CHD remains the leading cause of death in the USA( Reference Go, Mozaffarian and Roger 1 ), and dietary intake is an important attributable factor. Secular changes in cardiovascular and CHD death( Reference Ergin, Muntner and Sherwin 2 ) and food consumption( Reference Kant and Graubard 3 ) strongly suggest nation-wide common environmental rather than genetic influences on these phenotypes. Twin studies have demonstrated the genetic impact on CHD( Reference Carmelli, Selby and Quiroga 4 , Reference Wu, Neale and Acton 5 ), and dietary intake( Reference Hasselbalch, Heitmann and Kyvik 6 ), as well as influences of familial environment( Reference Carmelli, Selby and Quiroga 4 , Reference Pimpin, Ambrosini and Llewellyn 7 ) and shared non-familial environment( Reference Carmelli, Selby and Quiroga 4 , Reference Dubois, Diasparra and Bedard 8 , Reference Hammar, Hakala and Jorgensen 9 ) on diet( Reference Pimpin, Ambrosini and Llewellyn 7 – Reference Hammar, Hakala and Jorgensen 9 ) and CHD( Reference Carmelli, Selby and Quiroga 4 , Reference Hedlund, Kaprio and Lange 10 ). To date, there is no evidence from a 40-year dietary randomised controlled trial targeting CHD. Therefore, it remains unclear whether diet related to very long-term (like 40 years in the reported study) CHD mortality risk is genetically, common-environmentally or familially independent.
The nature of diet is complex. Humans eat a variety of foods containing numerous dietary compounds. Food items can be processed or cooked in diverse ways. A combination of all consumed foods and beverages with the synergy( Reference Jacobs and Tapsell 11 ) and the antagonism among them when interacting with human bodies, defined as a whole diet, affect health and disease. Whole diet can be evaluated through dietary pattern or diet quality in a population study, as reviewed previously( Reference Dai 12 ). There are two limitations in previously traditional observational studies of whole diet( Reference Trichopoulou, Costacou and Bamia 13 – Reference Gardener, Wright and Gu 16 ): potential confounding from genetic and environmental influences was not well controlled, and moderate consumption was not considered in the evaluation of whole diet in relation to long-term CHD.
To overcome these two limitations, we created the plant-food-dominant dietary pattern( Reference Trichopoulou, Costacou and Bamia 13 – Reference Sofi, Macchi and Abbate 20 ) with an attempt to incorporate the well-known nutritional concept of moderate consumption into the quantification of a whole diet( Reference Rumawas, Dwyer and McKeown 21 ). Next, using a co-twin control design, we aimed to address whether this dietary pattern was prospectively related to long-term CHD mortality risk, independent of genetic factors and common environment, or familial predisposition, and we tested the contribution from genetic and environmental factors to the association. Our outcome was time-to-event, where the primary event was CHD deaths and the secondary event was deaths from total CVD and all causes.
Methods
Study population
As widely described( Reference Dai, Krasnow and Liu 22 – Reference Dai, Mukamal and Krasnow 24 ), initiated in 1969, the prospective National Heart, Lung, and Blood Institute (NHLBI) Twin Study enrolled 514 middle-aged, white male veteran twin pairs (254 monozygotic (MZ) and 260 dizygotic (DZ) twin pairs( Reference Dai, Krasnow and Liu 22 )) who were born between 1917 and 1927 and aged 42–55 years at the baseline examination (1969–1973). All twins were physically examined at baseline and during follow-up investigations. Zygosity was ascertained by eight RBC antigen groups and later variable number of tandem repeat DNA markers( Reference Reed, Carmelli and Christian 25 ). The Institutional Review Board at each examination site approved the study protocol, and all twins gave written informed consent. Exclusion criteria a priori were as follows: (1) no dietary data available, and (2) daily total energy intake <2092 kJ (500 kcal) or >25 104 kJ (6000 kcal)( Reference Hauret, Bostick and Matthews 26 ). Baseline dietary data were not collected among fifty-nine twin pairs. No twins had implausible total energy intake. A total of 910 twins (234 MZ and 221 DZ pairs) were included in the analyses with cumulative 26 672 person-years during the 40-year follow-up period.
Baseline diet assessment
Usual dietary data were obtained through a standardised nutritionist-administered, cross-checked, dietary history interview( Reference Feinleib, Garrison and Fabsitz 27 ) validated in the Framingham Study( Reference Mann, Pearson and Gordon 28 , Reference Dawber, Pearson and Anderson 29 ). Daily food intake amount was calculated from food intake frequency and the serving size. Daily energy intake and daily nutrient intake in gram weight were calculated.
Moderation-quantified healthy diet score
With an attempt to incorporate the well-known nutritional concept of moderate consumption, we used a scoring strategy modified from Rumawas et al.( Reference Rumawas, Dwyer and McKeown 21 ). The moderation-quantified healthy diet (MQHD) score contained fourteen components (Table 1): total grains, fruits, vegetables, dairy products, alcohol( Reference Steffen, Van Horn and Daviglus 30 ), fish, poultry, red meats, nuts and legumes, potatoes, eggs, sweets, ratio of fried meat:non-fried meat and ratio of unsaturated fatty acids:SFA( Reference Dai, Su and Bartell 31 ). The amount of a food item or group was measured in servings, and was adjusted for 10 460 kJ/d (2500 kcal/d). Thus, the individualised food consumption amount could be calculated as ‘the number of servings of a food group for the reference value for maximal component score (Table 1)×individualised total energy requirement in kJ (kcal)/(10460 kJ or 2500 kcal)’. A value for each component was calculated with a penalty for excessive intake( Reference Rumawas, Dwyer and McKeown 21 ), except for the ratios. If the amount of each component was below the reference, a value ranging from 0 to 10 was calculated as ‘10×the consumption amount divided by the reference’. If the consumption amount of each component was greater than the reference, a value was given as ‘10−(10×(the consumption amount−the reference)/the reference)’. If the value was negative, it was converted into 0. A value of 0 was assigned for a ratio of fried meat:non-fried meat >0·8. If the ratio of fried meat:non-fried meat was ≤0·8, a value was calculated as ‘10−10×the ratio divided by the reference’. For ratio of unsaturated fatty acids:SFA, if the ratio was ≤4, a value from 0 to 10 was calculated as ‘10×the ratio divided by 4’( Reference Dai, Su and Bartell 31 ); if the ratio was >4, a value of 0 was assigned because of the concern for oxidative stress. A total score was generated through summing the value from each of fourteen components.
Table 1 The reference value for the maximal score of 10 for each component of moderation-quantified healthy diet score and alternative moderation-quantified healthy diet (MQHD) score among 910 twinsFootnote * (Medians and interquartile ranges (IQR))

aMQHD, alternative modified Mediterranean-style diet.
* Servings were based on daily 10 460 kJ (2500 kcal).
† Dairy products referred to all milk and milk products including whole milk, skimmed milk, chocolate milk, ice cream, milk-containing products such as pudding, butter and cheese.
‡ Dairy products the same as defined above, with exclusion of butter.
§ Fried food included fried potatoes, potato chips, fried eggs, fried meat, fried chicken, fried fish and fried shellfish.
‖ Servings/week.
¶ Servings/d.
** White meat includes seafood, fish and poultry.
We constructed a slightly more food-specified version: alternative moderation-quantified healthy diet (aMQHD). The aMQHD score included four additional components: the presence of lamb or veal consumption, the presence of skimmed milk consumption, ratio of white meat:red meat and ratio of ice cream:dairy product consumption. Addition of these four components that were relevant to what Americans consumed might help dietary or eating planning to a certain degree, as such planning often needs detailed food specification. A value of 10 was assigned if lamb or veal was consumed, skimmed milk was consumed, if the ratio of white meat:red meat was ≥1 or if the ratio of ice cream consumption:total dairy product consumption was ≤0·1. Otherwise, a value of 0 was given. A total score was the sum of values from each of eighteen components. The aMQHD score was the standardised total score with a theoretical range from 0 to 100. Because of the enhanced specification, it was less generalisable than MQHD.
Both MQHD and aMQHD scores were standardised to a 0 to 100 scale as ‘100×total score divided by the theoretical maximum score of 140 for MQHD and 180 for aMQHD’ with a theoretical range from 0 to 100. A higher value indicates greater conformity to the healthy whole diet. Because of the penalty for excessive intake and the assignment of a low value for the intake below the recommended consumption amount( Reference Rumawas, Dwyer and McKeown 21 ), we translated the nutritional concept of ‘moderate consumption’ to our scoring algorithm.
Other baseline variables
As described previously( Reference Dai, Krasnow and Liu 22 ), through in-person interview and physical examination, data were recorded for demographic, socio-economic, lifestyle, familial, anthropometric, biochemical and clinical factors. Information on current use of medications was collected. Heart disease and other CVD were diagnosed by the physician at baseline( Reference Mikulec, Holloway and Krasnow 32 ).
Assessment of end points and follow-up
Vital status and the cause and date of death were ascertained through medical records in four active follow-up examinations and later on using death certificates or the National Death Index through 31 December 2009( Reference Mikulec, Holloway and Krasnow 32 ). As previously described( Reference Dai, Krasnow and Liu 22 , Reference Mikulec, Holloway and Krasnow 32 ), physicians assigned corresponding International Classification of Diseases, Ninth Revision codes for morbidity outcomes. Death certificates or the National Death Index coded to the ninth revision codes were obtained for decedents. The primary end point was death from CHD (410–414). Secondary end points were death from all CVD (390–398, 402, 404, 410–438) and all causes. Subjects were considered lost to follow-up if a death certificate or coding from the National Death Index could not be traced. The lost-to-follow-up twins were included in this study and treated as if they were alive at the date of the end of the study( Reference Dai, Krasnow and Liu 22 ). The follow-up was terminated at the date of death, end of follow-up or loss to follow-up, whichever occurred first.
Statistical analysis
To avoid model overfitting, particularly for analyses among MZ twins, we constructed a modified Framingham Risk Score( Reference Dai, Krasnow and Liu 22 , Reference Wilson, D’Agostino and Levy 33 ). This risk score consisted of seven cardiovascular risk factors (age, smoking, systolic and diastolic blood pressure, cholesterol in HDL and LDL and diabetic status( Reference Wilson, D’Agostino and Levy 33 )), and was associated with coronary heart mortality risk in our cohort (hazard ratio (HR) per unit increment in the risk score, 1·08; 95 % CI, 1·05, 1·11). Intraclass correlation coefficient for the diet score was estimated with a random coefficient model. Heritability was calculated using Falconer’s method( Reference Falconer 34 ). Factors shared between co-twins could be categorised into shared genetic and common environmental factors between co-twins, or into familial factors (a combination of genes and familial environment) and environment shared outside of family (i.e. shared non-familial environment, such as local food supply/accessibility/processing/service/retail settings, recreational facilities, school and workplaces( Reference Dai, Krasnow and Liu 22 )) (Fig. 1).

Fig. 1 Interpretation of factors shared between co-twins. MZ, monozygotic twins; DZ, dizygotic twins.
We used a frailty survival model to estimate HR( Reference Dai, Krasnow and Liu 22 , Reference Gharibvand and Liu 35 ) to account for clustering within a twin pair( Reference Dai, Krasnow and Liu 22 ). In all models, the outcome was time-to-event. In the overall association analysis, we treated the study population as a general population through accounting for clustering. The exposure variable was a diet score and was used as a continuous variable after we confirmed the linearity using restricted cubic splines( Reference Harrell 36 , 37 ). In analyses of the association between the diet score and the outcome controlling for familial predisposition and shared non-familial environment (a matched analysis for the co-twin control design), we used a within-pair and between-pair effect model( Reference Carlin, Gurrin and Sterne 38 ). The within-pair effect variable was the exposure. The between-pair effect variable was both a predictor and a matching factor representing a combination of factors shared between co-twins (Fig. 1). The within-pair effect of diet score was calculated as the deviation of a twin’s diet score from the mean diet score of his twin pair, and controls for confounding from shared factors (Fig. 1)( Reference Carlin, Gurrin and Sterne 38 ). If the interaction was not statistically significant, we used data pooled by zygosity. If otherwise, zygosity-specific analyses would be considered.
Other individual-level covariates/predictors were controlled in the model. We controlled for total energy intake (continuous) in the basic model. Then, we additionally adjusted for known CHD risk factors, including socio-economic factors (years of education (continuous)( Reference Winkleby, Jatulis and Frank 39 )), lifestyle factors (marital status (never, not married currently and married currently), BMI (continuous)), modified Framingham Risk Score (continuous) and use of antihypertensives (yes/no). Multiple imputations were used to account for missing data and to obtain fully adjusted parameter estimates.
We performed a confirmatory analysis, as a single dietary measure could attenuate the association because of the misclassification. Given the comparable classification between a multi-measure and a single measure for those at extreme intakes( Reference Freudenheim, Johnson and Wardrop 40 ), we compared those in the top score quartile with those in the bottom one in relation to coronary heart death risk. Sensitivity analyses were performed using several slightly modified versions of diet scores, and after exclusion of twins with baseline diabetes and CHD. We also performed secondary analyses using the Mediterranean diet score constructed according to the algorithm published by Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 13 ).
All analyses were conducted with SAS software version 9.2 (SAS Institute). Significance levels were set at 0·05 (two-sided).
Results
Components of whole diet
Table 1 shows each score component, food items consisting of each component, the reference value for the maximal score of 10 for each component and the median value of each component in the studied cohort.
Characteristics of the study participants
A total of 910 twins (455 twin pairs) were followed up to 40 years with a median of 32·0 (interquartile range 23·3–37·3) years. There were 113 CHD deaths, 198 cardiovascular deaths and 610 all-cause deaths. The mean age at baseline was 48·0 (range 42–55) years. MQHD score ranged from 10 to 57·1. Men with a higher diet score had a lower energy intake, higher percentages of energy content from both protein and carbohydrates, a lower percentage of energy content from dietary total fat, SFA and MUFA, more years of education and higher systolic blood pressure (Table 2). In all, nineteen twins out of the 910 twins were lost to follow-up. The lost-to-follow-up twins were similar to other twins, except that they were less likely to be currently married (data not shown).
Table 2 Baseline characteristics of twins in relation to a continuous moderation-quantified healthy diet (MQHD) score in the whole cohort pooled by zygosityFootnote * (Numbers and percentages; mean values with their standard errors

Ref., referent value.
* All P values were corrected for clustering within a twin pair using linear mixed models for continuous variables. Raw values for continuous variables are presented. Diet scores were continuous dependent variables. A regression coefficient was explained as the expected change in a diet score for 1-unit increment in an independent variable (continuous) or the exposure relative to non-exposure group.
Overall associations (i.e. general population associations)
Table 3 shows that a greater MQHD score, meaning a dietary pattern that would be considered related to better health than a lower score, was statistically significantly associated with a lower mortality risk from specific and all causes after energy intake adjustment in the whole cohort (i.e. MZ and DZ combined). Multivariable adjustment did not materially change the association for specific and all causes: HR for each 10-unit increment in MQHD score was 0·76 (95 % CI 0·66, 0·88) for CHD mortality risk, 0·87 (95 % CI 0·79, 0·96) for cardiovascular mortality risk and 0·95 (95 % CI 0·91, 0·996) for all causes (Table 3).
Table 3 Overall and within-pair associations of moderation-quantified healthy diet (MQHD) score with the risk of mortality from specific and all causes in the whole cohort pooled by zygosityFootnote * (Hazard ratios and 95 % confidence intervals per 10-unit increment; n 910)

MZ, monozygotic twins; DZ, dizygotic twins.
* Overall associations were equivalent to general population associations, and their HR and 95 % CI were estimated for each 10-unit increment in diet score (continuous variable). Within-pair associations were additionally controlled for genetic and common environmental factors, and their HR were estimated for per within-pair 10-unit difference in diet score (continuous variable). Between-pair associations were the associations between familial predisposition and other environmental factors shared between co-twins and outcomes, and their HR were estimated for per 10-unit increment in the average of diet score of the twin pair through frailty survival model. Frailty survival models were used for analyses to account for within-pair clustering, in which the frailty was a random effect to account for the clustering. Energy-adjusted model controlled for total energy intake (continuous). Multivariable-adjusted covariates included total energy intake (continuous), marital status (never, not married currently and married currently), years of education (continuous), BMI (continuous), modified Framingham Risk Score (continuous) and antihypertensives (yes/no).
The heritability was 21 % for MQHD score and 4·5 % for aMQHD (online Supplementary Table S1). Variation in factors shared between co-twins (i.e. shared genetic and common environmental factors including familial predisposition factors and shared non-familial environment in Fig. 1) explained 24 % of variation in MQHD score and 27 % in aMQHD score (online Supplementary Table S1). These shared factors that made co-twins similar were next controlled in within-pair analyses.
Within-pair associations
The interaction between within-pair effects and zygosity was not significant for specific or all causes (Table 3). In the whole cohort pooled by zygosity (i.e. MZ and DZ combined), for MQHD score, the within-pair association was statistically significant for specific causes after both energy and multivariable adjustment: multivariable-adjusted HR for a twin with a 10-unit higher MQHD score than his co-twin brother was 0·79 (95 % CI 0·64, 0·96) for CHD mortality risk and 0·87 (95 % CI 0·76, 0·998) for cardiovascular mortality risk (Table 3).
Between-pair associations
Table 3 shows that the combined familial and shared non-familial factors (i.e. genetic and common environmental factors) significantly influenced the association between diet score and CHD death risk.
The confirmatory analysis demonstrated that multivariable-adjusted HR for twins in the top quartile relative to the bottom quartile for MQHD score for CHD was statistically significant (Table 4), supporting our primary results. Similar results were obtained for aMQHD (online Supplementary Table S2).
Table 4 Overall associations of moderation-quantified healthy diet (MQHD) and alternative moderation-quantified healthy diet with coronary heart mortality risk comparing the top quartile with the bottom quartileFootnote * (Hazard ratios (HR) and 95 % confidence intervals)
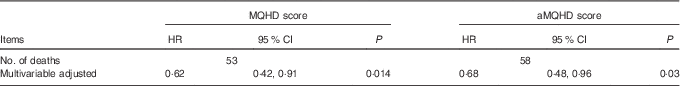
aMQHD, alternative modified Mediterranean-style diet.
* HR was estimated through frailty survival model. Multivariable-adjusted covariates included total energy intake (continuous), marital status (never, not married currently and married currently), years of education (continuous), BMI (continuous), modified Framingham Risk Score (continuous) and antihypertensives (yes/no).
We performed sensitivity analyses using several versions of slightly modified diet scores. Results were roughly similar after use of one weekly serving of red meat as the cutoff for the maximal score, exclusion of butter from dairy products, exclusion of both fat-rich potatoes (fried potatoes and potato/maize chips) from potato products and sweet grain products (donuts, cakes, pies and pastries) from grain products, separately (data not shown), and exclusion of alcohol intake as a diet score component (online Supplementary Table S3). The excluded food item(s) or groups were controlled for in the model. In another sensitivity analysis of twins after exclusion of those with baseline diabetes and CHD, similar results were obtained (data not shown).
We performed secondary analysis of intakes of nutrients, as shown in Table 5. As secondary analyses, the overall association was significant between Trichopoulou’s Mediterranean diet score and the long-term risk of death from CHD and total CVD (online Supplementary Tables S4 and S5), but both within-pair and between-pair effects were important in the overall associations (online Supplementary Table S4).
Table 5 Macronutrient intake among twins (Mean values with their standard errors estimated from mixed model; n 910)

Discussion
A greater MQHD score was associated with a lower 40-year mortality risk from specific and all causes after adjustment for known CHD risk factors including socio-economic, lifestyle and clinical risk factors; however, after additional controlling for familial and shared non-familial factors, the association remained robust for CHD and cardiovascular mortality risk. In brief, the cardio-protective environmental factors that we demonstrated were predominantly nutritional.
Interpretation of associations between whole diet and outcomes
Our overall association was equivalent to that found in the general population. Different dietary patterns as previously reviewed( Reference Dai 12 ), including Mediterranean diet( Reference Trichopoulou, Costacou and Bamia 13 , Reference Knoops, de Groot and Kromhout 41 ), alternative Mediterranean diet, Dietary Approaches to Stop Hypertension diet( Reference Salehi-Abargouei, Maghsoudi and Shirani 14 ) and animal protein diet( Reference Chen, McClintock and Segers 42 ), had been linked to the risk of mortality from CHD( Reference Trichopoulou, Costacou and Bamia 13 – Reference Struijk, May and Wezenbeek 15 , Reference Knoops, de Groot and Kromhout 41 , Reference Chen, McClintock and Segers 42 ), CVD( Reference Salehi-Abargouei, Maghsoudi and Shirani 14 – Reference Gardener, Wright and Gu 16 , Reference Knoops, de Groot and Kromhout 41 – Reference Tognon, Lissner and Saebye 43 ) and all causes( Reference Trichopoulou, Costacou and Bamia 13 , Reference Knoops, de Groot and Kromhout 41 , Reference Tognon, Lissner and Saebye 43 ). Our findings were generally consistent with previous studies in supporting the influence of a whole diet on specific and all-cause mortality risk; however, we provided long-term time-to-death evidence with the novel moderation-quantified healthy dietary pattern.
More importantly, using the co-twin control-matched design, to our knowledge, we elucidated for the first time that the association between scales representative of a whole diet and the long-term mortality risk from CHD was free of potential confounding from familial factors. We additionally controlled for environmental factors shared between co-twins outside of family.
Potential mechanisms underlying the association
The underlying mechanism had been explored from various perspectives, including individual food components( Reference Mente, de Koning and Shannon 44 ), psychosocial health( Reference Ferranti, Dunbar and Higgins 45 , Reference Chocano-Bedoya, O’Reilly and Lucas 46 ) and pathophysiological pathways such as inflammation( Reference Dai, Miller and Bremner 47 , Reference Lopez-Garcia, Schulze and Fung 48 ), oxidative stress( Reference Dai, Jones and Goldberg 49 ), hypertension( Reference Weng, Steffen and Szklo 50 ), autonomic( Reference Dai, Lampert and Wilson 51 ) and endothelial dysfunction( Reference Lopez-Garcia, Schulze and Fung 48 , Reference Dai, Lampert and Wilson 51 ). It is well known that a diet rich in plant foods and fish is associated with a low risk for CHD and CVD( Reference McEvoy, Temple and Woodside 52 ). However, our data supported the crucial influence of whole diet rather than just its individual components on the mortality risk.
Limitations and strengths
There are several limitations in our study. The NHLBI Twin Study only collected baseline dietary data. This would unlikely alter our findings, as a single measure would attenuate the association( Reference Willett 53 ). Similar to other diet score constructions( Reference Trichopoulou, Costacou and Bamia 13 – Reference Gardener, Wright and Gu 16 , Reference Dai, Miller and Bremner 47 , Reference Weng, Steffen and Szklo 50 ), our cutoff points for scoring and the penalty assignment were arbitrary. Our findings supported that whole diet incorporating the quantified concept of moderate consumption was cardio-protective. Although alcohol is a component of a whole diet, the American Heart Association( 54 ) cautions people not to start drinking if they do not already drink alcohol. No baseline physical activity data were collected. Alternatively, we controlled for baseline BMI, an indicator for medium- to long-term nutritional status, and activity level reflecting dietary consumption relative to physical activity. The participants in our study were white male twins only. There should be caution in generalising our findings to females and other racial groups.
Our study has several advantages. By taking advantage of the accuracy of our dietary data, we were able to provide a quantitative reference for the moderate consumption in the practical implementation. In dietary planning and implementation, some of the energy content from solid fats and added sugars may be used for alcohol consumption instead( 17 ). Because each twin pair member shares the same birth date and co-twins are subject to the same age and period changes during follow-up, within-pair associations are controlled for age, cohort, period effects and secular trends as unmeasured (latent) environmental factors.
In conclusion, the association of MQHD and aMQHD with a lower long-term CHD mortality risk is affected by nutritional and familial factors, supporting their use for dietary planning to prevent CHD mortality. Our findings support the current healthy diet concept, including moderate consumption of food items/groups, with an emphasis on whole diet.
Acknowledgements
Part of this work was performed when J. D. worked at Indiana University School of Public Health-Bloomington, and at the Division of Epidemiology, the Department of Medicine and Vanderbilt Center for Translational and Clinical Cardiovascular Research in Vanderbilt University Medical Center.
This study was supported by the American Heart Association (Scientist Development grant no. 10SDG2630182 to J. D.), and NHLBI grant (HL51429 to the NHLBI Twin Study).
J. D. designed the research, analysed the data and wrote the paper, and had primary responsibility for the final content of the manuscript. T. R. provided data from the NHLBI Twin Study and R. E. K. provided data on vital status and causes of death. All authors provided critical revisions to the manuscript for important intellectual contents; satisfied the authorship criteria of the International Committee of Medical Journal Editors; conducted research and provided essential materials; and read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114516001914











