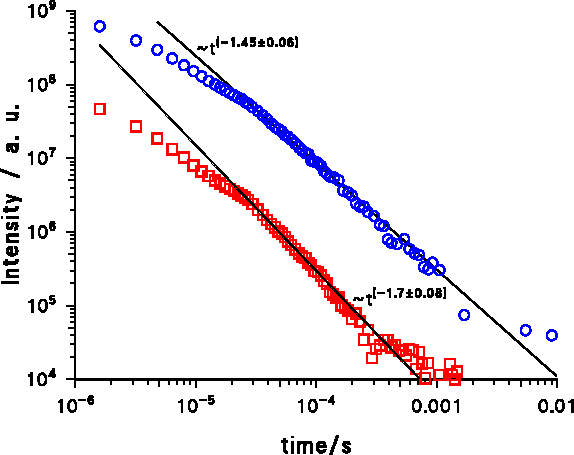
Figure 4. Decay of the violet Cathodoluminescence of AlN at 77 K, sample LS12: 575 nm. Squares: At 315 nm wavelength; Circles: At 470 nm wavelength. Both measurements where made at nearly the same intensity and the maximum intensity was at 360 nm.
At room temperature the decay is much faster at short times but much slower at medium and long times (Figure 5). The decay is significantly slower than ~t−1 at medium and long times but at very long times the decay must be faster as ~t−1 because the energy of all photons from the VL, E VL = Σ i h · f i , with the Planck constant h and frequency fi of the photon number i, is finite. If the decay was not faster than ~t−1 , then the sum would be proportional or greater than the harmonic series and therefore infinite.
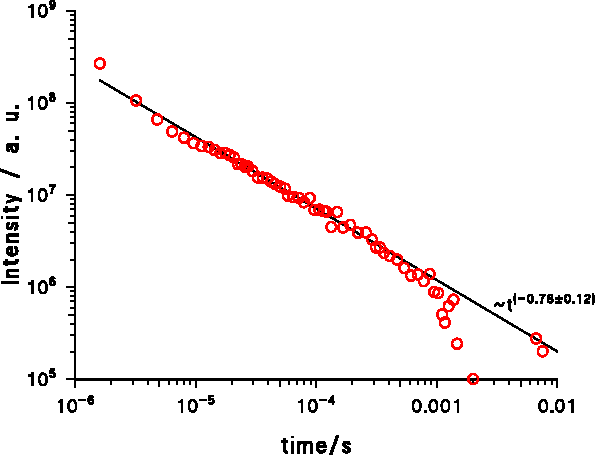
Figure 5. Decay of the violet cathodoluminescence from AlN on SiC at 453 nm and 300 K (sample LS12: 575 nm MBE-ALN on SiC)
With increasing temperature the intensity of the VL decreases but the relative spectrum is nearly unchanged; it has only a weak red-shift.
3. Conclusion
The algebraic decay of the violet luminescence at low temperatures indicates donor-acceptor pair transitions Reference Thomas, Hopfield and Augustyniak[5]. This is consistent with the observation that the luminescence at the long wave edge of the band decays slower than at the short wave edge. The donor-acceptor pair transitions can also explain the weak red shift of the VL with the weak shrinking of the bandgap with increasing temperature. At room temperature the VL is much weaker due to stronger non-radiative recombination processes, and the smaller decay exponent indicates that these non-radiative processes are much faster than the radiative recombination. The related material GaN shows a similar behavior at low temperatures (<80 K), which is not surprising because the yellow luminescence (YL) of GaN shifts with increasing Al fraction of AlxGa1−xN continuously to the violet luminescence (VL) of AlN. But at room temperature the non-radiative recombination processes in GaN are not so strong as in AlN.








