Introduction
The transition from preantral (multilaminar follicles) to early antral follicles requires the action of local factors, hormones, and efficient bidirectional communication between granulosa cells and oocytes. Several attempts to promote in vitro growth of preantral follicles up to maturation have been reported in various domestic species (bovine: Paulino et al., Reference Paulino, Cunha, Barbalho Silva, Souza, Lopes, Donato, Peixoto, Matos-Brito, van den Hurk and Silva2018; Antonino et al., Reference Antonino, Soares, Júnior, de Alvarenga, Mohallem, Rocha, Vieira, de Souza, Beletti, Alves, Jacomini, Goulart and Alves2019; Bezerra et al., Reference Bezerra, Lima, Paulino, Silva, Silva, Souza, van den Hurk and Silva2019a; Paulino et al., Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020; Vasconcelos et al., Reference Vasconcelos, Costa, Azevedo, Barroso, de Assis, Paulino, Silva, Silva, Souza and Silva2021; caprine: Ferreira et al., Reference Ferreira, Cadenas, Sá, Correia, Guerreiro, Lobo, Alves, Maside, Gastal, Rodrigues and Figueiredo2018; Soares-Costa et al., Reference Soares-Costa, Costa, Silva, Cunha, Paulino, Silva, Silva, van den Hurk and Silva2018; Pontes et al., Reference Pontes, Gouveia, Menezes, de Barros, Barberino, do Monte, Donfack, Celestino, Salgueiro, de Figueiredo and de Matos2019; ovine: Macedo et al., Reference Macedo, Santos, Bezerra, Menezes, Gouveia, Barbosa, Lins, Monte, Barberino, Batista, Barros, Wischral, Queiroz, Araújo and Matos2019; Mbemya et al., Reference Mbemya, de Sá, Guerreiro, de Sousa, Nguedia, Alves, Santos, Pessoa, Comizzoli, Figueiredo and Rodrigues2019; Barros et al., Reference Barros, Monte, Santos, Lins, Cavalcante, Gouveia, Müller, Oliveira, Donfack, Araújo and Matos2020; Silva et al., Reference Silva, Araújo, Silva, Gouveia, Barberino, Lins, Monte, Macedo, Santos, Menezes, Silva and Matos2021; Gomes et al., Reference Gomes, de Brito, de Sá, Ñaupas, Palomino, da Silva, Lopes, Mbemya, Alves, Zelinski, de Figueiredo and Rodrigues2022; and swine: de Lima et al., Reference de Lima, Rubessa, Rocha, Winters, Milner, Campello, Figueiredo and Wheeler2017; Kere et al., Reference Kere, Liu, Chen, Chao, Tsai, Yeh, Siriboon, Intawicha, Lo, Chiang, Fan and Ju2020). Different from mice (Eppig and O’Brien, Reference Eppig and O’Brien1996; O’Brien et al., Reference O’Brien, Pendola and Eppig2003), embryo production from in vitro cultured preantral follicles from human and domestic animals has not yet, however, been reported in the literature (Wu and Tian, Reference Wu and Tian2007; Arunakumari et al., Reference Arunakumari, Shanmugasundaram and Rao2010; de Figueiredo et al., Reference de Figueiredo, de Lima, Silva and Santos2018, Reference de Figueiredo, Cadenas, de Lima and Santos2020; Paulino et al., Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022). The maintenance of bidirectional communication between the oocyte and the granulosa cells in cultured follicles, as well as the large quantity of messenger RNA (mRNA) and proteins that the oocyte needs to synthesize during its growth make it difficult the complete their development in vitro (Alam and Miyano, Reference Alam and Miyano2020; de Figueiredo et al., Reference de Figueiredo, Cadenas, de Lima and Santos2020; Paulino et al., Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022).
The endocrine and paracrine control that regulates the transition from preantral to antral follicles is complex and involves a precise interaction of several factors (Araújo et al., Reference Araújo, Gastal, Figueiredo and Gastal2014; de Figueiredo et al., Reference de Figueiredo, de Lima, Silva and Santos2018, Reference de Figueiredo, Cadenas, de Lima and Santos2020). Any interference with this control can lead to ovarian disorders such as polycystic ovary syndrome (Abdel Aziz et al., Reference Abdel Aziz, Abdel-Wahab, Ibrahim and Kasimanickam2021; Asghari et al., Reference Asghari, Shokri-Asl, Rezaei, Tavallaie, Khafaei, Abdolmaleki and Majdi Seghinsara2021). The present review highlights the mechanisms involved in endocrine and paracrine control during the transition from preantral to early antral follicles, as well as the importance of granulosa cell proliferation, antral cavity formation, and estradiol production. The strategies to promote in vitro growth of preantral follicles are also discussed.
Endocrine and paracrine control during the transition from preantral to early antral follicles
The transition from preantral to early antral follicles is a step in which follicle development is regulated by intraovarian factors, but the follicles are responsive to gonadotropins. The slow growth of preantral and early antral follicles is gonadotropin independent, but progression to a late antral follicular state requires follicle-stimulating hormone (FSH; Iber and Geyter, Reference Iber and Geyter2013). FSH is the main endocrine regulator of follicle development (Paulino et al., Reference Paulino, Cunha, Barbalho Silva, Souza, Lopes, Donato, Peixoto, Matos-Brito, van den Hurk and Silva2018, Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020; Vasconcelos et al., Reference Vasconcelos, Costa, Azevedo, Barroso, de Assis, Paulino, Silva, Silva, Souza and Silva2021) and its receptors are detectable in granulosa cells of preantral follicles in various species (murine: Camp et al., Reference Camp, Rahal and Mayo1991; human: Oktay et al., Reference Oktay, Briggs and Gosden1997; Méduri et al., Reference Méduri, Charnaux, Driancourt, Combettes, Granet, Vannier, Loosfelt and Milgrom2002; porcine: Durlej et al., Reference Durlej, Knapczyk-Stwora, Duda, Galas and Slomczynska2011; bubaline: Sharma et al., Reference Sharma, Dubey and Kumar2011; caprine: Saraiva et al., Reference Saraiva, Celestino, Araújo, Chaves, Almeida, Lima-Verde, Duarte, Silva, Martins, Bruno, Matos, Campello, Silva and Figueiredo2011; Barros et al., Reference Barros, Cavalcante, Macedo, Barberino, Lins, Gouveia, Menezes, Queiroz, Araújo, Palheta, Leite and Matos2013; Ferreira et al., Reference Ferreira, Cadenas, Sá, Correia, Guerreiro, Lobo, Alves, Maside, Gastal, Rodrigues and Figueiredo2018). This hormone stimulates granulosa cell proliferation and promotes follicular growth and antrum formation (Ferreira et al., Reference Ferreira, Cadenas, Sá, Correia, Guerreiro, Lobo, Alves, Maside, Gastal, Rodrigues and Figueiredo2018; de Figueiredo et al., Reference de Figueiredo, Cadenas, de Lima and Santos2020; Fushii et al., Reference Fushii, Yamada and Miyano2021). Other endocrine factors, such as melatonin have a functional role in preantral follicles by influencing their development, increasing the production of active mitochondria in oocytes and steroidogenesis in granulosa cells (Barros et al., Reference Barros, Cavalcante, Macedo, Barberino, Lins, Gouveia, Menezes, Queiroz, Araújo, Palheta, Leite and Matos2013; Riaz et al., Reference Riaz, Yousuf, Liang, Hua and Yang2019; Barros et al., Reference Barros, Monte, Santos, Lins, Cavalcante, Gouveia, Müller, Oliveira, Donfack, Araújo and Matos2020). It is well established that FSH stimulates the production of aromatase (Cyp19a1), which synthesizes 17β-estradiol, an important hormone for granulosa cell proliferation (Fitzpatrick and Richards, Reference Fitzpatrick and Richards1994; Bishonga et al., Reference Bishonga, Takahashi, Katagiri, Nagano and Ishikawa2001). Anti-Müllerian hormone (AMH) is also produced by granulosa cells of preantral and antral follicles (Umer et al., Reference Umer, Zhao, Sammad, Weldegebriall Sahlu, Yunwei and Zhu2019; Gautam et al., Reference Gautam, Vats, Pal, Haldar and De2021). It is already well established that this hormone prevents early depletion of follicles, but there is still much to elucidate in the role of this hormone during folliculogenesis. Rocha et al. (Reference Rocha, Rubessa, de Lima, da Silva, Winters, Polkoff, Milner, Campello, de Figueiredo and Wheeler2021) have shown that there is an interaction between AMH and FSH, in which AMH reduces FSH-induced estradiol and progesterone production. Tanimoto et al. (Reference Tanimoto, Sekii, Morohaku, Li, Pépin and Obata2021) showed that, for the development of a viable follicle, blockage of AMH production by oestrogen is needed.
Oocyte-derived factors, such as growth differentiation factor-9 (GDF-9), bone morphogenetic protein 15 (BMP-15), and fibroblast growth factor-2 (FGF-2) are important regulators of preantral follicle growth by inducing granulosa cell proliferation and differentiation, and antral cavity formation (Reineri et al., Reference Reineri, Coria, Barrionuevo, Hernández, Callejas and Palma2018; Monte et al., Reference Monte, Santos, Menezes, Gouveia, Lins, Barberino, Oliveira, Donfack and Matos2019). Other factors, such as epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1), influence the development of preantral follicles by expanding oocyte diameter and inducing granulosa cell proliferation (Bezerra et al., Reference Bezerra, Monte, Barberino, Lins, Oliveira Junior, Santos, Bezerra, Neves, Silva, Carvalho and Matos2019b; Paulino et al., Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020). Vascular endothelial growth factor (VEGF) is an important angiogenic factor that induces granulosa cell proliferation, an essential characteristic for the transition from preantral to antral follicles, and improves oocyte maturation (Araújo et al., Reference Araújo, Silva, Duarte, Magalhães, Almeida, Gonçalves, Bruno, Silva, Campello, Rodrigues and Figueiredo2011; da Silva et al., Reference da Silva, Rossetto, Chaves, Duarte, Araújo, Feltrin, Bernuci, Anselmo-Franci, Xu, Woodruff, Campello and Figueiredo2015; Cadenas et al., Reference Cadenas, Leiva-Revilla, Vieira, Apolloni, Aguiar, Alves, Lobo, Rodrigues, Apgar, Smitz, Figueiredo and Maside2017). Activin is another intraovarian factor that accelerates the growth of preantral follicles, estradiol synthesis, and mRNA expression for FSH receptor in rat granulosa cells (Tanaka et al., Reference Tanaka, Matsuzaki, Tanaka, Iwasa, Kuwahara and Irahara2019). Conversely, in bovine species, activin decreases the FSH stimulating action in the bovine preantral follicle cultured in vitro, which was associated with decreased levels of transcripts for hyaluronan synthases (HAS-1, HAS-2) and proliferating cell nuclear antigen (PCNA; Silva et al., Reference Silva, Bezerra, Costa, Rossi, Passos, Vasconcelos, Rossetto, Donato, Magalhães-Padilha, Campello, Saraiva, Figueiredo, Peixoto, Van den Hurk and Silva2014).
Granulosa cell proliferation and oocyte–granulosa cell interaction during the transition from preantral to early antral follicles
Granulosa cells form a favourable metabolic and hormonal environment for oocyte growth and maturation (Baumgarten and Stocco, Reference Baumgarten, Stocco and Skinner2018). Similarly, the oocyte can influence the proliferation of granulosa cells, providing follicular development through the production of growth factors such as BMP-15 and GDF-9 (de Figueiredo and de Lima, Reference de Figueiredo and de Lima2017; Baumgarten and Stocco, Reference Baumgarten, Stocco and Skinner2018). Orisaka et al. (Reference Orisaka, Orisaka, Jiang, Craig, Wang, Kotsuji and Tsang2006) showed that GDF-9 controls follicular fate by promoting its survival and growth during the preantral to early antral transition, suppressing granulosa cell apoptosis and follicular atresia through PI3K/Akt activation.
During the development of the preantral follicle, GDF-9 and BMP-15 continue to stimulate the proliferation of granulosa cells, however, they decrease the production of progesterone and increase the expansion and reorganization of granulosa cells to form the antrum cavity. In addition, GDF-9 stimulates the expression of FSH receptors in granulosa cells, which become responsive to gonadotropins and influence the differentiation and recruitment of theca cells, which form a scaffold structure, supporting the vascular system (De Conto et al., Reference De Conto, Matte and Cunha-Filho2021). Theca cells begin to produce luteinizing hormone (LH) receptors, steroidogenic enzymes and androgens (Young and McNeilly, Reference Young and McNeilly2010), while LH stimulates the production of androgens by theca cells, which are used by granulosa cells to produce 17β-estradiol (Xavier and Freitas, Reference Xavier and Freitas2021). 17β-Estradiol increases the sensitivity of granulosa cells to gonadotropins and IGF-1, which stimulates follicular steroidogenesis (Ginther et al., Reference Ginther, Beg, Bergfelt, Donadeu and Kot2001). BMP-4 and FSH also play an important regulatory role in the growth and steroidogenesis of preantral follicles. According to Sakaguchi et al. (Reference Sakaguchi, Huang, Yang, Yanagawa and Nagano2017), BMP-4 inhibits the luteinization of granulosa cells, while FSH increases their proliferation and the viability of the oocyte–cumulus–granulosa complex.
Granulosa cells produce several autocrine and paracrine factors that may be involved in the initiation of antrum formation such as kit ligand, activins, inhibins, hyaluronan, Versican, AMH and transforming growth factor α (TGFα). These factors also synchronize oocyte growth, granulosa cell proliferation and theca cell differentiation (Vasconcelos et al., Reference Vasconcelos, Saraiva, Costa, Passos, Silva, Rossi, Portela, Duarte, Magalhães-Padilha, Campelo, Figueiredo, Van den Hurk and Silva2013; Dumesic et al., Reference Dumesic, Meldrum, Katz-Jaffe, Krisher and Schoolcraft2015). In this sense, the interaction between the oocyte and granulosa cells, as well as the differentiation and proliferation of theca cells, can be determinants for the progress of preantral follicle growth, steroidogenesis and oocyte maturation (Chu et al., Reference Chu, Xu, Yang and Sun2018; Alam and Miyano, Reference Alam and Miyano2020). AMH plays an inhibitory role in modulating the responsiveness of granulosa and theca cells to gonadotrophic stimuli, thereby regulating follicular progression from the gonadotropin-responsive to the gonadotropin-dependent stage (Campbell et al., Reference Campbell, Clinton and Webb2012).
After antrum formation, granulosa cells are physically separated into mural granulosa cells, which organize along the follicle wall, and cumulus granulosa cells, which surround the oocyte (Baumgarten and Stocco, Reference Baumgarten, Stocco and Skinner2018; Zhang, Reference Zhang and Skinner2018). Cumulus granulosa cells nourish the oocyte by providing specific amino acids, glycolysis products and cholesterol biosynthesis substrates through gap junctions (Baumgarten and Stocco, Reference Baumgarten, Stocco and Skinner2018). In addition, they prevent premature oocyte maturation and resumption of meiosis in the oocyte by maintaining high levels of cAMP in the oocyte (Russell and Robker, Reference Russell, Robker and Skinner2018; Zhang, Reference Zhang and Skinner2018). The mechanisms that control the transition from preantral to early antral follicles are shown in Figure 1 and Table 1.

Figure 1. Factors that control the transition from preantral to early antral follicle.
Table 1. Role of growth factors in oocyte, granulosa and theca cells during the transition from preantral to early antral follicle

Mechanisms of antrum formation in preantral follicles
When the distance between the mural granulosa and the cumulus cells increases, the formation of the antral cavity occurs, marking the preantral to early antral follicles transition (Chu et al., Reference Chu, Xu, Yang and Sun2018). The antral cavity is formed between the granulosa cells and requires a fluid ingress from the vascularization of theca cells via membrane proteins such as aquaporins (AQPs; Kawashima and Kawamura, Reference Kawashima and Kawamura2018; Paz et al., Reference Paz, de Sousa, Alves, Lobo, Sales, Tavares, de Sá, Guerreiro, Maside, Rocha, Bertolini, Bordignon, de Figueiredo and Rodrigues2018). Several AQPs, such as AQP5, AQP7, AQP8, and AQP9, are related to the influx of water through the follicle wall to form follicular fluid. These membrane proteins are expressed in the granulosa cells of different species (swine: Skowronski et al., Reference Skowronski, Kwon and Nielsen2009; ovine: Sales et al., Reference Sales, Duarte, Rodrigues, Lima, Silva, Carvalho, Brito, da Maranguape, Lobo, Aragão, Moura, Figueiredo and Rodrigues2015; bovine: Ishibashi et al., Reference Ishibashi, Hara and Kondo2009). Paz et al. (Reference Paz, de Sousa, Alves, Lobo, Sales, Tavares, de Sá, Guerreiro, Maside, Rocha, Bertolini, Bordignon, de Figueiredo and Rodrigues2018) also demonstrated the presence of AQP3 in granulosa cells, and is also involved in the expansion of the antral cavity during the transition from preantral to the antral follicle. Antrum formation depends on the stimulation of local factors for the production and secretion of polysaccharides and proteins by the granulosa cells, which accumulate and generate an osmotic gradient that attracts fluid from the thecal vasculature (Baumgarten and Stocco, Reference Baumgarten, Stocco and Skinner2018). It is recognized that granulosa cells express the enzymes that synthesize hyaluronic acid, Versican and perlecan, which are responsible for the formation of follicular fluid (Schoenfelder and Einspanier, Reference Schoenfelder and Einspanier2003; Clarke et al., Reference Clarke, Hope, Byers and Rodgers2006; Vasconcelos et al., Reference Vasconcelos, Saraiva, Costa, Passos, Silva, Rossi, Portela, Duarte, Magalhães-Padilha, Campelo, Figueiredo, Van den Hurk and Silva2013; Nagyova et al., Reference Nagyova, Salustri, Nemcova, Scsukova, Kalous and Camaioni2020).
The development of the antral cavity is intensified by granulosa cell activity. Alam et al. (Reference Alam, Lee and Miyano2018) showed that, even without the presence of an oocyte, GDF-9 and BMP-15 influence the production of antrum-like structures. GDF-9 is known to stimulate Versican and perlecan expression and interacts favourably with FSH to increase hyaluronan synthetase 2 expression (Vasconcelos et al., Reference Vasconcelos, Saraiva, Costa, Passos, Silva, Rossi, Portela, Duarte, Magalhães-Padilha, Campelo, Figueiredo, Van den Hurk and Silva2013; Silva et al., Reference Silva, Bezerra, Costa, Rossi, Passos, Vasconcelos, Rossetto, Donato, Magalhães-Padilha, Campello, Saraiva, Figueiredo, Peixoto, Van den Hurk and Silva2014). Hyaluronan, proteoglycans and their glycosaminoglycan side chains are osmotic solutes that act to increase the osmotic pressure inside the follicle, resulting in fluid accumulation (Rodgers and Irving-Rodgers, Reference Rodgers and Irving-Rodgers2010). Some studies have also demonstrated that EGF increases mRNA levels for GDF-9 in in vitro cultured preantral follicles (Alam et al., Reference Alam, Lee and Miyano2018; Paulino et al., Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020), showing that EGF acts via GDF-9 to promote antral cavity formation. The mechanisms involved in the formation of antral cavity formation are shown in Figure 2.

Figure 2. Hormones and growth factors that control the formation of the antral cavity during the transition from preantral to early antral follicles.
Production of estradiol during the transition from preantral to early antral follicles
When preantral follicles reach six or seven layers of granulosa cells, the inner layer of theca becomes active and the formation of the antral cavity begins. Increased 17β-estradiol in follicular fluid is associated with increased mRNA expression for CYP19A1 in granulosa cells (García-Guerra et al., Reference García-Guerra, Canavessi, Monteiro, Mezera, Sartori, Kirkpatrick and Wiltbank2018).
As a growing follicle acquires sufficient aromatase activity as a result of FSH stimulation, estradiol production suppresses FSH secretion below that necessary to support the development of less mature follicles, which consequently suffer atresia (Zeleznik, Reference Zeleznik2004). Therefore, estradiol biosynthesis is a tightly regulated molecular process, dependent on the expression of key steroidogenic enzymes by FSH and intraovarian signalling molecules, including beta-catenin, an essential co-transcription factor for maximal induction of the aromatase mRNA transcript and subsequent estradiol production (Forrest et al., Reference Forrest, Flores, Gurule, Soto-Navarro, Shuster, Gifford and Gifford2022). β-Catenin regulates the transformation of androgen to estradiol by increasing the transcription of CYP19A1 through functional interactions with steroidogenic factor-1 (SF1; Forrest et al., Reference Forrest, Flores, Gurule, Soto-Navarro, Shuster, Gifford and Gifford2022). Furthermore, LH promotes preantral to antral follicular transition by upregulating follicular androgen biosynthesis (Orisaka et al., Reference Orisaka, Hattori, Fukuda, Mizutani, Miyamoto, Sato, Tsang, Kotsuji and Yoshida2013).
Expression of luteinizing hormone/choriogonadotropin receptor (LHCGR) and cytochrome P450 family 17, subfamily A, member 1 (CYP17A1) mRNAs appear in large preantral follicles, concomitantly with theca differentiation. Followed by the expression of steroidogenic acute regulatory protein (StAR) in 1.0 mm antral follicles, granulosa cells from preantral and early antral follicles do not express StAR. Therefore, steroidogenesis in bovine follicles potentially begins in follicles ≥ 1.0 mm. Furthermore, the mRNA for CYP17A1 was located exclusively in theca internal cells, which indicates that the conversion of progestogens to androgens occurs only in the theca interna (Braw-Tal and Roth, Reference Braw-Tal and Roth2005). Furthermore, the neonatal rat ovary is completely devoid of antral follicles at birth. By day 12 of age, small-sized to medium-sized antral follicles are present, in addition to follicles at all preceding stages of development. During the intervening period the ovary becomes steroidogenically active, and responsive to gonadotrophins on days 7–9 of age, suggesting that granulosa and theca cells become active at that time (Carson and Smith, Reference Carson and Smith1986).
The expression of key enzymes involved in steroidogenesis is crucial for the proper development of the follicles. It has been observed that the mRNA encoding P450arom was not detectable until early antral cavity formation, in addition to being expressed only in granulosa cells (Yuan et al., Reference Yuan, Wang, Lan, Sui, Yu and Tan2008). The mechanisms involved in the production of estradiol during the transition from preantral to early antral follicles are shown in Figure 2.
Follicle atresia during the transition from preantral to early antral follicles
Follicular atresia is characterized by morphological changes in the oocyte, granulosa, and theca cells that culminate with follicular death and, consequently, regulate the capacity of females to generate mature gametes during the fertile period (Makarevich et al., Reference Makarevich, Földešiová, Pivko, Kubovičová and Chrenek2018). This process promotes a drastic reduction in the ovarian follicle population during the reproductive lifespan. According to Zhang et al. (Reference Zhang, Liu, Yao, Li, Liu and Pan2018), crosstalk among cell apoptosis, autophagy and ferroptosis is involved in the control of atresia in ovarian follicles. In preantral follicles, degeneration initially occurs in the oocyte and, subsequently, in granulosa cells (Meng et al., Reference Meng, Jan, Hamer, van Pelt, van der Stelt, Keijer and Teerds2018). The first signs of atresia in the oocyte are the retraction of nuclear chromatin and oocyte fragmentation, which triggers the process of irreversible elimination of ovarian follicles at this stage of development. In the granulosa cells of preantral follicles, changes are rarely observed.
Zoheir et al. (Reference Zoheir, Darwish, Liguo and Ashour2021) showed that cellular DNA fragmentation is an important biochemical marker of apoptosis during follicular atresia. Other evidence confirmed that apoptosis is not the only death pathway active in the ovary. Gannon et al. (Reference Gannon, Stämpfli and Foster2012) reported a decrease in preantral follicle numbers without a concomitant increase in apoptosis, and no change in apoptosis markers caspase 3 and TUNEL. Recent studies have demonstrated that, in granulosa cells from cultured preantral follicles of buffaloes, transmembrane protein AQP8 is involved in the regulation of cycle progression and apoptosis (Cao et al., Reference Cao, Li, Huang, Shi and Li2021). Furthermore, miRNAs have been shown to control several fundamental biological processes, including follicular atresia through their target genes and signalling pathways, and play a central role in the regulation of autophagy (Ma et al., Reference Ma, Tang, Guo, Liang, Zhang and Jiang2020). Gannon et al. (Reference Gannon, Stämpfli and Foster2012) showed that degeneration of preantral follicles is associated with the activation of the autophagy cascade. Meng et al. (Reference Meng, Jan, Hamer, van Pelt, van der Stelt, Keijer and Teerds2018) reported that the standard pathway of degeneration in preantral follicles is through autophagy, and that the activation of this pathway occurs under normal physiological conditions. Considering that, in vivo, follicular atresia during preantral to early antral follicles transition is controlled by various intrafollicular regulators such as growth factors, cytokines, and steroids (Orisaka et al., Reference Orisaka, Jiang, Orisaka, Kotsuji and Tsang2009a), the development of strategies to interrupt atresia is a difficult task.
Despite early antral follicles (∼2.0 mm in diameter – human species) are still not dependent on gonadotropins, Orisaka et al. (Reference Orisaka, Tajima, Tsang and Kotsuji2009b) showed that they are susceptible to apoptotic signals. In these follicles, with the progression of atresia, there is a reduction in the number of layers of granulosa cells, and invasion of fibroblasts and macrophages (Seneda et al., Reference Seneda, Bergamo, González, Zangirolamo and Morotti2021). Meng et al. (Reference Meng, Jan, Hamer, van Pelt, van der Stelt, Keijer and Teerds2018) reported that atresia in these follicles was associated with the activation of the autophagy cascade. Increasing the levels of microtubule-associated light chain protein 3 (LC3) and of autophagy homeostasis-associated protein BECLIN1 is linked with the death of preantral and early antral follicles by the autophagy cascade (Gordy and He, Reference Gordy and He2012). Activation of this pathway occurs under normal physiological conditions and the presence of LC3, sequestosome 1 (SQSTM1/P62) and autophagy-related protein 7 (ATG7) are markers for autophagy (Gannon et al., Reference Gannon, Stämpfli and Foster2012). During atresia of those follicles, Meng et al. (Reference Meng, Jan, Hamer, van Pelt, van der Stelt, Keijer and Teerds2018) also reported that a loss in mitochondrial antioxidant capacity in granulosa cells led to the activation of AMP-activated protein kinase alpha 1 (AMPK-α1) and AMPK-α2, while at the same time the expression of protein kinase B (AKT) and mammalian target survival of the rapamycin complex 1 (mTORC1) was decreased. AMPK is an important regulator of metabolism, an inhibitor of the mTORC1 complex, and a direct activator of autophagy (Emerling et al., Reference Emerling, Weinberg, Snyder, Burgess, Mutlu, Viollet, Budinger and Chandel2009; Egan et al., Reference Egan, Shackelford, Mihaylova, Gelino, Kohnz, Mair, Vasquez, Joshi, Gwinn, Taylor, Asara, Fitzpatrick, Dillin, Viollet, Kundu, Hansen and Shaw2011; Kim et al., Reference Kim, Kundu, Viollet and Guan2011). Oxidative stress is able to reduce superoxide dismutase (SOD) expression in granulosa cells of preantral follicles, and to activate AMPK, which leads to the activation of autophagy. The mechanisms that regulate the preantral and early antral follicles atresia are shown in Figure 3.
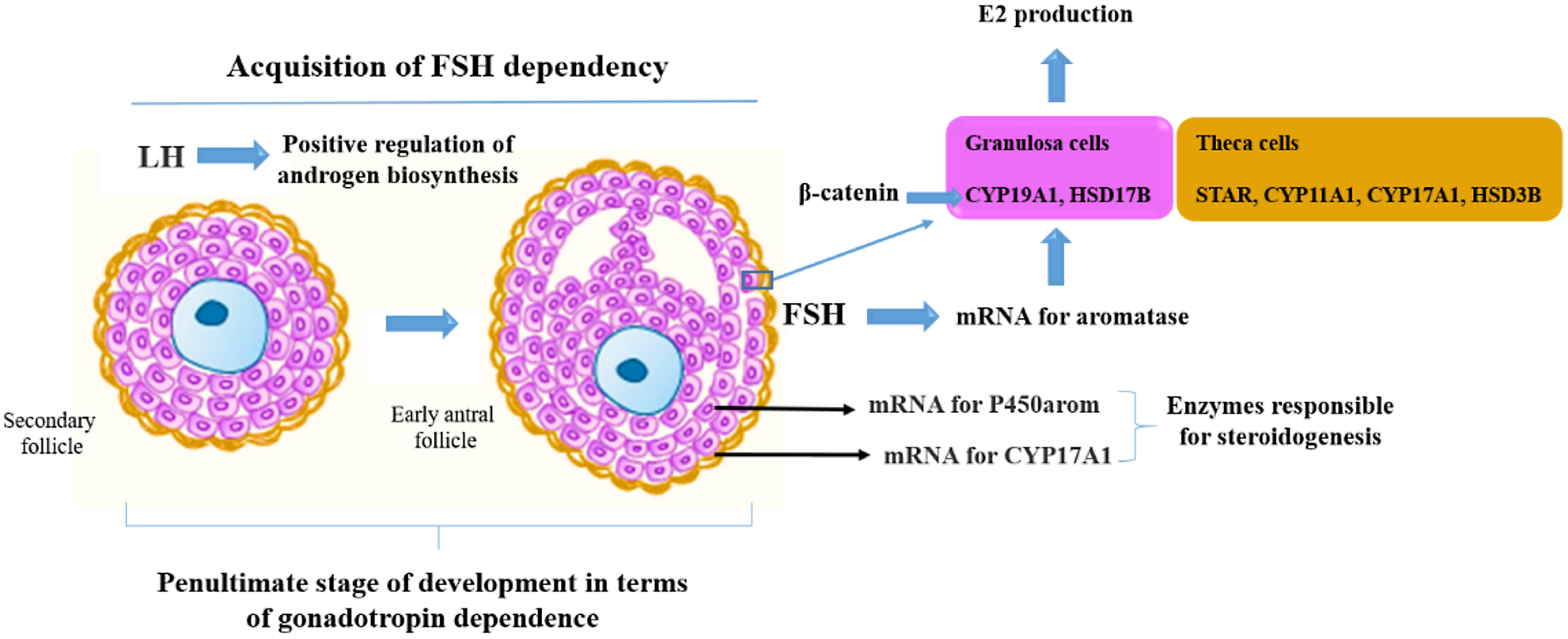
Figure 3. Mechanisms that control production of estradiol (E2) during the transition from preantral to early antral follicles.
Strategies to promote in vitro growth of preantral follicles
Preantral follicles represent 90% of the ovarian follicle population and, therefore, the development of effective culture systems to promote their growth in vitro is an interesting field of study in regard to avoiding the process of atresia that naturally occurs in vivo during the late antral stages of development. Follicular growth, antrum formation and acquisition of oocyte competence have been obtained in some species through the addition of different substances such as hormones, growth factors and cytokines to the culture medium in species that require short culture periods, such as murine (O’Brien et al., Reference O’Brien, Pendola and Eppig2003). However, in human and large animal species, the development of primordial to preovulatory follicles is a very long process (∼6 months; Paulino et al., Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022) and various researchers have been working to develop efficient culture systems.
The limitations of in vitro culture of preantral follicles come from the difficulty of maintaining the three-dimensional structure of follicles, and the many signals necessary to coordinate the stimulation of follicular growth (Paulino et al., Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022). Although bi-dimensional (2D) follicle culture has been successfully performed in many studies (da Cunha et al., Reference da Cunha, Melo, Sousa, Araújo, Vasconcelos, Silva and Silva2018; Paulino et al., Reference Paulino, Cunha, Barbalho Silva, Souza, Lopes, Donato, Peixoto, Matos-Brito, van den Hurk and Silva2018, Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020; Vasconcelos et al., Reference Vasconcelos, Costa, Azevedo, Barroso, de Assis, Paulino, Silva, Silva, Souza and Silva2021), a major limitation of these systems is their inability to maintain follicle architecture, with the oocyte surrounded by granulosa cells. This is particularly problematic with follicles from large mammalian species that require longer term cultures (Simon et al., Reference Simon, Kumar and Duncan2020). Given the importance of maintaining the follicle complex architecture, three-dimensional (3D) culture systems can help to maintain follicle complex architecture (Simon et al., Reference Simon, Kumar and Duncan2020). For this system, various types of matrices are used to encapsulate follicles to maintain follicle architecture and cell–cell interactions, thereby creating a microenvironment similar to that of the in vivo ovary, improving somatic cell proliferation and oocyte growth (Belli et al., Reference Belli, Vigone, Merico, Redi, Zuccotti and Garagna2012). In general, matrices have been engineered from natural components such as collagen and alginate (Healy et al., Reference Healy, Dolitsky, Villancio-Wolter, Raghavan, Tillman, Morgan, DeCherney, Park and Wolff2021), or Matrigel (Hao et al., Reference Hao, Tuck, Prakash, Damdimopoulos, Sjödin, Lindberg, Niklasson, Pettersson, Hovatta and Damdimopoulou2020), or from synthetic components such as polyethylene glycol (Green and Shikanov, Reference Green and Shikanov2016; Tomaszewski et al., Reference Tomaszewski, DiLillo, Baker, Arnold and Shikanov2021). Maintaining the follicular shape along with regulating the physical and chemical features of the microenvironment can be used as a tool to understand the underlying biology of follicle growth and maturation (Ghorbani et al., Reference Ghorbani, Eyni, Norahan, Zarrintaj, Urban, Mohammadzadeh, Mostafavi and Sutherland2022; Khunmanee and Park, Reference Khunmanee and Park2022).
Preantral follicles cultured in the 3D system had greater homogeneity of daily growth, higher rates of viability and antrum formation, as well as low rates of degeneration (Antonino et al., Reference Antonino, Soares, Júnior, de Alvarenga, Mohallem, Rocha, Vieira, de Souza, Beletti, Alves, Jacomini, Goulart and Alves2019; Panta et al., Reference Panta, Silva, Padilha, Correia, Rondina, Figueiredo and Magalhães Padilha2019). Preantral follicles from other species have been successfully cultured in a three-dimensional culture system using alginate and fibrin (monkey: Xu et al., Reference Xu, Xu, Bernuci, Fisher, Shea, Woodruff, Zelinski and Stouffer2013; Bulgarelli et al., Reference Bulgarelli, Ting, Gordon, de Sá Rosa-e-Silva and Zelinski2018; cat: Chansaenroj et al., Reference Chansaenroj, Songsasen and Chatdarong2019; human: Chiti et al., Reference Chiti, Dolmans, Hobeika, Cernogoraz, Donnez and Amorim2017).
Multistep culture systems have also been developed to further mimic the physiologic environment of developing follicles (Green and Shikanov, Reference Green and Shikanov2016; Simon et al., Reference Simon, Kumar and Duncan2020). These systems have been used for culturing early preantral follicles. The multistep method starts with the culture of ovarian cortical tissue for 3 weeks to initiate primordial follicle activation and to support follicle growth to the preantral stage. At the end of ovarian tissue culture, preantral follicles are isolated mechanically and cultured individually or in a group for 6 weeks (Xu et al., Reference Xu, Lawson, Bean, Ting, Pejovic, De Geest, Moffitt, Mitalipov and Xu2021). In this system, Xu et al. (Reference Xu, Lawson, Bean, Ting, Pejovic, De Geest, Moffitt, Mitalipov and Xu2021) demonstrated that ∼50% of human follicles survived for 6 weeks. Most surviving follicles grew to the antral stage and produced the ovarian steroid hormones estradiol and progesterone, in addition to paracrine factors such as activin A, IGF-2 and VEGF. In addition, the cultured preantral follicles exhibited morphology similar to that of human follicles developed in vivo.
Many efforts have been made to elucidate the mechanisms involved in the growth of preantral follicles, as reviewed recently by Paulino et al. (Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022). Although comprehension of the molecular regulation and composition of the microenvironment coordinating the events in preantral follicles remains incomplete, over time many studies have been conducted to optimize culture systems to support follicular growth (Healy et al., Reference Healy, Dolitsky, Villancio-Wolter, Raghavan, Tillman, Morgan, DeCherney, Park and Wolff2021). Preantral follicles from human and animal species require the development of a tightly regulated culture system with adequate nutrients, cytokines, growth factors, and developmental stage-dependent hormones to support cell survival and proliferation, as well as cellular function, which changes as follicles grow and oocytes mature (Simon et al., Reference Simon, Kumar and Duncan2020; Paulino et al., Reference Paulino, de Assis, Azevedo, Silva, da Cunha and Silva2022). Therefore, understanding the influence of several compounds for supplementation of culture medium for preantral follicles is fundamental. In this context, melatonin increases follicular and oocyte diameters, forms the antral cavity and preserves high rates of morphologically intact sheep preantral follicles for up to 18 days of culture (Barros et al., Reference Barros, Monte, Santos, Lins, Cavalcante, Gouveia, Müller, Oliveira, Donfack, Araújo and Matos2020).
Another alternative to improve follicular development in vitro is to supplement the culture medium with EGF, whose signalling regulates many cellular processes associated with survival (Sabbah et al., Reference Sabbah, Hajjo and Sweidan2020). EGF has an important role in folliculogenesis, by promoting several processes, such as granulosa and theca cell proliferation (Jachter et al., Reference Jachter, Simmons, Estill, Xu and Bishop2022). The effects of adding EGF to the culture medium are directly associated with improved survival of bovine preantral follicles. In general, EGF-treated follicles reach a larger diameter at the end of the culture period (Paulino et al., Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020; Jachter et al., Reference Jachter, Simmons, Estill, Xu and Bishop2022). Hormones, such as progesterone, and cytokines, such as TNF-α, are able to maintain healthy follicle morphology and positively influence follicular growth and antrum formation in cattle (Paulino et al., Reference Paulino, Cunha, Barbalho Silva, Souza, Lopes, Donato, Peixoto, Matos-Brito, van den Hurk and Silva2018, Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020). Also in cattle, preantral follicles cultured in the presence of BMP-2 or BMP-4 showed a significant increase in follicular diameter and greater average daily growth (da Cunha et al., Reference da Cunha, Melo, Sousa, Araújo, Vasconcelos, Silva and Silva2018).
Understanding the mRNA transcription of preantral follicles can provide important insights to detect follicular gene expression at several critical stages of its development under different culture conditions. Therefore, the identification of genes differentially expressed at each of growth and follicular stage development can be used to elucidate the processes involved in follicular growth (Paulini et al., Reference Paulini, Araujo, Silva and Lucci2022). In bovine species, an overexpression of mRNA for oocyte maturation factor Mos (c-MOS) and GDF-9 was observed when oocytes from preantral follicles were cultured in the presence of EGF. Furthermore, higher levels of cyclin B1 and GDF9 mRNA were observed in oocytes from follicles cultured with progesterone (Paulino et al., Reference Paulino, Barroso, Silva, Souza, Bezerra, Silva, Donato, Peixoto and Silva2020). In addition, bovine preantral follicles cultured in the presence of alpha-lipoic acid expressed higher levels of transcripts for the FSH receptor, LHCGR, IGF-1, BMPR1a, TGFβ1, TGFβR1, ActRIIB, GDF9, and activin. The expression of pro-apoptotic genes BCL2 associated X (BAX) and c-Myc were also downregulated (Zoheir et al., Reference Zoheir, Harisa, Allam, Yang, Li, Liang, Abd-Rabou and Harrath2017).
To mitigate the effects of oxidative stress induced by culture conditions, such as lower quality oocytes, follicular cell death, inactivation of antioxidant enzymes, and mitochondrial damage, there has been an increasing interest in the antioxidant potential of natural compounds (Lins et al., Reference Lins, Cavalcante, Santos, Menezes, Barros, Barberino, Bezerra, Macedo and Matos2017). Preantral follicles from sheep cultured in medium supplemented with kaempferol showed high percentages of follicular survival, antrum formation, and greater follicular diameter. In addition, kaempferol preserves higher levels of metabolically active mitochondria (Santos et al., Reference Santos, Monte, Lins, Barberino, Menezes, Gouveia, Macedo, Oliveira Júnior, Donfack and Matos2019). In cattle, the presence of eugenol in a culture medium promoted higher daily growth rates of bovine preantral follicles, in addition to stimulating the expression of mRNA for glutathione peroxidase 1 (GPX1; Vasconcelos et al., Reference Vasconcelos, Costa, Azevedo, Barroso, de Assis, Paulino, Silva, Silva, Souza and Silva2021). The strategies to promote in vitro growth of preantral follicles are represented in Figure 4.

Figure 4. Strategies to promote in vitro growth of preantral follicles.
Final considerations
The transition from preantral to antral requires intense and precise granulosa–oocyte interaction, any dysregulation can interfere with the acquisition of oocyte competence. Endocrine, paracrine and autocrine factors are essential for follicular growth, antral cavity formation, granulosa cell proliferation, differentiation, and future gonadotropin dependence. In vitro studies have contributed to a better understanding of the roles of hormones and growth factors during follicular development, addressing molecular events and bidirectional communications between the oocyte and surrounding granulosa cells.
Data availability
All data searched in this study are included in this publication.
Acknowledgements
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarships. J. R. V. Silva is a researcher of Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (grant number 308737/2018-0).
Conflict of interest
The authors declare that they have no competing interests.








