Introduction
Snail-borne parasitic diseases including most trematodiasis and some nematodiasis diseases pose high risks to human health worldwide. Many parasitic diseases are important public health issues, particularly in tropical and subtropical countries (Lu et al., Reference Lu, Gu, Limpanont, Song, Wu, Okanurak and Lv2018). Freshwater snails have received considerable attention because of their involvement with some species as intermediate hosts for trematodes. This has become an issue of significant medical and/or veterinary importance with regard to the cause of parasitic diseases in people and animals, and is especially true in diseases associated with various food-borne trematodes (Madsen & Hung, Reference Madsen and Hung2014). Several indigenous South-East Asian freshwater snail species are widely consumed by local populations, particularly snails in the families Viviparidae, Ampullariidae and Pachychilidae. In the Indo-Burma region, many freshwater snails are harvested for human consumption by local communities. The shells are sold for decorative purposes or as construction materials and have also been used in artwork (Nagachinta et al., Reference Nagachinta, Piamtipmanus, Jivaluk, Punyaganok and Totanapoka2005; Köhler et al., Reference Köhler, Seddon, Bogan, Tu, Sri-Aroon, Allen, Allen, Smith and Darwall2012).
Viviparid snails are considered common in South-East Asia and are particularly prevalent in Thailand, where various species are consumed as food. In Thailand, there are eight genera of the family Viviparidae, while only two genera (Filopaludina and Eyriesia) are recognized as being of medical importance (Brandt, Reference Brandt1974; Tropmed Technical Group, 1986). These viviparid snails are commonly captured for human consumption and used to feed poultry. However, they are also known to transmit the trematode parasite of Echinostomatidae, specifically Echinostoma revolutum (Chantima et al., Reference Chantima, Chai and Wongsawad2013; Noikong & Wongsawad, Reference Noikong and Wongsawad2014; Noikong et al., Reference Noikong, Wongsawad, Chai, Saenphet and Trudgett2014).
In Northern Thailand, more than 50% of the stool samples collected from area residents were found to contain echinostome eggs (Sornmani, Reference Sornmani1969). Raw snails, fish and tadpoles were the suspected sources of these human echinostomiasis cases in this area (Sornmani, Reference Sornmani1969; Chai & Jung, Reference Chai and Jung2020). Infections are most prevalent in areas where traditional cultural practices encourage the ingestion of raw or insufficiently cooked snails, fish and tadpoles. Generally, echinostomiasis is considered a minor disease that is confined to low-income areas. However, geographical boundaries and the populations that are at risk continue to expand and change, resulting in higher rates of infection. A number of other factors contribute to the current situation such as widening international markets, improved transportation systems, changing eating habits in developed countries and various other demographic aspects (Toledo & Esteban, Reference Toledo and Esteban2016). Therefore, the aim of this study was to identify the viviparid snail species intended for public consumption in the local wet markets of Thailand, as well as investigating the prevalence of echinostome metacercariae infection among these snails. The association between the prevalence and intensity of echinostome metacercariae infections and host size, as well as the relationship to the host species, was also determined.
Materials and methods
Study area, snail collection and identification
Viviparid snail specimens were sampled for 8 months (May–December 2018) from local wet markets in 11 Districts of Chiang Rai Province, Northern Thailand (19°57′40″–20°07′10″N and 99°45′51″–99°57′24″E). This area is known for high consumption of viviparid snails. Fresh viviparid snails were purchased from local wet markets and transported to the laboratory. The snails were maintained on lettuce in aquaria containing dechlorinated tap water and aerated at room temperature under a natural light–dark cycle. Examinations were usually performed within 72 h after collection. Snail species were identified according to the morphological features described by Brandt (Reference Brandt1974).
Examination for parasite infection
Snails were examined for infection by shedding and crushing methods. For shedding, individual specimens were placed in 10-ml bottles half filled with dechlorinated tap water and left for 24 h to allow for shedding. Snails were checked for the shedding of cercariae by exposure to artificial light for about 3 h. Snails were individually screened for a period of 24 h. Before crushing, the snails were frozen for anaesthetization. The shell of each snail was broken and the whole body was then crushed in a Petri dish. Larval trematode infections were detected by examining the tissue microscopically after crushing. Larval trematodes were identified based on morphological features and counted according to snail species. The prevalence and intensity were also determined.
Statistical analysis
Statistical analyses were conducted using SPSS 26. Descriptive statistics were used to summarize the prevalence and intensity of larval trematode infections by snail species and shell size. Shell lengths of viviparid snails were divided into three groups: <20.0 mm, 20.0–<30.0 mm and ≥30.0 mm. An exact logistic regression was employed to analyse the association of echinostome metacercariae infection in snails according to species and shell size. Negative binomial regression was used to analyse the association of echinostome metacercariae infections and intensity according to species and shell size. This method was preferred over the Poisson regression based on the likelihood ratio test. Statistical significance was set at P ≤ 0.05.
Results and discussion
This was the first survey to elucidate the distribution of edible snail species and related incidence rate of parasitic infection in urban settings of Chiang Rai Province, Northern Thailand. Four species including Cipangopaludina annandalei, Filopaludina martensi martensi, F. sumatrensis polygramma and F. doliaris (fig. 1) were identified among 1100 viviparid snails from local wet markets in Chiang Rai Province, and their distributions in 11 different settings were identified for the first time. Viviparid snails intended for human consumption, particularly Filopaludina spp., were found in almost all of the markets. Thus, local wet markets of Chiang Rai Province abundantly support the trade of viviparid snail species as a rich and cheap source of protein for the residents of local communities. Filopaludina was identified as the preferred snail species used to prepare a popular traditional dish in Thailand (Nagachinta et al., Reference Nagachinta, Piamtipmanus, Jivaluk, Punyaganok and Totanapoka2005). The indigenous people of north-eastern Thailand also enjoy eating a locally popular snail dish called Koi Hoi, which is usually prepared from chopped raw snail meat and flavoured with seasonings and vegetables. This dish is typically consumed immediately after preparation (Eamsobhana et al., Reference Eamsobhana, Yoolek, Punthuprapasa and Yong2009). Interestingly, C. annandalei identified in this study has never previously been considered as a source of food (Nagachinta et al., Reference Nagachinta, Piamtipmanus, Jivaluk, Punyaganok and Totanapoka2005). Limited research has been conducted on C. annandalei, which was originally discovered and described by Brandt (Reference Brandt1974). The limited amount of information on C. annandalei has hindered the categorization of its species conservation status in Thailand, and identification of C. annandalei in local wet markets of Chiang Rai Province has raised interesting questions concerning its distribution under natural conditions in Chiang Rai Province and throughout Thailand. Further research is needed to accurately evaluate the conservation status of this snail species and its importance.

Fig. 1. Edible viviparid snails collected from local wet markets in Chiang Rai Province, Northern Thailand: (a) Cipangopaludina annandalei; (b) Filopaludina martensi martensi; (c) F. sumatrensis polygramma; (d) F. doliaris. Scale bar = 1 cm.
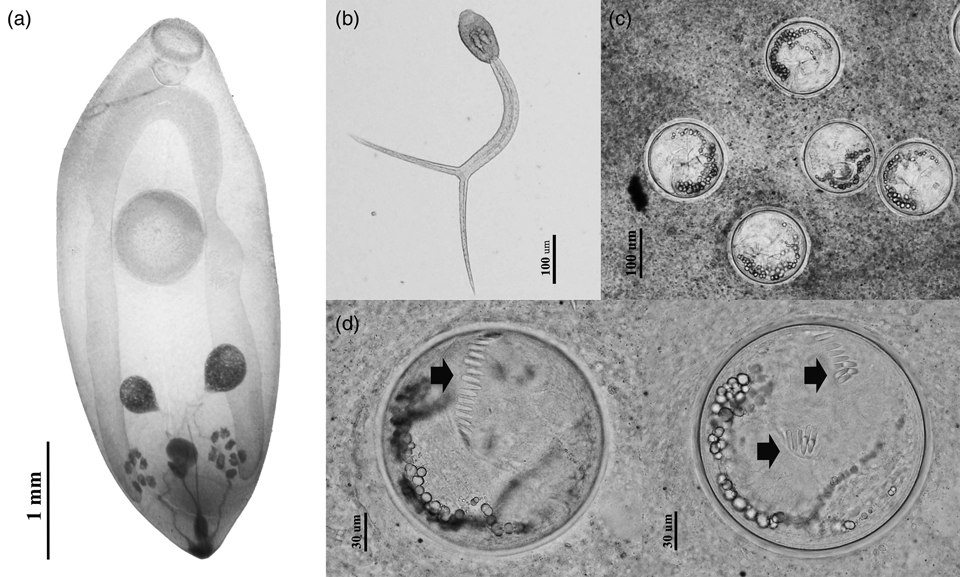
Findings revealed that viviparid snails were commonly infected with echinostome metacercariae and the metacercariae of the avian trematode that were identified as Thapariella anastomusa based on their morphological features (fig. 2). A very small number of the snails were found to be shedding cercariae. Notably, only furcocercous cercaria (fig. 2b) were recorded from C. annandalei. Overall prevalence of parasitic infection in viviparid snails was low (16.9%), although levels within individual snail species were as high as 55.5% (F. doliaris) (table 1). Throughout our survey, a total of 2976 echinostome metacercariae were recovered from all snail species with mean intensity of 16.4. Co-infections between echinostome metacercariae and T. anastomusa were found in all Filopaludina spp. snails, concurring with previous studies conducted in Northern Thailand (Chantima et al., Reference Chantima, Suk-ueng and Kampan2018; Phalee et al., Reference Phalee, Phalee and Wongsawad2018) that found echinostome metacercariae in three snail families indigenous to Thailand, i.e. Viviparidae, Bithyniidae and Buccinidae (Brandt, Reference Brandt1974; Burch & Lohachit, Reference Burch and Lohachit1983; Chantima et al., Reference Chantima, Chai and Wongsawad2013). Thus, viviparid snails play an important role as second intermediate hosts of echinostomes. The three Filopaludina snail species are known to be susceptible to Echinostoma spp., particularly E. revolutum (Chantima et al., Reference Chantima, Chai and Wongsawad2013; Noikong & Wongsawad, Reference Noikong and Wongsawad2014; Noikong et al., Reference Noikong, Wongsawad, Chai, Saenphet and Trudgett2014). Here, only morphological characteristics were used to identify echinostome metacercariae, while metacercariae could not be identified to the species level. Therefore, further research is required to intensively investigate the adult morphology and applying molecular markers to identify these echinostomes. Prevalence of echinostome metacercariae in Filopaludina spp. was relatively low and ranged from 12.8% to 51.9% in Chiang Rai local wet markets compared with the findings of previous studies undertaken in other regions of Northern Thailand including Chiang Mai, Lumphun and Lampang Provinces (Chantima et al., Reference Chantima, Chai and Wongsawad2013; Noikong & Wongsawad, Reference Noikong and Wongsawad2014; Sakda et al., Reference Sakda, Wongsawad and Wongsawad2018). Most Echinostoma spp. metacercariae infected snails belonged to the family Viviparidae, particularly Filopaludina spp. This significant evidence supported the epidemiological situation of Echinostoma spp. and their intermediate hosts in Northern Thailand. Many viviparid snail species are eaten by the local people and they could also serve as intermediate hosts for human trematode infections if consumed when insufficiently cooked.

Fig. 2. Morphology of larval trematodes recorded from viviparid snails in Chiang Rai local wet markets. (a) Metacercaria of Thapariella anastomusa; (b) Furcocercous cercaria; (c) Echinostome metacercarial cysts were clumped together in the pericardial sac and compressed under a cover slip; (d) Isolated echinostome metacercaria showing collar spines (arrow).
Table 1. Prevalence and intensity of echinostome and Thapariella anastomusa metacercariae parasitizing viviparid snails in wet markets of Chiang Rai.

Interestingly, C. annandalei was infected with echinostome metacercariae at a high intensity. These snails had not previously been considered to be of medical importance (Brandt, Reference Brandt1974). Notably, the metacercariae of Echinostoma spp. were previously found in the same genus of this snail collected from a market in Vientiane Municipality, Lao PDR (Sohn et al., Reference Sohn, Chai, Na, Yong, Eom, Park, Min and Rim2013), in C. chinensis malleata snails collected from Xiengkhuang Province, Lao PDR (Sohn et al., Reference Sohn, Na, Lee, Eom, Yong, Chai and Min2019), and in snails indigenous to Korea (Sohn & Na, Reference Sohn and Na2017). Conversely, C. annandalei was not known to play a role as a first or second intermediate host of trematodes, while the distribution of this snail species was only known from the type locality in Chiang Rai Province. Hence, this is the first report on the detection of echinostome metacercariae in C. annandalei. Furthermore, this study is the first to record echinostome metacercariae prevalence within C. annandalei snails. Our findings indicate that echinostome metacercariae are well suited to infect and develop within C. annandalei snail populations. This suggests a significant potential for C. annandalei snails to become a problematic second intermediate host of parasites in Chiang Rai Province. If C. annandalei snails are found to be infected with echinostome metacercariae in Chiang Rai Province, as well as in other potentially novel geographical areas, this could have major implications in terms of future echinostomiasis risks. In many developing countries, the prevalence of echinostomiasis is known to be aggravated by a range of socioeconomic factors and an explosively growing free-food market. This situation is exacerbated by the lack of food inspection policies and poor sanitation practices (Graczyk & Fried, Reference Graczyk and Fried1998). Consumption of freshwater snails as food and exposure to echinostome metacercariae through parasitized snails are important factors in human parasitization. Distribution systems to domestic, public and international markets also play a major role in exposure to food-borne parasites and the emergence of parasites in different areas (Toledo et al., Reference Toledo, Esteban and Fried2012). According to Toledo & Esteban (Reference Toledo and Esteban2016), the geographical boundaries and populations at risk are currently expanding and changing in relation to other relevant factors such as growing international markets, improved transportation systems, new eating habits that have been adopted in developed countries and a range of other demographic changes. Nevertheless, our study results remain unclear as to whether the C. annandalei snail is a new second intermediate host of Echinostomatidae within Thailand. Identification of infected C. annandalei snails is the only confirmation of potential transmission of the disease; however, our findings suggest that a cautious interpretation of transmission based on this snail infection is necessary.
In snail-trematode systems, infection intensity by Echinostoma spp. metacercariae among snails and/or bivalves is significantly correlated with host size. Furthermore, host size is considered another relevant factor in determining the mortality associated with echinostome cercarial penetration and encystment in the second intermediate host (Morley et al., Reference Morley, Lewis and Adam2004; Ponder & Fried, Reference Ponder and Fried2004; Schneck & Fried, Reference Schneck and Fried2004; Leung & Poulin, Reference Leung and Poulin2008). Our field study determined that smaller snails displayed higher echinostome metacercarial infection prevalence and intensity levels than larger snails. However, associations between snail size and the prevalence of echinostomes, as well as the intensity of metacercariae were not significant (P > 0.05) (tables 2 and 3). These findings correlated with Christensen et al. (Reference Christensen, Frandsen and Roushdy1980) with regard to Echinostoma liei, those of Sullivand (Reference Sullivand1985) with regard to E. liei and Echinoparyphium spp., and those of Detwiler & Minchella (Reference Detwiler and Minchella2009) with regard to E. revolutum and Echinoparyphium spp. To the best of our knowledge, results obtained from our field study suggest that the pattern of high parasite prevalence and intensity in smaller, field-collected snails is not due to their more refractory nature to infection. Hence, the specific cause of the pattern of parasitic infection across snail size classes requires further investigation. By contrast, snail species were found to be significantly associated with the prevalence and intensity of echinostome metacercariae, particularly C. annandalei (P ≤ 0.05). Importantly, the C. annandalei snail was significantly species-specific at the level of the second intermediate host of these echinostomes. However, previous reports found that the specificity of echinostomes toward the second intermediate host was low and usually associated with numerous other host species (Huffman & Fried Reference Huffman and Fried1990). Second intermediate hosts for Echinostomatidae range from invertebrates to vertebrates, while natural products such as snail mucus may serve as second intermediate hosts of echinostomes (Huffman & Fried Reference Huffman and Fried1990; Esteban & Munoz-Antoli Reference Esteban, Munoz-Antoli, Fried and Toledo2009). Nevertheless, there are differences in the susceptibility of second intermediate hosts for cercarial penetration by Echinostoma spp. (Christensen et al., Reference Christensen, Frandsen and Roushdy1980; Anderson & Fried, Reference Anderson and Fried1987). Thus, results presented here suggest that echinostome infections can result in a complex systemic host response that exhibits both specific and nonspecific effector mechanisms. Coustau et al. (Reference Coustau, Gourbal, Mitta, Adema, Fried and Toledo2009) and Detwiler & Minchella (Reference Detwiler and Minchella2009) suggested that many host-related factors, such as species distribution and host behaviour, may influence the probability of contact between host and parasite. Patterns of host specificity can also result from the open or closed nature of the encounter and compatibility filters. Accordingly, further studies are important to elucidate the various interactions observed with regard to echinostome infection, especially pairing field and laboratory studies to enable a better understanding of the underlying mechanisms and patterns of parasitic infection.
Table 2. Prevalence of echinostome metacercariae infection in viviparid snails according to species and shell size.

Note: OR, odds ratio obtained from logistic regression; CI, confidence interval.
Table 3. Intensity of echinostome metacercariae parasitizing viviparid snails in local wet markets of Chiang Rai as a function of snail species and shell size.

Note: IRR, incidence rate ratio obtained from univariate negative binomial regression; CI, confidence interval; SD, standard deviation.
Our results confirmed the role of C. annandalei as the second intermediate snail host of echinostomes, and the first study to identify the presence of echinostomes in this snail. Our findings also demonstrated the role of the second intermediate snail hosts for echinostomes with regard to a wider range of viviparid snails. Notably, these snails belong to the preferred snail species used to prepare a very popular traditional dish in Chiang Rai Province. The high prevalence of these zoonotic echinostomes in viviparid snails, i.e. F. doliaris and C. annandalei, suggests their role as reservoir hosts in Thailand. The prevalence of echinostomes should be a matter of concern for public health authorities in areas where these snails are routinely consumed. Local populations must be educated on the risks associated with this practice as a constituent of any incorporated food-borne zoonotic trematode prevention and control program. Our findings also suggest that echinostome infection in viviparid snails may result from a complex system of host–parasite response. However, further investigations are needed to fully understand the interactions between viviparid snails and this trematode.
Acknowledgements
The authors acknowledge Chiang Rai Rajabhat University for financial support and the Environmental Science Research Laboratory, Energy and Environment Program and Biological Science Program, Faculty of Science and Technology, Chiang Rai Rajabhat University for providing the necessary research facilities. We express our sincere gratitude to anonymous reviewers for their useful suggestions on the earlier version of the manuscript. Finally, we would like to thank Dr. Russell Kirk Hollis and Mr. Peter Charge for proofreading the manuscript.
Financial support
This work was supported financially by Chiang Rai Rajabhat University, Thailand.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
Animal experiments were not conducted in this research study; however, some animals were included in our surveyed research. Fieldwork, including collection of animals in the field and specimen examination, was authorized by the Institute of Animals for Scientific Purpose Development (IAD), National Research Council of Thailand (permit number U1-03137-2559, issued to Kittichai Chantima). This study complied with all the relevant national regulations and institutional policies for the humane care and use of animals.