Numerous definitions are in use in relation to CVD but in broad terms it could be regarded as that which describes a range of diseases affecting the heart and vasculature( 1 , 2 ). These may include coronary artery disease, myocardial infarction (MI), stroke/cerebrovascular disease, peripheral artery disease, atherosclerosis, hypertension, arrhythmias, heart failure and other vascular disorders( 1 ). CVD is a major public health concern and is associated with an enormous burden of illness, disability and mortality. It is the biggest killer in the UK and Ireland( 2 , 3 ). In 2009, approximately one-third of all deaths in the UK were due to CVD( 2 ). Of these, over 82 000 deaths were caused by CHD, and about 49 000 were caused by stroke( 2 ). Similarly, in Ireland, CVD resulted in 35% of all deaths in 2006( 3 ). Clearly, reducing the incidence of CVD is a high priority for health agencies and authorities in most Westernised countries.
There are many risk factors for CVD, some of which are non-modifiable (such as increasing age, sex, family history and ethnicity( Reference Thom, Haase and Rosamond 4 )), whereas others are modifiable (such as abnormal blood lipids, smoking, diabetes, elevated blood pressure, abdominal obesity, lack of physical exercise, diet, stress and overconsumption of alcohol( Reference Yusuf, Hawken and Ounpuu 5 )). In the last 30 years, various lines of evidence point to the suggestion that low vitamin D status (as reflected by serum/plasma 25-hydroxyvitamin D (25(OH)D) concentration below a defined threshold; see later) is an additional risk factor for CVD( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Lee, O'Keefe and Bell 7 ). Ecological data from Scragg in 1981 suggested that the rate of CVD-related death is elevated at higher latitudes( Reference Scragg 8 ), and higher latitudes are associated with greater periods of the year during which dermal synthesis of vitamin D is not possible due to limited UVB sunlight in these extended winters( Reference Cashman 9 ). Similar ecological data exist between higher latitudes and CVD risk factors such as hypertension( Reference Rostand 10 ) and type 1 diabetes mellitus( Reference Mohr, Garland and Gorham 11 ). At a cellular level, both cardiac myocytes and fibroblasts express key components of the vitamin D metabolism machinery, such as vitamin D receptors as well as the 1α-hydroxylase and 24-hydroxylase enzymes, which are responsible for the activation and deactivation steps of vitamin D metabolism, respectively( Reference Bouillon, Carmeliet and Verlinden 12 – Reference Thadhani 15 ). Furthermore, vitamin D receptors and 1α-hydroxylase knockout mice display myocardial hypertrophy, which persists even after normalisation of calcium and phosphorus levels, and myocardial dysfunction characterised by increased contractility and impaired systolic function become evident in the vitamin D receptors and 1α-hydroxylase knockout mice, respectively( Reference Bouillon, Carmeliet and Verlinden 12 – Reference Thadhani 15 ). These mechanistic findings of a role for vitamin D in CVD risk are supported by observational data in human subjects (see later); however, the issue of causality remains an important research question and a persisting knowledge gap. If low serum/plasma 25(OH)D concentration is causally linked to risk of CVD then this is important not only because low vitamin D status is quite common particularly in winter in countries above 40°N( Reference Cashman, Fitzgerald and Kiely 16 ) but is also of key relevance since such low vitamin D status can be improved by a number of food-based strategies( Reference Cashman 9 , Reference Kiely and Black 17 ). This review will firstly very briefly overview how vitamin D may play a role in CVD, which provides the biological plausibility for how a low vitamin D status may increase risk of CVD; it will then review the current evidence-base to support a link between low vitamin D status and CVD risk, with particular emphasis on data from randomised control trials (RCT), cohort studies and recent meta-analysis data. Reference will be made to the conclusions of a number of authoritative agencies/bodies so as to help distill the existing data that links low vitamin D status to CVD risk. Finally, the review will summarise current serum 25(OH)D concentrations within a select number of adult populations in the context of different definitions of vitamin D status proposed recently, and then briefly highlight food-based strategies for increasing vitamin D intake and status, which could ultimately lower the risk of CVD should the causality between vitamin D and CVD be affirmed.
Role of vitamin D and CVD: the mechanisms of action and biological plausibility
As mentioned earlier, both the heart and the vasculature possess vitamin D receptors as well as the 1α–hydroxylase enzyme( Reference Bouillon, Carmeliet and Verlinden 12 – Reference Thadhani 15 ) to activate 25(OH)D to 1,25-dihydroxyvitamin D (1,25(OH)2D), and thus are important target tissues for vitamin D. In particular, as activation of 25(OH)D to 1,25(OH)2D in extra-renal tissues is highly dependent on substrate availability, then as along with circulating 1,25(OH)2D concentrations, the concentration of serum/plasma 25(OH)D might be an important determinant of vitamin D effects in the heart and vessels( Reference Pilz, Tomaschitz and Drechsler 18 ). The mechanistic evidence on the effect of vitamin D status on myocardial diseases and CVD risk factors has been expertly reviewed elsewhere( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Lee, O'Keefe and Bell 7 , Reference Pilz, Tomaschitz and Drechsler 18 – Reference Muldowney and Kiely 21 ). To aid the reader, these will only be briefly highlighted here, and interested readers are referred to these comprehensive reviews for detailed explanations. As can be seen in Table 1, there may be multiple potential effects of vitamin D on the heart and vasculature. Several vitamin D effects on the electrophysiology, contractility and structure of the heart suggest that vitamin D deficiency might be a causal factor for myocardial diseases( Reference Pilz, Tomaschitz and Drechsler 18 , Reference Pilz, Tomaschitz and März 19 ). Most of these will be affected through the actions of 1,25(OH)2D, which has been suggested to possess antihypertrophic effects on cardiomyocytes, to be able to suppress the cardiac renin–angiotensin system (important in regulating blood pressure and mineral metabolism, an increased activation of which has been linked to the development of myocardial hypertrophy) and the natriuretic peptides (secreted by cardiomyocytes due to volume overload and increased atrial and/or ventricular pressure), which together may at least in part mediate antihypertrophic effects of vitamin D( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Pilz, Tomaschitz and Drechsler 18 , Reference Muldowney and Kiely 21 ). Calcium flux and calcium homoeostasis are also important for electrophysiology and contractility of the heart( Reference Pilz, Tomaschitz and Drechsler 18 ), and 1,25(OH)2D plays an important regulatory role in cellular calcium handling. Myocardial extracellular matrix turnover is also regulated by vitamin D effects on the expression of matrix metalloproteinases (which hydrolyse these matrix metalloproteinases) and tissue inhibitors of these metalloproteinases, which if not in concert can lead to initiation and progression of both diastolic and systolic heart failure( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Pilz, Tomaschitz and Drechsler 18 , Reference Muldowney and Kiely 21 ). 1,25(OH)2D may have effects through other molecular targets also( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Pilz, Tomaschitz and Drechsler 18 ).
Table 1. Effects of vitamin D on the heart and vessel

RAS, renin–angiotensin system; PTH, parathyroid hormone; Gla, gamma-carboxylated proteins.
The Table is generated from reviews( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Pilz, Tomaschitz and Drechsler 18 , Reference Pilz, Tomaschitz and März 19 ).
Vitamin D deficiency might also contribute to myocardial diseases by various indirect effects, which are related to disturbances of calcium homoeostasis, elevated parathyroid hormone concentrations (which may elevate blood pressure as well as contribute to myocardial hypertrophy and pro-arrhythmia), infections, autoimmunological processes, endothelial dysfunction, as well as evidence that vitamin D may exert beneficial effects on other well-reported cardiovascular risk factors such as arterial hypertension, diabetes mellitus, dyslipidaemia, atherosclerosis, obesity and inflammation. These have been reviewed elsewhere in detail( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Lee, O'Keefe and Bell 7 , Reference Pilz, Tomaschitz and Drechsler 18 – Reference Muldowney and Kiely 21 ). In relation to the latter two, recent data on genetic variants in vitamin D metabolism-related genes and those of the BMI( Reference Wang, Zhang and Richards 22 – Reference Vimaleswaran, Berry and Lu 24 ), seem to suggest that obesity contributes to vitamin D deficiency and not that vitamin D deficiency causes obesity. For example, in these Mendelian randomisation studies genetic variants in some vitamin D metabolism-related genes are not associated with increased risk of obesity, but genetic variants in obesity-related genes are associated with increased risk of vitamin D deficiency. This is not unexpected as adipose tissue has an ability to sequester vitamin D and thus limit the synthesis of 25(OH)D leading to lower vitamin D status in overweight and obese subjects. Thus, obesity can have a confounding effect on the association between vitamin D status and CVD. Similarly, while increased inflammation plays an important role in CVD and vitamin D (as 1,25(OH)2D) has been suggested as possessing anti-inflammatory effects( Reference Querfeld 25 ), recent data show that systemic inflammation arising from elective knee surgery has a dramatic lowering effect on serum 25(OH)D, an effect evident even months later( Reference Reid, Toole and Knox 26 ). The potential for confounding in existing human data, particularly observational data will be mentioned again later.
Is low vitamin D status a risk factor for CVD?: the evidence-base
In reviewing the evidence for low vitamin D status as a risk factor for CVD, it may be useful to consider the evidence in a weighted manner as often used for example by the Institute of Medicine( 1 ) and the European Food Safety Agency( 27 ). RCT data are generally weighted as the strongest type of evidence, at least for development of dietary reference values/intakes( 1 , 27 ). Observational evidence, while it can support associative relationships between vitamin D and a health outcome such as CVD, is considered as second tier evidence( 1 ). Among the observational studies, data from cohort studies are the strongest, followed then in order of strength by case–control, cross-sectional and finally ecological studies( 1 ). Thus, priority is given in this review to the RCT and cohort data linking low vitamin D status to CVD, where possible. Meta-analysis of data arising from prospective studies and the RCT can also provide a useful distillation of findings from available studies, some of which are often mixed. The review will begin with the lowest level of evidence and systematically work towards the highest, i.e. data from the RCT.
Observational data
Epidemiological studies of different designs have reported reduced serum/plasma 25(OH)D concentrations and increased risk of MI and early mortality due to CVD (for reviews, see( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Lee, O'Keefe and Bell 7 , Reference Pilz, Tomaschitz and Drechsler 18 – Reference Muldowney and Kiely 21 , Reference Lamberg-Allardt, Brustad and Meyer 28 , Reference Elamin, Abu Elnour and Elamin 29 )). In particular, these include at least twenty population-based follow-up studies of the association of 25(OH)D and cardiovascular events and mortality (details of these studies have been provided recently in a meta-analysis by Wang et al.( Reference Wang, Song and Manson 30 )). As shown by Wang et al.( Reference Wang, Song and Manson 30 ) some of these studies report an association between ‘poor’ vitamin D status and increased risk of cardiovascular events and mortality, while others do not. Interestingly, in those that do find an association, adjusted relative risk ratios of between 1·7 and 5·3 have been reported for those with ‘low’ (typically serum 25(OH)D<26–37·3 nmol/l) v. ‘higher’ vitamin D status (typically serum 25(OH)D <34–75 nmol/l), while similar types of 25(OH)D categories have been used in studies where the association was NS( Reference Pilz, Tomaschitz and März 19 , Reference Wang, Song and Manson 30 ). As mentioned earlier, however, the potential effect of confounding across these various studies and adjustment for these on the divergent findings is likely to be significant and needs to be borne in mind in relation to their use in meta-analyses. These individual studies have been reviewed in detail elsewhere( 1 , Reference Pilz, Tomaschitz and Drechsler 18 – Reference Muldowney and Kiely 21 , Reference Lamberg-Allardt, Brustad and Meyer 28 , Reference Wang, Song and Manson 30 , Reference Chung, Balk and Brendel 34 ). It is worth noting also that the study by Melamed et al.( Reference Melamed, Michos and Post 31 ), using the third National Health and Nutrition Examination Survey cohort, while CVD risk was not statistically significant, the all-cause mortality data suggested a U- or reverse J-shaped dose-relationship, with increased total mortality for both the lowest and highest serum 25(OH)D concentrations (<44·4 and >80·1 nmol/l, respectively) in this cohort followed for 9 years. These J-shaped dose-relationship findings were extended upon and supported in a recent follow-up of the same cohort after an additional 6 years (15 years of follow-up in total)( Reference Sempos, Durazo-Arvizu and Dawson-Hughes 32 ). Interestingly, meta-analyses of such studies seem to support the finding of ‘low’ serum 25(OH)D concentrations being associated with incident CVD( Reference Wang, Song and Manson 30 , Reference Grandi, Breitling and Brenner 33 ). For example, Grandi et al.( Reference Grandi, Breitling and Brenner 33 ) reported a hazard ratio for cardiovascular mortality of 1·83 (95% CI 1·19, 2·80) in individuals with serum 25(OH)D concentrations below a threshold range of approximately 25–50 nmol/l. More recently, the meta-analysis by Wang et al.( Reference Wang, Song and Manson 30 ) of nineteen independent prospective cohort studies (which amassed to 6123 CVD cases in 65 994 participants) reported an inverse association between serum 25(OH)D concentrations and risk of CVD outcome, but with considerable heterogeneity among studies. The pooled relative risk when comparing the lowest with highest serum 25(OH)D concentration categories within the studies was 1·52 (95% CI 1·30, 1·77) for total CVD, 1·42 (95% CI 1·19, 1·71) for CVD mortality and 1·38 (95% CI 1·21, 1·57) for CHD. Interestingly, the CVD risk tended to increase in a linear manner across decreasing serum 25(OH)D concentrations below approximately 60 nmol/l with a pooled relative risk of 1·03 (95% CI 1·00, 1·06) per 25 nmol/l decrease in serum 25(OH)D concentrations( Reference Wang, Song and Manson 30 ).
The North American Institute of Medicine in reviewing the Dietary Reference Intakes (DRI) for calcium and vitamin D( 1 ) used data from a comprehensive systematic evidence-based review (referred to the Agency for Healthcare Research and Quality's Tufts report) of the calcium and/or vitamin D and multiple health outcomes, including cardiovascular health( Reference Chung, Balk and Brendel 34 ). This systematic review provided an important evidence-base by which the DRI committee were able to address a critical question, namely whether there is enough evidence to support the non-skeletal health effects of vitamin D and calcium in terms of setting new DRI? The DRI committee pointed towards the conclusion of the Agency for Healthcare Research and Quality's -Tufts report that the evidence was insufficient to support a relationship between vitamin D (or calcium) and risk for CVD( 1 ). The DRI committee, taking into account the data from the Agency for Healthcare Research and Quality's -Tufts report and also further data which they identified, did acknowledge that observational evidence supports a relationship between serum 25(OH)D concentrations and the presence of CVD; however, it does not show a relationship with risk of developing CVD( 1 ). Interestingly, the Nordic Nutrition Recommendation group who have a draft vitamin D recommendation out for public consultation( 35 ) also had a working group undertake a systematic review which concluded that in general, systematic reviews based on cohorts or case-controlled studies have consistently found an association between low serum 25(OH)D (mostly below 37·5 nmol/l or below 50 nmol/l) and increased risk of CVD( Reference Lamberg-Allardt, Brustad and Meyer 28 ).
Cross-sectional and prospective studies have similarly produced evidence of associations between vitamin D intake/status and blood pressure, inflammation and diabetes, with less convincing data for dyslipaemia (for reviews, see( Reference Zittermann, Schleithoff and Koerfer 6 , Reference Lee, O'Keefe and Bell 7 , Reference Pilz, Tomaschitz and Drechsler 18 – Reference Muldowney and Kiely 21 , Reference Lamberg-Allardt, Brustad and Meyer 28 )). It is also worth stressing that when interpreting the findings of such observational studies it is important to bear in mind the possible reciprocal influences of vitamin D deficiency, CVD and cardiovascular risk factors. Pilz et al.( Reference Pilz, Kienreich and Tomaschitz 20 ) illustrate this issue of reverse causation and possible reciprocal influences nicely by use of two examples, (i) low serum 25(OH)D concentrations may hypothetically contribute to myocardial dysfunction but heart failure associated limitations in mobility may reduce outdoor activities and sunlight exposure thus contributing to low serum 25(OH)D concentrations; (ii) on the other hand, heart failure may also cause wasting with weight loss and subsequently beneficial effects on metabolic risk factors (e.g. improved glucose homoeostasis) but also higher serum 25(OH)D concentrations due to lower BMI. Clearly, data from the RCT would be important to provide evidence of causality in relation to low vitamin D status and CVD.
Data from the randomised control trials
In contrast to the many observational studies examining the association between vitamin D status and CVD, the number of intervention studies is quite limited. A UK-based RCT originally designed for fracture prevention, showed no effect of 2500 μg (100 000 IU) of vitamin D3 every 4 months (which would equate to approximately 20 μg/d (800 IU/d) of vitamin D3) over a 5-year follow-up period on incidence of CVD events or mortality in elderly adults (n 2686)( Reference Trivedi, Doll and Khaw 36 ). Avenell et al.( Reference Avenell, MacLennan and Jenkinson 37 ), in their long-term follow up for vascular disease mortality (as well as total mortality and that from cancer) of elderly subjects (aged >70 years, n 5292) in the RECORD RCT trial, reported a hazard ratio of 0·91 (95% CI 0·79, 1·05) for vascular disease between those that received vitamin D3 (20 μg/d (800 IU/d) for 24–62 months) and those that received placebo. In the USA, the Women's Health Initiative trial tested the effect of vitamin D, in combination with calcium supplementation, on risk of coronary and cerebrovascular events in postmenopausal women aged 50–79 years (n 36 282)( Reference Hsia, Heiss and Ren 38 ). Women received either the active treatment (10 μg/d (400 IU/d) vitamin D3 plus 1000 mg calcium daily) or placebo and after 7 years of follow-up there was no effect on risk evident( Reference Hsia, Heiss and Ren 38 ). The Women's Health Initiative trial has been much commented on in relation to issues of compliance, allowable use of non-protocol supplements, numbers of subjects in which serum 25(OH)D analysis was undertaken and these need to be considered when interpreting the findings of the trial. Several other trials which used vitamin D and calcium, and had CVD as a secondary outcome measure, also found no significant treatment effect of vitamin D on CVD risk (for reviews, see( 1 , Reference Lamberg-Allardt, Brustad and Meyer 28 , Reference Elamin, Abu Elnour and Elamin 29 , Reference Chung, Balk and Brendel 34 )). The inter-relationship between vitamin D and calcium for many health outcomes, not just CVD, is a key consideration and one which adds a further level of complexity in delineating the effect of both nutrients in isolation on health( Reference Cashman and Kiely 39 ). In a secondary analysis of their RCT (n 1471), Bolland et al.( Reference Bolland, Barber and Doughty 40 ) found that compared with those taking placebo, postmenopausal women taking 1000 mg elemental calcium had a significantly higher risk of MI and a composite CVD endpoint of MI, stroke and sudden death. It has been suggested that caution may be warranted because the effect of calcium may possibly attenuate a potential beneficial effect of vitamin D alone( Reference Pilz, Tomaschitz and Drechsler 18 , Reference Muldowney and Kiely 21 ). However, this effect of calcium on CVD risk is not found in all trials (for reviews, see( 1 , Reference Lamberg-Allardt, Brustad and Meyer 28 )) and the vascular disease mortality hazard ratio (1·07, 95% CI 0·92, 1·24) for calcium supplementation (1000 mg/d) over placebo was NS in the RECORD trial follow-up study( Reference Avenell, MacLennan and Jenkinson 37 ). Rejnmark et al.( Reference Rejnmark, Avenell and Masud 41 ) recently showed in an individual patient data-level pooled analysis of 70 528 patients (86·8% females) from eight major vitamin D trials that vitamin D with calcium reduces mortality (hazard ratio, 0·91 (95% CI 0·84, 0·98)) in the elderly, whereas the available data did not support an effect of vitamin D alone. Again, with the RCT data, and especially when combining data from across studies, the issue of confounding rises again. Muldowney and Kiely( Reference Muldowney and Kiely 21 ) have suggested that data from the rather limited number of RCT are confounded by inappropriate study design, inadequate characterisation of subjects, lack of data on season and sunshine exposure and interventions combining calcium and vitamin D.
The Nordic Nutrition Recommendation group( 35 ) summarised in their draft report that there is probable evidence for an inverse association between low vitamin D status and increased CVD risk. However, they suggested that the data are insufficient to establish a precise cut-off for an increased risk( 35 ). The North American DRI committee concluded in relation to data from the RCT that the totality of the evidence does not support an interaction between vitamin D (or calcium) and risk of CVD, including hypertension( 1 ). Furthermore, in their concluding statement they suggest that a review of the available evidence from both the RCT and the observational studies on associations between vitamin D and calcium intake and risk of CVD shows that although observational evidence supports a relationship between serum 25(OH)D concentrations and the presence of CVD, it does not show a relationship with risk of developing CVD, and evidence was not found for a causal relationship between vitamin D intake and development of disease( 1 ). Furthermore, due to the lack of statistically significant evidence supporting associations between vitamin D intake or serum 25(OH)D concentration and risk for CVD and the further lack of evidence on CVD as a primary outcome of treatment in RCT with vitamin D and/or calcium, the DRI committee could not draw an inference about the efficacy of CVD to support DRI development for vitamin D (and ultimately used bone health for the same)( 1 ). An Endocrine Task Force briefed with developing a clinical practice guideline on the evaluation, treatment and prevention of vitamin D deficiency (with emphasis on care of patients who are at risk for deficiency)( Reference Holick, Binkley and Bischoff-Ferrari 42 ) commissioned the conduct of a systematic review of the literature on vitamin D supplementation and cardiovascular outcomes( Reference Elamin, Abu Elnour and Elamin 29 ). This systematic review concluded that there was no significant effect on MI, stroke, lipid fractions, glucose or blood pressure; blood pressure results were inconsistent across studies, and the pooled estimates were trivial in absolute terms( Reference Elamin, Abu Elnour and Elamin 29 ).
A brief consideration of vitamin D status classification as relates to CVD and implications
In relation to the important research field of vitamin D and CVD, as well as the many other health (skeletal and non-skeletal) outcomes, it is important to briefly highlight the need for agreement on definitions of vitamin D status. In a recent review of vitamin D, CVD and mortality, it has been proposed that vitamin D ‘deficiency’, ‘insufficiency’, ‘optimal range’, ‘sufficiency’ and ‘intoxication’ be defined as serum 25(OH)D concentrations <50, 50–74, 75–100, 75–250 and >375–500 nmol/l, respectively( Reference Pilz, Tomaschitz and März 19 ). Some of these proposed cut-off concentrations align with those of the Endocrine Task Force( Reference Holick, Binkley and Bischoff-Ferrari 42 ) which suggest vitamin D ‘deficiency’, ‘insufficiency’ and ‘sufficiency’ as serum 25(OH)D <50, 52·5–72·5 and 75–250 nmol/l, respectively. In their review, Pilz et al.( Reference Pilz, Tomaschitz and März 19 ) make a critically important point which is that any vitamin D status classification is debatable unless sufficient data from the RCT are available to support that targeting certain 25(OH)D ranges reduces hard endpoints such as CVD or mortality. As outlined earlier, RCT evidence is lacking in relation to vitamin D and CVD and thus it is not surprising that the North American Institute of Medicine based their serum 25(OH)D thresholds (<30 nmol/l as risk of vitamin D deficiency, 40 nmol/l representing the average requirements and 50 nmol/l representing the needs of 97·5% of the population) on the basis of bone health outcomes( 1 ). The 30 and 50 nmol/l serum 25(OH)D concentrations were also recently proposed by the Nordic Nutrition Recommendation for the Nordic population( 35 ).
The implications of these definitions become extremely evident when one uses them to define ‘low’ or ‘good’ vitamin D status in the population. As an example, data from the National Adult Nutrition Survey would suggest that about 18, 40, 60 and 84% of Irish adults aged 18+ years have winter-time serum 25(OH)D <30, 40, 50 and 75 nmol/l, respectively( Reference Cashman, Kiely and Kinsella 43 , Reference Cashman, Muldowney and McNulty 44 ). In the UK, data from the National Diet and Nutrition Survey of adults aged 19–64 years show that about 36, 59, 73 and 96% of adults in the UK have winter-time serum 25(OH)D <30, 40, 50 and 80 nmol/l, respectively ( Reference Ruston, Hoare and Henderson 45 ). The implication of the definition of vitamin D status as might relate to CVD, or any other health outcome(s), from a public health perspective is also critical in terms of defining the associated dietary reference values (or DRI), the vitamin D intake values for population health. To illustrate this, Table 2 shows the vitamin D intake estimates using data from several vitamin D RCT aimed at defining the distribution of dietary vitamin D requirements during winter but at different classifications of vitamin D deficiency( Reference Cashman, Hill and Lucey 46 – Reference Cashman, FitzGerald and Viljakainen 48 ) as outlined earlier. The vitamin D intake estimates to maintain 97·5% of a population life-stage group (Recommended Daily Allowance or Reference Nutrition Intake (in the UK) or Population Reference Intake (in the EU)) over these various serum thresholds during winter, which is the nadir of the year in terms of vitamin D status at least in countries above and below 40°, varies from about 8–28 μg/d (320–1120 IU/d) vitamin D, depending on life-stage group and threshold concentration.
Table 2. Estimated dietary requirements for vitamin D at the 97·5th percentile in adolescent girls (mean age 11·3 years), adults (aged 20–40 years) and elderly (aged 64+ years) to maintain serum 25(OH)D above selected concentrations during winter*
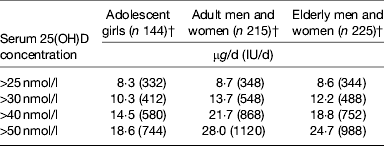
* The vitamin D intake value that will maintain serum 25(OH)D concentrations in 97·5% of subjects above the indicated cut-off level during winter.
Dietary strategies for increasing vitamin D intake and status
Irrespective of which definition of ‘low’ vitamin D status one chooses, the data in Table 2 clearly show that the habitual mean vitamin D intakes, in the range 2–7 μg/d (80–280 IU/d) by European populations( Reference Cashman 9 , Reference Cashman, Fitzgerald and Kiely 16 , Reference Kiely and Black 17 ), are below any of these estimates of intake requirement. However, the selection of the definition becomes important in terms of devising strategies to bridge the gap between current intake (2–7 μg/d (80–280 IU/d) on average) and vitamin D intake requirement estimates of ∼10 v. ∼25 μg/d (∼400 v. 1000 IU/d); for serum 25(OH)D > 30 and 50 nmol/l, respectively).
It has been emphasised and re-emphasised that there are only a limited number of public health strategies available to correct low dietary vitamin D intake, and these have been reviewed in detail elsewhere( Reference Cashman 9 , Reference Kiely and Black 17 , Reference Black, Seamans and Cashman 49 ), so only a brief overview will be provided here:
-
1. Improving intake of naturally occurring vitamin D-rich foods. This is the least likely strategy to counteract low dietary vitamin D intake due to the fact that there are very few food sources that are rich in vitamin D. Furthermore, most of these are not frequently consumed by many in the population( Reference Henderson, Irving and Gregory 50 ).
-
2. Vitamin D supplementation. Supplementation with vitamin D has been shown to significantly improve vitamin D intake across a variety of age, race, ethnic and gender groups as well as improving vitamin D status per se (whose efficacy is dependent on dose)( Reference Calvo and Whiting 51 , Reference Seamans and Cashman 52 ). However, evidence seems to suggest that the population intake of vitamin D from supplements is quite low( Reference Flynn, Hirvonen and Mensink 53 ). This is a function mainly of the relatively low vitamin D content of most supplements in some countries relative to the requirement as discussed earlier. Recent data from the National Adult Nutrition Survey in Ireland showed that despite conferring a benefit in terms of higher mean serum 25(OH)D concentrations in those adults who used vitamin D-containing supplements, only 17·5% of the Irish adults aged 18–84 years (16% of those aged 18–64 years) consumed them( Reference Cashman, Muldowney and McNulty 44 ). Some are of the view that while not highly effective at a population level, vitamin D supplementation may be appropriate in high-risk groups such as the elderly( 54 , Reference Viljakainen, Palssa and Karkkainen 55 ).
-
3. Vitamin D fortification (mandatory or voluntarily) of food. This has been viewed by some as a feasible and effective measure once applied in an evidence-based approach. In response to concerns about widespread vitamin D deficiency, many countries have implemented either mandatory or discretionary food fortification( Reference Cashman 9 , Reference Kiely and Black 17 ). Fortification of foods with vitamin D in the USA and Canada has an important effect on the mean daily intake of vitamin D by the average adult; however, Calvo and Whiting( Reference Calvo and Whiting 51 ) suggest that the current level of fortification in the USA and Canada is not effective in reaching the required levels of vitamin D intake. This may relate to the level of fortification, types and choice of food vehicles and the issue of mandatory or optional/voluntary fortification( Reference Cashman 9 , Reference Kiely and Black 17 ). Flynn et al.( Reference Flynn, Hirvonen and Mensink 53 ) have recently shown that the 95th percentile of intake of vitamin D from voluntary fortified foods in Europe is low. Thus, from a European perspective, we need to model European food and vitamin D intake data to ascertain which food vehicles and what level of vitamin D addition will ensure an effective but safe rise in serum 25(OH)D concentration in European populations. This research will be undertaken in a recently funded EU Framework 7 (FP7) collaborative project called ‘ODIN; Food-based solutions for Optimal vitamin D Nutrition and health through the life cycle’ (www.ODIN-vitD.eu) commencing in Autumn 2013.
It is also important to stress that food-based strategies that are devised to maintain serum 25(OH)D over 25/30 nmol/l (the definition of vitamin D deficiency on the basis of bone health outcomes) will shift the vitamin D intake distribution to the right and consequently will also shift the serum 25(OH)D distribution to the right, which will have benefits for many in the population in relation to CVD, if there is indeed causality.
Conclusion
While epidemiological associations between low vitamin D status and CVD are convincing and supported by mechanistic data, data from the RCT (as top tier evidence) are lacking at present. It should be noted however that several large RCT are underway which aim to test the effect of vitamin D supplementation on CVD (but also other health outcomes) in the general population( Reference Kupferschmidt 56 ). It will take between 4 and 7 years for data from these RCT to become available and we will be certain whether or not if the data can lend support to the positive epidemiological associations. Worryingly, Pilz et al.( Reference Pilz, Kienreich and Tomaschitz 20 ) have also suggested that certain design limitations within these RCT, such as inclusion of study participants regardless of their baseline serum 25(OH)D concentrations, may limit their ability to detect the beneficial effects of vitamin D on cardiovascular morbidity and mortality in high-risk vitamin D deficient individuals. Only one of the five RCT has more than one dose of vitamin D. While there is a strong need to perform further well-designed RCT on vitamin D and cardiovascular events and its risk factors, there are some important knowledge gaps in this area that would be important to address before conducting further RCT. Drawbacks to previous meta-analyses will be addressed in the FP7 ODIN project. For example, it will include standardised 25(OH)D data and undertake the harmonisation of clinically validated endpoints and intermediate risk biomarkers, both of which will substantially improve the overall quality and reliability of the outcomes. While epidemiological studies suggest that the association between serum 25(OH)D and cardiovascular risk is particularly strong at very low serum 25(OH)D levels, there exist no sufficient data from the meta-analyses of the RCT to confirm if potential cardiovascular effects of vitamin D are restricted to vitamin D-deficient individuals. A planned individual patient data meta-analysis on the relationship between serum 25(OH)D and cardiovascular events and its risk factors, within the ODIN project, will significantly add to the existing literature that is currently mainly based on unstandardised 25(OH)D concentrations. The issue of standardised 25(OH)D data has been highlighted recently in the National Adult Nutrition Survey whereby the estimates of vitamin D deficiency and inadequacy derived from immunoassay and liquid chromatography tandem MS analysis of the same sample, were dramatically different (percentage <30 nmol/l throughout the year increased from about 6–11% when the serum 25(OH)D analytical platform switched from immunoassay to liquid chromatography tandem MS( Reference Cashman, Kiely and Kinsella 43 ). Individual patient data meta-analysis of vitamin D RCT within ODIN will also provide novel data on whether there are effects of vitamin D supplementation on cardiovascular risk factors in individuals with vitamin D deficiency, classified according to serum 25(OH)D. Thus, while at present the jury is still out in relation to vitamin D and CVD, largely due to lack of required evidence, new RCT and meta-analysis evidence will emerge over the coming years which may help the jury in drawing a clearer judgment.
Acknowledgements
None.
Financial support
There was no financial support for this review.
Conflicts of interest
None.
Authorship
K. D. C. was the sole author of the manuscript.






