The prevalence of the metabolic syndrome, as defined by the combination of abdominal obesity, impaired glucose tolerance, dyslipidaemia and hypertension(Reference Joy, Lahiry and Pollex1), is increasing throughout the world. This syndrome is associated with an increased risk of CVD and type 2 diabetes(Reference Joy, Lahiry and Pollex1). The pathophysiology of the metabolic syndrome is clearly multifactorial, and besides the direct impact of dietary imbalance, there is considerable evidence that adverse environmental influences during early development may increase disease risk in later life(Reference Godfrey, Lillycrop and Burdge2).
A prebiotic is a non-viable food component that confers a health benefit on the host associated with modulation of the microbiota(3). Prebiotic consumption may be associated with various health benefits including a reduction of colorectal cancer risk(Reference Key and Spencer4), a reduction of atopic dermatitis incidence in formula-fed, high-risk infants(Reference Moro, Arslanoglu and Stahl5) and the prevention of type 2 diabetes(Reference de Munter, Hu and Spiegelman6).
Several studies(Reference Cani, Dewever and Delzenne7, Reference Cani, Neyrinck and Maton8) have described the effect of prebiotic supplementation on body weight and fat mass in adult experimental animal models. In some studies(Reference Cani, Possemiers and Van de Wiele9), the decrease in overall fat mass, and in the various deposits of white adipose tissue, is not associated with any effect on body weight. A recent study has shown that supplementation with a mix of inulin and fructo-oligosaccharides had a significant benefit on the maintenance of an appropriate BMI and fat mass accretion in non-obese adolescents(Reference Abrams, Griffin and Hawthorne10). However, very little is known about the effects of consuming a high-fibre diet during pregnancy and the development of the offspring(Reference Champ and Hoebler11).
Methods
Experimental procedure
The experimental protocol was approved by Pays de la Loire Animal Care Committee. Female BALB/cj (Janvier, Le Genest Saint Isle, France) mice, 8 weeks old, were housed in a temperature- and humidity-controlled room with a 12 h light–12 h dark cycle. Female mice were randomised to receive either a control diet (purified diet 210; SAFE, Augy, France) or a diet supplemented with a prebiotic (purified diet 210 modified with 4 % prebiotic, w/w) diet. The prebiotic diet used a combination of galacto-oligosaccharides (GOS; Laiterie de Montaigu, Montaigu, France) and inulin (9:1, w/w). The GOS concentrate contained 45 % GOS, 37 % glucose and 18 % lactose. To have the same energy (14 370 kJ/kg) and nutrient contribution in both diets, the introduction of GOS concentrate was made at the expense of cellulose (1·8 %) and glucose (2·2 %) of the control diet.
On the day of impregnation, two female mice were housed with one male per cage. The offspring remained with their dam until weaning. After weaning, young male mice were separated from their mothers, and kept on the same diet as their dam throughout the end of the study.
Offspring body weight and food intake were determined at weaning, and then 2, 40 and 48 d after weaning. Mice were killed 48 d after weaning. Their length was measured from the tail to the snout and the selected organs (spleen, stomach, colon and thigh muscle) were weighed, and peripheral blood was collected.
Leptin assay
Plasma leptin concentrations in mice were measured using an ELISA kit (R&D system, Abingdon, UK), according to the manufacturer's instructions.
Statistics
All data are presented as mean values with their standard errors. The non-parametric Mann–Whitney test was used to compare groups. Differences were considered significant when P < 0·05.
Results
Prebiotic effects on the weight and behaviour of pregnant mice
During pregnancy, weight gain did not differ between females of both control (n 13) and prebiotic (n 12) groups (Fig. 1). Food intake was similar for both groups (2·4 (se 0·1) g/d in the control group v. 2·5 (se 0·3) g/d in the prebiotic group) during pregnancy.
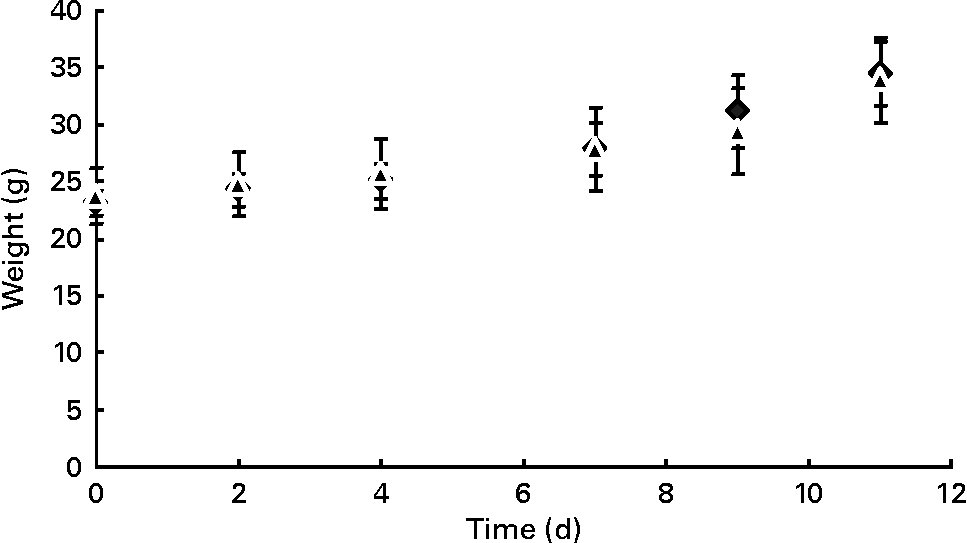
Fig. 1 Time course of maternal body weight of control (◆) and prebiotic-fed (▲) mice throughout pregnancy. Values are means, with their standard errors represented by vertical bars.
At birth, the number of offspring per female was not different between the control (5·6 (se 3·4)) and prebiotic (4·1 (se 2·6)) groups. After birth, cannibalism occurred more frequently in the control group (42·4 %) than in the prebiotic group (18·3 %; P = 0·047).
Prebiotic effects on pup growth
Table 1 summarises the results obtained on body weight and food intake during the growth of the offspring. Offspring length, tissue and organ weight, and blood leptin concentration, 48 d after weaning (killed), are also shown in Table 1.
Table 1 Organ weights (expressed in mg/g total body weight) and plasma leptin concentration in male offspring
(Mean values with their standard errors, n 5)
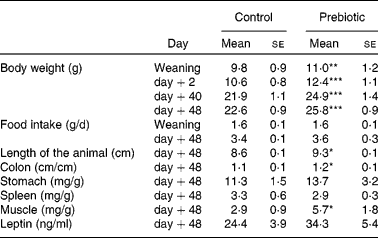
Mean values were significantly different from those of the control group: *P < 0·05, **P < 0·01, ***P < 0·001.
At weaning, body weight was significantly higher in the prebiotic group than in the controls. The difference in body weight between the two groups persisted throughout the end of the experiment and was 13·0, 16·9, 13·4 and 14·2 % at 0, 2, 40 and 48 d after weaning, respectively.
Upon killing 48 d after weaning, we did not observe any difference in spleen and stomach weights, whereas significant differences were found in colon length and thigh muscle weight between the prebiotic and control groups (Table 1). There was a non-significant trend towards higher leptin concentrations in the prebiotic group.
Discussion
The present study addressed the effect of prebiotic supplementation on pregnant mice and the development of their offspring in the perinatal period. The control and prebiotic-supplemented diets were carefully matched for nutrient and energy content, and the only difference was the presence of GOS–inulin in the ‘prebiotic diet’ replacing equivalent amounts of dietary fibre and sugars from the control diet. Thus, differences observed between the groups can be attributed to the prebiotic.
To the best of our knowledge, the present study is the first to provide evidence for the beneficial effects of a prebiotic diet administered during gestation. The prebiotic diet indeed(Reference Joy, Lahiry and Pollex1) did not alter food intake nor weight gain during pregnancy, compared with the control diet(Reference Godfrey, Lillycrop and Burdge2), did not alter the number of offspring per dam, but(3) it decreased the incidence of cannibalism. In addition, prebiotic supplementation had a significant impact on offspring body weight at weaning. Body weight could have been different between the groups of the offspring at the time of birth, but we chose not to weigh pups from birth until weaning in an effort to minimise stress in the dams and their offspring. The offspring of dams fed a prebiotic diet were heavier at 21 d (weaning) but their weight gain from weaning to the end of the study (27 d after weaning) was the same as that of the offspring of dams fed a control diet. This difference in body weight was not associated with an increase in the weight of several splanchnic organs (e.g. stomach or spleen) but was associated with an increase in colon length. Most intriguing was the rise in muscle mass, without any rise in fat mass, as reflected by the unaltered leptin level.
Several studies(Reference Smith, Hulsey and Goodnight12, Reference Stotland, Cheng and Hopkins13) have shown that excessive weight gain during pregnancy increases the risk of developing hyperlipidaemia, insulin resistance and obesity in childhood. In the present study, the prebiotic diet affected neither body-weight gain nor food intake during pregnancy. The 2-fold reduction in the incidence of cannibalism during lactation in the ‘prebiotic group’, compared with the control group, clearly warrants further investigation, including, for instance, the determination of stress hormones such as corticosterone in pregnant and lactating mice.
Very few studies have explored the effects of prebiotics on the development of offspring. In a rat pup model, Maurer et al. (Reference Maurer, Eller and Hallam14) did not observe any difference in body-weight gain between the offspring of dams fed either a high-fibre diet, a high-protein diet or a control diet. The difference in prebiotic composition could contribute to this discrepancy. In our case, we used a mix of GOS–inulin with a 9:1 (w/w) ratio, whereas Maurer et al. (Reference Maurer, Eller and Hallam14) used a mix of oligofructose–inulin with a 1:1 (w/w) ratio.
It is interesting to note that, in the present study, the effects observed in pups occurred via indirect exposure to the prebiotic-enriched diet. As little is known on the effect of prebiotic supplementation on the oligosaccharide content of breast milk, investigating whether prebiotics fed to lactating dams cross into rat milk deserves further study.
In numerous studies on adult rodent models, the decrease in body weight and fat mass following feeding with prebiotics was associated with a reduction of food/energy intake(Reference Cani, Possemiers and Van de Wiele9, Reference Daubioul, Rousseau and Demeure15). In the present study, we did not observe any decrease in food intake in either pregnant mice or their offspring, but observed an increase in body-weight gain in prebiotic offspring at the time of weaning, without any obvious impact on fat mass, as serum leptin level remained unaltered.
Accordingly, prebiotic supplementation increased muscle mass without any increase in body fat mass. The mechanisms involved remain to be explored, and could involve the secretion of hormones known to promote protein anabolism such as insulin, growth hormone and insulin-like growth factor 1. Regardless of its mechanism(s), such protein anabolic effect of prebiotics, if confirmed in human infants, would be of potential relevance to neonatal care. In fact, enhancing early growth, and particularly lean body mass accretion, has long been the ultimate goal of neonatologists. The high-protein formulas that have been routinely used to achieve that goal, however, may expose to the risk of excess fat mass deposition and/or the metabolic syndrome in the long run(Reference Singhal, Fewtrell and Cole16, Reference Koletzko, von Kries and Closa17). Promoting lean body mass without enhancing fat mass accretion in the perinatal period through prebiotic supplementation therefore warrants further investigation.
Finally, in the present study, prebiotic supplementation was associated with an increase in the pup's colon length. The present results are consistent with earlier data on adult rats fed with fibre-enriched diets(Reference Reimer and McBurney18–Reference Reimer, Thomson and Rajotte20), and with the study in rat pups born from dams fed a high-fibre diet(Reference Maurer and Reimer21). Such a trophic effect on the offspring's colon suggests that the intestinal microbiota was affected in pups. Whether alterations in intestinal microbiota mediate the effects observed on offspring body composition through metabolic and/or hormonal modulation remains to be explored in our model.
In conclusion, increasing the fibre content of the maternal diet during pregnancy and lactation allowed improvement of body-weight gain in the offspring without any impact on fat mass but with an increase in muscle mass. Such a promising finding suggests that the supplementation of the maternal diet during the perinatal period should be explored as a promising, novel strategy to enhance the growth of infants with intra-uterine-to-extra-uterine growth restriction.
Acknowledgements
The authors thank the Laiterie of Montaigu (France) for providing the GOS concentrate. They also thank Jean Louis Lescure for his efficient technical help in the animal experimentation. The present study was supported by la region Pays de la Loire through the ‘Perinatal Nutrition and Metabolic Imprinting’ (NUPEM) research grant. All authors significantly contributed to the study and read and approved the final content of the manuscript. N. D. contributed to the data acquisition and analysis and was the main contributor to the writing of the manuscript. P. G. and M. B. contributed to the study design, data acquisition and discussion, and the writing of the manuscript. V. H.-M. contributed to the data acquisition and discussion. D. D. contributed to the data discussion and the writing of the manuscript. M. C. contributed to the study design, data discussion and the writing of the manuscript. The authors declare that there are no conflicts of interest.






