Increasing evidence points to the role of certain dietary factors as key players in metabolic abnormalities, not only as contributors to body weight, a prominent risk factor for type 2 diabetes, but also as independent risk factors. For example, studies support the beneficial role of dietary fibre in reducing postprandial glycaemic response, improving insulin resistance and reducing inflammation( Reference Slyper 1 , Reference Cho, Qi and Fahey 2 ). Conversely, high-glycaemic index (GI) foods induce postprandial hyperglycaemia, which has been linked to type 2 diabetes risk( Reference Aston 3 , Reference Dong, Zhang and Zhang 4 ). Evidence also shows that increased fat intake can promote insulin resistance and inflammatory responses( Reference Nappo, Esposito and Cioffi 5 , Reference Esposito, Nappo and Giugliano 6 ). However, these dietary factors have been rarely examined simultaneously in relation to type 2 diabetes risk.
Over the past decade, dietary pattern analyses have increasingly been used to study associations between diet and disease risk. Dietary patterns may better describe the ‘real-world’ eating habits of free-living people, where nutrients are consumed together, and not in isolation( Reference Hu 7 , Reference Van Dam 8 ), and can therefore be used to create food-based public health guidance that is easier to interpret than nutrient-based advice.
Empirically defined dietary patterns defined as ‘healthy’ and high in fruit, vegetables and whole-grain foods, and low in red meat, added sugar and fried foods, have been linked with a reduced type 2 diabetes risk( Reference Brunner, Mosdol and Witte 9 – Reference Villegas, Yang and Gao 13 ); however, the mechanisms or pathways between ‘healthy’ dietary patterns and type 2 diabetes risk are, as yet, unclear. Reduced rank regression (RRR)( Reference Hoffmann, Schulze and Schienkiewitz 14 ) is a hypothesis-based statistical approach to identifying dietary patterns. The few studies so far that have applied RRR to examine diet and type 2 diabetes risk have mainly investigated dietary patterns related to inflammatory pathways( Reference Schulze, Hoffmann and Manson 15 – Reference Liese, Weis and Schulz 17 ). To our knowledge, no study has used RRR to investigate dietary patterns characterised by dietary GI, fibre and fat intake to date, yet separately these dietary factors have been linked with diabetes risk. Furthermore, despite the increasing popularity of studying dietary patterns, most cohort studies use only a single measure of dietary intake at baseline. It is important to study how changes in these patterns over the life-course affect disease risk and to what extent changing diet alters disease risk.
The aim of this study was to identify an RRR-derived dietary pattern characterised according to dietary fibre, GI and dietary fat, as these have been independently linked to increased type 2 diabetes risk, and to assess its longitudinal association with type 2 diabetes risk in the MRC National Survey of Health and Development (NSHD). It was hypothesised that repeated measures of a dietary pattern characterised by high intakes of fibre and low intakes of fat and low GI would be longitudinally and positively associated with type 2 diabetes risk over the life course, and independently of body weight and waist circumference (WC).
Methods
Participants
The MRC NSHD is a socially stratified sample of 5362 individuals (2547 male and 2815 female) born during one week in March 1946 in England, Scotland and Wales. The cohort has been followed-up twenty-three times to date, and the response rate throughout the study has been good, ranging between 78 % at the age of 16–35 years and 95 % at the age of 0–4 years( Reference Wadsworth, Kuh and Richards 18 ). At the latest data collection in 2006–2010 at the age of 60–64 years, 53 % of the original cohort (n 2856) was eligible for inclusion after exclusion of those who had died (n 778), lived abroad (n 584), had previously refused consent (n 594) or were untraceable (n 550). The 2661 individuals who responded (49 % of the original cohort and 84 % of the target sample) had remained broadly representative of the white British population born in the early post-war years( Reference Stafford, Black and Shah 19 ). The present analysis includes data on diet at 36, 43 and 53 years of age and incident type 2 diabetes diagnosed between 53 and 60–64 years of age. Survey respondents who maintained at least a 3-d food record were included in these analyses. The number of respondents completing diet diaries for at least 3 d was 2441 at the age of 36 years, 3187 at the age of 43 years and 1776 at the age of 53 years corresponding to 45, 59 and 33 %, respectively, of the original cohort. At all ages, individuals who completed diet diaries were more likely to be female, to be more educated, less likely to be in manual employment and to be smokers. We restricted all analyses to individuals with complete data on diet, as well as all variables needed. Complete data on diet, diabetes and all covariables were available for 1804 individuals at the age of 36 years, 2267 at the age of 43 years and 1478 at the age of 53 years.
Dietary data
Study members were asked to complete a 5-d food diary at 36, 43 and 53 years of age, detailing all foods and drinks consumed over 5 consecutive days( Reference Price, Paul and Key 20 ). Survey members were given guidance on household measures and photographs of portion sizes to aid completion by a research nurse who visited them at home. Food diaries were checked before coding and calculation of average daily nutrient intakes using an in-house program developed at the MRC Human Nutrition Research Unit( Reference Fitt, Cole and Ziauddeen 21 ), which linked food diaries with contemporaneous British food composition data. Food intakes were collapsed into forty-five food groups defined according to differences in GI and content of fat and fibre (Table 1). Dietary fibre density (g/4184 kJ or 1000 kcal) and fat density (g/4184 kJ or 1000 kcal) were calculated as total daily fibre (g; NSP) or fat (g) divided by total daily energy intake (EI; kJ/kcal) and multiplied by 1000. GI values were assigned to each food using the methodology described in detail by Aston et al. ( Reference Aston, Jackson and Monsheimer 22 ). Briefly, all food codes with total carbohydrate >0·1 g/100 g were assigned a GI value, based on five levels of data confidence relating to source of the data used, with level 1 being the highest. The average GI of the daily diet was calculated by assigning a glycaemic load (GL) value for each food item, then summing the GL values for the day and dividing this by the total daily carbohydrates (g)( Reference Wolever and Jenkins 23 ).
Table 1 Description of food groups included in the dietary pattern analyses

To assess dietary misreporting, the ratio of EI:estimated energy requirement (EER) was calculated according to an individualised method( Reference Rennie, Coward and Jebb 24 ). EER based on individual physical activity levels were calculated using equations from the Institute of Medicine of the National Academies( Reference Trumbo, Schlicker and Yates 25 ). To account for the variability of the methods used to estimate EI and EER, a 95 % CI for EI:EER was calculated( Reference Black and Cole 26 ). The 95 % CI of EI:EER for the NSHD was 0·54 and 1·46. Individuals reporting EI <54 % of their EER were classified as under-reporters, and those reporting >146 % were classified as over-reporters. The percentage of plausible EI reporters was 83 % at the age of 36 years, 84 % at the age of 43 years and 88 % at the age of 53 years. EI under-reporting was higher among overweight people and decreased with higher dietary pattern z-score. In all, 22 (125/581), 16 (168/1046) and 4 % (38/875) of overweight or obese people under-reported their EI at 36, 43 and 53 years of age, respectively, compared with 9, 9 and 2 % of normal-weight people. At the age of 43 years, EI under-reporting was higher among those diagnosed with type 2 diabetes between 53 and 60–64 years of age (20 % compared with 13 % of the remaining sample). Therefore, EI misreporting was included as a covariable in all analyses.
Type 2 diabetes
Ascertainment of type 2 diabetes at the age of 53 years was based on validated self-report. Self-reported diabetes was determined in response to a direct question and from all relevant medical information that study members reported (hospital attendances and medications). The validity of self-reported diabetes was assessed using general practitioners records, with a positive predictive value of 95 %( Reference Pastorino, Richards and Hardy 27 ). In all, 100 cases of prevalent diabetes at the age of 53 years were excluded from these analyses. At the age of 60–64 years, diabetes was ascertained by both self-reported information and by analyses of fasting blood glucose and HbA1c from 50-ml blood samples collected between 2006 and 2011 in five clinical research facilities. A diagnosis of diabetes was established if fasting plasma glucose was ≥7 mmol/l or HbA1c was ≥6·5 % (48 mmol/mol)( 28 ).
Covariables
Occupational social class, educational attainment, smoking and physical activity, based on interview and questionnaire data, were included as possible confounding factors. BMI and WC were included as mediating variables, as it was hypothesised that body weight would partially explain the association between diet and type 2 diabetes.
Data on lifetime occupational head of household social class at the age of 53 years (or earlier if this was unavailable) according to the UK Registrar-General( 29 ) was coded into six categories: (I) professional, (II) managerial and technical, (III) skilled non-manual, (IV) skilled manual, (V) partly skilled manual and (VI) unskilled manual. The highest level of educational qualification achieved by the age of 26 years was grouped into three categories: none (none attempted), intermediate (GCE ‘O’ level or equivalent, or vocational) or advanced (GCE ‘A’ level or equivalent, or degree or equivalent).
Physical activity at 36, 43 and 53 years of age was coded as inactive (no participation), moderately active (participated one to four times) and most active (participated five or more times), in the previous month (36 years), per month (43 years) and in the previous 4 weeks (53 years). Smoking at each follow-up was categorised as current, ex and never smoker. The use of prescribed medicines was assessed at each follow-up by a questionnaire. The latest information on prescribed medication for hypertension and dyslipidaemia was available at the age of 53 years. At all ages (36, 43, 53 years), a trained research nurse measured height, weight and WC using standard protocols. BMI was calculated from weight (kg) divided by height squared (m2).
Statistical analyses
RRR was used to identify dietary pattern z-scores. RRR derives dietary patterns by extracting successive linear combinations of predictor variables (food groups) that explain as much variation as possible in another set of response variables, which are hypothesised to be on the pathway between the predictor variables and the outcome( Reference Hoffmann, Schulze and Schienkiewitz 14 ). Dietary fibre density (g/4184 kJ or 1000 kcal), GI (units) and total dietary fat density (g/4184 kJ or 1000 kcal) were chosen as the response variables because, based on previous literature, they were hypothesised to be important dietary determinants of the risk of type 2 diabetes. The function PROC PLS in the software SAS was used to conduct all RRR analyses.
Initially, exploratory RRR analyses were conducted separately using dietary data collected at 36, 43 and 53 years of age. RRR derives as many dietary patterns as there are response variables, which in this case were three. At all ages the first dietary pattern derived from RRR analyses explained the greatest variation in all three response variables (29·8 % at age 36 years, 31·8 % at age 43 years and 37·9 % at age 53 years) compared with the second and third patterns, which explained approximately 12–15 and 5 %, respectively. Therefore, only the first dietary pattern was analysed further. Each study member received a z-score calculating the degree to which their dietary intake reflected this dietary pattern at 36, 43 and 53 years of age. To assess longitudinal associations between dietary patterns and type 2 diabetes, a z-score for exactly the same dietary pattern (based on the same covariance matrix) at 36, 43 and 53 years was required. To achieve this, confirmatory RRR analyses( Reference Imamura, Lichtenstein and Dallal 30 ) were used to calculate dietary pattern z-scores at 36 and 43 years of age using scoring weights from the first dietary pattern identified at 53 years. The first dietary pattern at the age of 53 years was chosen because it explained the most amount of total variation in all response variables, and the factor loadings (foods) on this dietary pattern were consistent at all ages (not shown but available on request).
Multivariable logistic regression models were used to examine prospective associations between quintiles of dietary pattern z-scores at 36, 43 and 53 years of age and type 2 diabetes risk between 53 and 60–64 years of age. The diet z-score quintiles were entered as a categorical variable, with the lowest quintile used as the reference category. Analyses were adjusted for social class, education, smoking, physical activity, medications for hypertension and dyslipidaemia, EI and EI misreporting (model 1), and subsequently for BMI and WC (model 2). Interactions between the dietary pattern z-score and sex were tested using multiplicative interaction models.
To examine changes in dietary pattern z-scores between periods over the life course in relation to type 2 diabetes risk, a conditional model of change( Reference Wills and Tilling 31 ) was used. Dietary pattern z-score changes for the periods 36–53, 36–43 and 43–53 years were calculated conditional on earlier z-score using the residual method. That is, dietary pattern z-score changes were estimated for each period by regressing each z-score measure on the earlier measures and saving the residuals; for example, the change between 36 and 43 years was estimated by regressing the z-score at 43 years on the z-score at 36 years. These residuals represent the change in dietary pattern z-score above or below what is expected given an earlier z-score. A positive change z-score value reflects a deterioration of diet quality; conversely, a negative change z-score represents an improvement of the diet. In a multivariate regression model, we modelled these residuals against the outcomes, adjusting for all the covariables described in model 1, as well as changes in BMI and WC during the same time period.
It has been reported that the detrimental effect of a high-GI diet might be more pronounced among overweight people who are often more insulin resistant than normal-weight individuals( Reference Schulze, Liu and Rimm 32 , Reference Villegas, Liu and Gao 33 ). Therefore, to test this hypothesis, interactions between the dietary pattern and BMI at 36 years were tested in multiplicative interaction models.
Results
Characteristics of the three RRR-derived dietary patterns at 36, 43 and 53 years of age are shown in the online Supplementary Table S1. The dietary pattern used for confirmatory analyses was negatively associated with dietary fibre density (r −0·70) and positively associated with fat density (r 0·44) and GI (r 0·55). A higher z-score for this dietary pattern signifies a diet higher in GI and fat and lower in fibre. Factors loadings for this dietary pattern are shown in Fig. 1. A positive factor loading indicated that as the intake of that food increased so did the dietary pattern z-score, whereas foods with a negative factor loading decreased the z-score. The dietary pattern was characterised by low intake of fruit, vegetables, low-fat yogurt, wholemeal bread, high-fibre cereals and high intakes of white bread, processed meat, fried potatoes, butter and animal fat and added sugar. In total, 57 % of the variation in dietary pattern z-score was explained by the top five and bottom five factor loadings, with fresh fruit explaining most of the variation (23 %), followed by white bread (8 %), vegetables (6 %), low-fat yogurt (5 %) and processed meat (4 %).

Fig. 1 Factor loadings for the high-fat, high-GI, low-fibre dietary pattern in the NSHD used in confirmatory dietary pattern analyses. GI, glycaemic index; NSHD, National Survey of Health and Development.
At all ages, people with higher z-scores for the high-fat, high-GI, low-fibre dietary pattern were significantly more likely to be in manual employment, to be smokers, physically inactive and to have no educational qualifications (Table 2). BMI and WC were positively associated with higher dietary pattern z-scores at the age of 53 years. Those with higher z-scores had greater intakes of energy (kJ/kcal), fat density, alcohol and a greater average daily GI, as well as lower intakes of dietary fibre density.
Table 2 Study population characteristics by quintiles (Q) of the high-fat, high-glycaemic index (GI), low-fibre dietary pattern z-score at age 36 years (n 1804), 43 years (n 2267) and 53 years (n 1478) (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))

SEP, socio-economic position; WC, waist circumference; EER, estimated energy requirement.
* Test for trend by linear or logistic regression with control for sex.
† Diagnosed between 53 and 60–64 years of age.
‡ Medians and IQR.
The number of incident cases of type 2 diabetes diagnosed between 53 and 60–64 years of age was 166 (ninety-four among men and seventy-two among women). Associations between diabetes risk and the dietary pattern are shown separately for men and women, as a significant interaction was observed between dietary pattern scores at the age of 43 years and sex on type 2 diabetes (P=0·02), although not at the age of 36 years (P=0·85) or 53 years (P=0·14). The dietary pattern was significantly associated with increased odds of diabetes among women at 43 and 53 years of age (Table 3). Among women, there was an increasing trend in OR for type 2 diabetes with increasing quintile of dietary pattern z-score. Those women in the highest z-score quintile at 43 years had an OR for type 2 diabetes of 5·45 (95 % CI 2·01, 14·79); women in the highest quintile at 53 years had an OR of 3·22 (95 % CI 1·08, 9·54). After adjustment for BMI and WC, the associations remained at the age of 43 years (P for trend across quintiles<0·01) but were no longer significant at the age of 53 years (P=0·05) (Table 3). No associations were observed for men.
Table 3 Associations at each age between a high-fat, high-glycaemic index, low-fibre dietary pattern z-score and incident type 2 diabetes between 53 and 60–64 years of age (Odds ratio (OR) and 95 % confidence intervals)

* P for trend across quintiles of z-score.
† Model 1: adjusted for socio-economic position, education, energy intake, energy under-reporting, smoking, physical activity, medications for hypertension and dyslipidaemia.
‡ Model 2: as model 1+adjusted for BMI and waist circumference.
Analyses of z-score changes in dietary pattern and type 2 diabetes were conducted for those who provided diet diaries at all three data collection years and had non-missing values for all covariables (n 1180). There were no significant differences in average score change between men and women (Fig. 2). However, people who developed type 2 diabetes between age 53 and 60–64 years increased their dietary pattern z-score on average, with an overall change between age 36 and 53 years of 0·27 sd units (95 % CI 0·036, 0·496), compared with a change of −0·06 sd units (95 % CI −0·125, 0·005) for the rest of the sample (P<0·01). No other statistically significant differences in score change were observed between other time points.

Fig. 2 Mean change in dietary pattern z-score across the adult life course (36–53 years) by type 2 diabetes diagnosis (diagnosed between 53 and 60–64 years of age) and sex. Student’s t test was used to test for differences in z-score changes; age 36–43 years (![]() ): P=0·50 for men and <0·01 for women; age 43–53 years (
): P=0·50 for men and <0·01 for women; age 43–53 years (![]() ): P=0·39 for men and <0·01 for women; age 36–53 years (
): P=0·39 for men and <0·01 for women; age 36–53 years (![]() ): P=0·29 for men and <0·001 for women.
): P=0·29 for men and <0·001 for women.
Multivariable regression models (Table 4) showed that, independently of simultaneous changes in BMI and WC, changes in dietary pattern z-scores between age 36 and 43 years were significantly associated with type 2 diabetes risk among women (OR 1·63; 95 %CI 1·08, 2·46) but not among men; changes between 43 and 53 years of age were of borderline significance among women. The test for an interaction between BMI and dietary pattern interaction was not significant (P>0·05); therefore, the results are presented without stratification for BMI.
Table 4 Associations between changes in dietary pattern z-score through the adult life course and type 2 diabetes between 53 and 60–64 years of ageFootnote * (Odds ratio and 95 % confidence intervals)
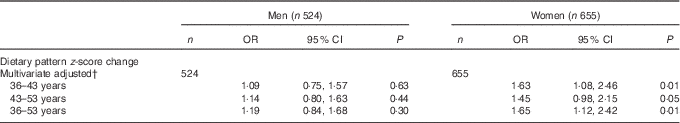
* OR of type 2 diabetes for a 1 sd increase in dietary patterns z-score in each interval conditional on previous dietary pattern z-score.
† Adjusted for socio-economic position, education, energy intake, energy under-reporting, smoking, physical activity, medications for hypertension and dyslipidaemia conditional BMI change and conditional waist circumference change.
Discussion
In this analysis of a large UK birth cohort, we identified a high-fat, high-GI, low-fibre dietary pattern that was prospectively associated with type 2 diabetes risk. This dietary pattern was characterised by a high consumption of white bread, processed meat, fried potatoes, butter, animal fats and added sugar, and a low intake of fruits, vegetables, low-fat yogurt and high-fibre cereals. Higher z-scores for this dietary pattern at 43 and 53 years of age were associated with an increased risk of type 2 diabetes diagnosed between 53 and 60–64 years of age among women, but not among men. Among women, a gradually increasing z-score representing an increasingly unhealthy diet over the life course (36–53 years) was strongly associated with type 2 diabetes. This association was independent of a wide range of potential confounders, including other health-related behaviours, and of the potential mediation of BMI and WC. Dietary GI and fibre act on satiety signals, whereas foods high in fat are very energy-dense, therefore affecting EI. Thus, it was expected that a dietary pattern high in fat and GI and low in fibre would act partly through its effect on EI and weight gain. The fact that an independent association between dietary pattern and diabetes remained after adjustment for EI and BMI and WC changes suggests that this pattern also acts through alternative pathways. The postprandial hyperglycaemia induced by high-GI foods can affect β-cell functions and insulin resistance both directly and indirectly by inducing a counter-regulatory hormone response, which increases circulating levels of free fatty acids( Reference Aston 3 , Reference Augustin, Franceschi and Jenkins 34 ). Free fatty acids, which are elevated when excess energy content and fat are consumed, increase insulin resistance by disrupting insulin signals in the gut and promote β-cell dysfunction through their lipotoxic effect in the pancreas( Reference Gastaldelli 35 ). Dietary fibre might reduce type 2 diabetes risk though its anti-inflammatory properties and its effect on glycaemia( Reference Kolb and Mandrup-Poulsen 36 ).
The positive associations between the dietary pattern and diabetes risk observed among women and not among men in this study may be explained because of several reasons. There might be biological sex differences in the responses to certain nutrients and the way these are disposed of and stored in the postprandial state. For example, it is known that sex-specific hormones can influence insulin receptors and lipid removal( Reference DeFronzo and Ferrannini 37 ), and that men oxidise a higher percentage of ingested fat than women( Reference Hebert, Ma and Clemow 38 ). It is unlikely that the sex difference could be because of different food choices, as there were no major sex differences in intake of the main foods characterising the dietary patterns. Hormonal changes associated with menopause might also explain the higher relative risk for type 2 diabetes with longer-term increases in dietary pattern z-score in women; it is possible that the cumulative influence of an unhealthy diet (as well as other lifestyle factors) on metabolic functions could come into play in the perimenopausal years, which is when women become more susceptible to chronic diseases associated with ageing( Reference Sowers, Derby and Jannausch 39 ).
Few cohort studies of this type have investigated men and women separately, and this is a strength of the current study. In the Melbourne Collaborative Cohort Study( Reference Montonen, Knekt and Harkanen 11 ), the association between a dietary pattern characterised by meats and fatty fried foods and diabetes was significantly stronger among women, whose risk for the disease was nearly 4-fold in the highest quintile of intake compared with the lowest quintile. Conversely, the risk among men in the highest quintile of intake was 2-fold compared with the lowest quintile and borderline significant( Reference Montonen, Knekt and Harkanen 11 ). In the Nurses’ Health Studies( Reference Schulze, Hoffmann and Manson 15 ), the relative risk of diabetes from intakes of an RRR-derived dietary pattern high in processed meat, refined grains and soft drinks was particularly high; on the other hand, a similarly characterised dietary pattern showed comparatively weaker associations in the Health Professionals Follow-up Study, which included male study members( Reference van Dam, Rimm and Willett 12 ).
Previous studies have found that protective dietary patterns identified with factor and cluster analyses, often labelled ‘healthy’ or ‘prudent’, tend to include fruits, vegetables, whole grains, whole bread and low-fat dairy products, whereas dietary patterns associated with increased type 2 diabetes risk tend to be high in red and processed meat, refined grains, fried foods, high-fat dairy products and sugar( Reference Brunner, Mosdol and Witte 9 – Reference Villegas, Yang and Gao 13 ). However, these dietary patterns were identified using purely exploratory methods, which do not necessarily identify disease-specific dietary patterns, and therefore their mechanisms of action may be difficult to elucidate. On the contrary, this study used RRR and incorporated hypothesised knowledge about pathways to disease, thus providing insight into the possible biological pathways that link these food groups with type 2 diabetes. This allowed us to investigate the synergistic action of dietary fibre, GI and dietary fat, individual factors for which there is increasing evidence of a link with type 2 diabetes. Furthermore, food-based public health recommendations based on key diabetes-relevant nutrients can be provided.
We should address various strengths and weaknesses of this study. Unlike most other prospective cohort studies, which rely on FFQ, the NSHD uses diet diaries, which do not rely on dietary recall. Prospectively recorded diet diaries correlate significantly better with biomarkers of intake, and are subject to substantially less regression dilution than FFQ( Reference Prentice 40 ). However, despite their value in providing detailed records of dietary intake, diet diaries, similar to all dietary assessments, are subject to error and dietary under-reporting. However, we attempted to adjust for dietary under-reporting in all our analyses using an accepted method. GI values were assigned by rigorous methodology and, where possible, GI values were sourced from the UK or from European studies. This ensured that the GI values in the NSHD were country-specific and as accurate as possible.
A particular strength was the use of repeated measures of dietary intake to investigate adult life-course changes in dietary patterns and type 2 diabetes risk; this has rarely been addressed in epidemiological studies, and most studies of dietary patterns assume that eating behaviours remain stable over the adult life course. Other strengths of this study were the use of a validated diabetes outcome measure.
On the other hand, loss to follow-up in NSHD might have introduced some degree of bias. Those providing dietary data were healthier and more likely to be women compared with those who did not complete diet diaries. Loss to follow-up of those less socially advantaged and less healthy may have resulted in under-estimation of effect sizes( Reference Kristman, Manno and Côté 41 ), although we have no reason to suspect that this would have altered the pattern of these associations. Reflecting the ethnic make-up of Britain in the 1940, the NSHD exclusively comprises Caucasians. Therefore, the findings from this paper might not be generalisable to cohorts of different ethnic groups. It is also important to recognise the potential measurement error associated with dietary assessment. The use of conditional change models might be associated with error when applied to repeated measures that are measured with some degree of error, as it is with diet.
In conclusion, a dietary pattern characterised by high-fat, high-GI and low-fibre intakes was prospectively associated with type 2 diabetes risk among women, and this association was independent of EI, BMI and WC. This association was robust when the dietary pattern was examined longitudinally over the life course (36–53 years), suggesting that the cumulative effects of changes in diet over a long-term period are particularly important for type 2 diabetes for women.
Acknowledgements
The authors thank the NSHD study members and the MRC staff responsible for the data collection of the NSHD over the past 68 years. The authors also thank the staff at MRC HNR, Cambridge for their collaboration in deriving GI values used in this study.
This study was conducted with funding from the Medical Research Council, UK (grant codes: MC_UU_12019/1 and 12019/4).
Authors’ contributions were as follows – S. P. and G. L. A. conceived and designed the study; S. P. analysed the data; all authors interpreted the data and oversaw the study; S. P. wrote the first draft of the manuscript. All authors revised and contributed to the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516000672










