Plant-derived dietary components play an important role in the prevention of CVD(Reference Block, Patterson and Subar1, Reference Willett2). The beneficial effects of these plant components have been attributed partly to the presence of numerous polyphenolic compounds, which have been shown to have antioxidant and/or free radical-scavenging properties in vitro (Reference Han, Shen and Lou3). Phenolic compounds comprise a group of natural substances found in plants. Polyphenols and tannic acid (TA) have been of great interest for many years, in part because of their impact on the colour, odour and flavour of foods and beverages(Reference Shahidi and Naczk4), and in part due to their health-promoting properties(Reference Higdon and Frei5). Based on their chemical structures, tannins are divided into four major groups: proanthocyanidins or condensed tannins, hydrolysable tannins, phlorotannins found in marine brown algae and complex tannins(Reference Santos-Buelga and Scalbert6, Reference Ragan and Glombitza7). The main components of hydrolysable tannins are gallotannins, also referred to as TA, and ellagitannins(Reference Clifford and Scalbert8). Hydrolysable tannin is made up of polymers of gallic or ellagic acid esterified to a core molecule, commonly glucose or a polyphenol such as catechin, and it exists in mixtures with many other classes of plant phenolic compounds(Reference Haslam9). The nutritional significance and potential effects of TA will mainly depend on its behaviour in the digestive tract and bioavailability. However, little is known about the absorption of tannin in the gasterointestinal tract and to what extent can it be retained in the body after absorption. Nevertheless, tannins exhibit multiple biological activities, including anticancer(Reference Okuda, Yoshida and Hatano10), antioxidant(Reference Hagerman, Riedl and Jones11, Reference Gyamifi and Aniya12) and antimicrobial activities(Reference Cowan13). In addition, most tannins exhibit marked antioxidant activity through the induction of enzymes that play a crucial role in cellular defences against reactive oxygen species. A growing number of studies support the many potential benefits of polyphenolic-mediated lipid regulation in vivo. Tannins have been shown to increase faecal fat excretion. The hypocholesterolaemic action of TA probably is mediated by an increased level of plasma HDL-cholesterol (HDL-C) and reduced LDL-cholesterol (LDL-C)(Reference Yugarani, Tan and Das14). However, the lipid-lowering action of TA in plasma and the liver has not yet been compared with that of clofibrate (CF), a hypolipidemic drug, in apo E-deficiency (apo E− / −) mice. Apo E− / − leads to an impaired hepatic uptake of remnant lipoproteins, thus causing severe hypercholesterolaemia and atherosclerosis in the mouse model. The process leading to atherosclerosis has been found to be very rapid when high-cholesterol diets are given to these mice(Reference Lardenoye, Delsing and de Vries15). Intervening in cholesterol synthesis and fatty acid β-oxidation could be an effective tool for the prevention of hyperlipidaemia in an atherogenic animal model. Fibrates activate PPAR-α and enhance both mitochondrial and peroxisomal β-oxidation(Reference Nagasawa, Inada and Nakano16). However, a hepatotoxic effect of CF, increased liver weight shown by CF supplementation, can probably be due to hepatocyte hyperplasia and hypertrophy associated with distinctive morphological effects in rodents(Reference Tosh, Alberti and Agius17). The molecular mechanisms underlying the TA supplement in mice, however, have been largely unknown. We hypothesised that TA may improve hyperlipidaemia and alter hepatic carnitine palmitoyl transferase (CPT) and β-oxidation, which may decrease hepatic lipid accumulation in atherogenic animals similar to the action of CF, but without altering liver weight. Accordingly, in the present study, we investigated the long-term effects of TA in comparison with CF on the alteration of plasma lipid profiles, hepatic lipid-regulating enzyme activities and the gene expression of cholesterol-regulating enzymes in apo E− / − mice fed a normal diet.
Materials and methods
Animals and diets
A total of thirty male apo E− / − mice (weighing 20–22 g) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The animals were housed individually with ad libitum feeding in a temperature-controlled room (20–24°C) with an automatic 12 h light–12 h dark cycle. After allowing 1 week for adaptation, all mice were randomly divided into three groups of ten mice each. The mice were fed an AIN-76 semi-synthetic diet with or without 0·02 % (w/w) CF (Sigma Chemical Company, St Louis, MO, USA) or 0·02 % (w/w) TA (Sigma Chemical Company) for 20 weeks. Food consumption and body weight were measured daily and weekly, respectively. At the end of the experimental period, all mice were anaesthetised with diethyl ether after a 12 h fast. Blood was taken from the inferior vena cava for the determination of plasma lipid levels at the end of the experimental period. The blood was centrifuged at 1000 g for 15 min at 4°C, and the plasma was separated. The livers were removed, rinsed with physiological saline, and weighed for enzyme activity and lipid measurement. All samples were stored at − 70°C until analysis. Studies were performed under protocols for animal studies approved by the Ethics Committee at Kyungpook National University, Daegu, Republic of Korea.
Analyses of plasma and hepatic lipids
Enzymatic assays for plasma total cholesterol (total-C), HDL-C and TAG were performed using enzymatic kits (Asan Pharm Company, Hwasung, Republic of Korea) based on a modification of the cholesterol oxidase and the lipase-glycerol phosphate oxidase methods. The HDL fractions were separated using the Asan kit according to the heparin-manganese precipitation procedure. Apo A-I and apo B levels were also measured using an enzymatic kit (Eiken, Japan). The hepatic lipids were extracted using a slightly modified version of the procedure developed by Bligh & Dyer(Reference Bligh and Dyer18). The hepatic tissues were homogenised in a 20 mm-potassium phosphate buffer (pH 7·4), and the hepatic lipids were extracted using a chloroform and methanol (1:1, v/v) solution. Triton X-100 and a sodium cholate solution were added to the dissolved lipid sample for emulsification. The dried lipid residues were dissolved in isopropanol for cholesterol, TAG and apo assays, which were carried out with the same enzymatic kit used in the plasma analyses.
Histopathological analyses
After TA supplementation for 20 weeks, the hepatic tissue was removed from each mouse and cleaned free of connective tissues. The aortic bulb was also removed, cleaned free of connective tissues and wrapped with saline-soaked gauze. It was fixed in 10 % (v/v) paraformaldehyde/PBS and embedded in paraffin for staining with haematoxylin and eosin. The stained area was then viewed using a microscope at a magnification of 200 × . Another section of the aortic bulb was cryo-sectioned and stained with Oil Red O solution as previously described(Reference Moghadasian, Mcmanus and Pritchard19).
Preparation of hepatic subcellular fractions
Hepatic microsomes were prepared according to the method described by Hulcher & Oleson(Reference Hulcher and Oleson20), with slight modifications. Half of 1 g of liver tissues was homogenised in 2 ml of ice-cold buffer (pH 7·0) containing 0·1 m-triethanolamine, 0·02 m-EDTA (pH 7·4) and 2 mm-dithiothreitol. The homogenates were centrifuged at 1000 g for 15 min at 4°C. The supernatants were centrifuged two additional times at 1000 g for 20 min and the obtained supernatants were centrifuged at 10 000 g for 20 min at 4°C. The mitochondrial pellet was then resuspended in a buffer for the measurement of CPT and β-oxidation activity. The supernatants were ultra-centrifuged at 105 000 g for 60 min at 4°C. The resulting cytosolic supernatants were used for the measurement of fatty acid synthase activity. The pellets were resuspended and ultra-centrifuged again. The microsomal pellets were then redissolved in 1 ml of homogenised buffer without dithiothreitol, and the microsomal protein concentrations were determined using the Bradford method and analysed for HMGR and acyl-CoA cholesterol acyltransferase (ACAT) activities.
Hepatic 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl-CoA cholesterol acyltransferase activities
Microsomal HMGR activity was measured using [14C]HMG-CoA as the substrate, based on a modification of the method described by Shapiro et al. (Reference Shapiro, Nordstrom and Mitschelen21). The incubation mixture containing the microsomes (100–200 μg), 500 nmol of NADPH and 50 nmol of [14C]HMG-CoA (2·09 × 109 Bq/mmol; PerkinElmer, Inc., Waltham, MA, USA) was pre-incubated at 37°C for 5 min. The reaction was terminated by the addition of 10 m-HCl, and the resultant reaction mixture was incubated at 37°C for an additional 15 min. The incubation mixture was then centrifuged at 10 000 g for 5 min, and the supernatant was spotted on a Silica Gel 60F254 TLC plate with mevalonate as the standard. The plate was developed in benzene–acetone (1:1, v/v). Finally, region R f 0·3–0·6 (the region containing mevalonate) was removed by scraping, and its 14C radioactivity was determined using a liquid scintillation counter (Packard Tricarb 1600TR; Packard Instrument Company, Meriden, CT, USA). HMGR activity was expressed as pmol of mevalonate synthesised/min per mg protein. The microsomal ACAT activity was determined using [14C]oleoyl-CoA according to the method described by Gillies et al. (Reference Gillies, Rathgeb and Perri22). To prepare the cholesterol substrate, cholesterol (1 mg/ml; Sigma Chemical Company) and Triton WR-1339 (10 %, v/v; Sigma Chemical Company) were each dissolved in the acetone, mixed well and completely dried in N2 gas. The dried substrate was then redissolved in distilled water to a final concentration of 300 μg cholesterol/ml. Next, the reaction mixtures containing the cholesterol solution, 1 m-potassium phosphate buffer (pH 7·4), 0·6 mm-bovine serum albumin, the microsomal fraction (4–8 mg protein) and distilled water were pre-incubated at 37°C for 30 min. The reaction was initiated by adding 5·62 nmol of [14C]oleoyl-CoA (2·15 × 109 Bq/mmol; PerkinElmer, Inc.); the reaction time was 30 min at 37°C. The reaction was stopped with the addition of isopropanol/heptane (4:1, v/v) and heptane. The reaction mixture was allowed to stand at room temperature for 2 min with 0·1 m-potassium phosphate buffer (pH 7·4). Finally, an aliquot of the supernatant was subjected to scintillation counting. The ACAT activity was expressed as pmol cholesteryl oleate synthesised/min per mg protein.
Enzymatic activity of hepatic fatty acid synthesis and β-oxidation
The fatty acid synthase activity in the liver tissue was determined by a spectrophotometric assay according to the method previously described by Carl et al. (Reference Carl, Lakshmanan and Porter23). The incubation mixture containing 125 mm of phosphate buffer (pH 7·0), 1 mm-EDTA-2Na, 1 mm-β-mercaptoethanol, 33 mm-acetyl-CoA, 100 mm-malonyl-CoA and 100 mm-NADPH+ was pre-incubated at 30°C for 5 min. The reaction was initiated by the addition of hepatic cytosol (50–100 μg), and the malonyl-CoA-dependent oxidation of NADPH was measured at 30°C for 2 min. One unit of enzyme activity is represented as nmol NADPH oxidised/min per mg protein. CPT activity was analysed according to the method described by Markwell et al. (Reference Markwell, McGroarty and Bieber24). The reaction mixture containing 116 mm-Tris–HCl buffer (pH 8·0), 0·09 % (v/v) Triton X-100, 1·1 mm-EDTA-2Na, 0·12 mm-DTNB and 1·1 mm-l-carnitine, and the hepatic mitochondria (50–100 μg) was pre-incubated at 25°C for 5 min. The reaction was initiated by the addition of 0·035 mm-palmitoyl-CoA, and the oxidation of palmitoyl-CoA was measured at 412 nm for 2 min. The enzyme activity was expressed as nmol coenzyme A with sulfhydryl functional group (CoASH) oxidised/min per mg protein. The fatty acid β-oxidation was determined using the method described by Lazarow(Reference Lazarow25) by monitoring the reduction of NAD to NADH at 340 nm; the activity was expressed as nmol NADH oxidised/min per mg protein. It was determined in the presence of the mitochondrial fraction containing 47 mm-Tris–HCl (pH 8·0), 1 mm-KCN, 6·6 mm-dithiothreitol, 0·0075 % (w/v) bovine serum albumin, 0·02 % (v/v) Triton X-100, 0·1 mm-CoA, 10 mm-FAD and 0·2 mm-NAD. After 5 min of pre-incubation, the reaction was initiated by the addition of 25 mm-palmitoyl-CoA. The reaction continued at 37°C and 340 nm for 5 min.
RNA isolation and mRNA expression analysis
The liver was homogenised in Trizol reagent (Invitrogen Life Technologies, Grand Island, NY, USA), and total RNA was isolated according to the manufacturer's specifications. The total RNA was reverse-transcribed into cDNA using oligo (dT) primers, deoxyribonucleoside triphosphates, Superscript II reverse transcriptase and 5 μg of total RNA from the RevertAid™ First-strand cDNA kit (Fermentas, Burlington, CA, USA). The RNA expression was quantified by real-time quantitative PCR using ABsolute™ QPCR SYBR Green Mixes (ABgene, Surrey, UK) and the SDS7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Each cDNA sample was amplified using primers labelled with SYBR Green dye for glyceraldehyde-3-phosphate dehydrogenase. The sequences of the primers were as follows: glyceraldehyde-3-phosphate dehydrogenase (sense, 5′-ACCACAGTCCATGCCATCAC-3′; antisense, 5′-TCCACCACCCTGTTGCTGTA-3′); HMGR (sense, 5′-CTCTGCTTGTGAGAAGGAAC-3′; antisense, 5′-AGTCTCTGCTTCCACCACTA-3′), ACAT (sense, 5′-AGAAATCAAGCAAAGATCCA-3′; antisense, 5′-AGGAGTCCTTGGGTAGTTGT-3′). The amplification was performed as follows: 10 min at 90°C, 15 s at 95°C and 60 s at 60°C for a total of forty cycles. The cycle threshold values obtained were those cycles at which a statistically significant increase in SYBR green emission intensity occurred. The fold changes were calculated using the 2-ΔΔCt method(Reference Livak and Schmittgen26).
Western blot analysis
The liver tissues were prepared according to Nathan et al. (Reference Nathan, Zdankiewicz and Wang27) with a slight modification. In brief, the tissue was homogenised with a 250 m-sucrose buffer (pH 7·4) containing 50 mm-triethanolamine, 150 mm-NaCl, 5 mm-EDTA, 10 % NP 40, 1 mm-PMSF, 1 mm-benzamidine, 1 mm-dithiothreitol and 1 % Triton X 100. The homogenates were then centrifuged at 10 000 g for 20 min at 4°C, and the supernatant was used for a PPAR-α assay. The total protein (100 μg) was electrophoresed on to 10 % SDS-polyacrylamide gel and transferred to polyvinylidene fluoride membranes (Milipore, Boronia, VIC, USA), blocked, and probed with rabbit anti-mouse PPAR-α (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and then with anti-rabbit IgG secondary antibody (1:1000; Pharmacia Biosciences, Amersham, Bucks, UK). The immunoreactive bands were visualized using the ECL kit (Pierce Chemical Company, Rockford, IL, USA) according to the manufacturer's instructions, quantified using a Bio Image Whole Band Analyzer (50S; B.I. System Company, Jackson, MI, USA).
Statistical analysis
The parameter values were all expressed as means with their standard error of the mean. Significant differences among the groups were determined by one-way ANOVA using the SPSS Program (SPSS, Inc., Chicago, IL, USA). The differences between the means were assessed using Duncan's multiple-range test, and the results were considered statistically significant at P < 0·05.
Results
Plasma lipids
Table 1(A) lists the effects of CF and TA on plasma lipid levels. The initial plasma total-C concentrations in the three groups were approximately the same (data not shown). After 20 weeks on the experimental diet, the plasma total-C level was significantly lowered in both the CF and TA groups compared with the control group (P < 0·05). The plasma LDL-C concentrations were also significantly lowered by 24 and 23 % in the CF and TA group, respectively. However, the concentrations of plasma HDL-C and apo A-I were markedly elevated in the CF and TA groups, with a concomitant increase in the HDL-C:total-C ratio in comparison with the control group. The plasma apo A-I levels were significantly higher in the CF and TA groups when compared with the control group, while the plasma apo B level was not different between the groups.
Table 1 Effects of tannic acid (TA) supplementation for 20 weeks on the plasma and hepatic lipid profiles in apo E−/− mice fed a normal diet
(Mean values with their standard errors, n 10)

C, control diet; CF-0·02, 0·02 % clofibrate-supplemented diet; TA-0·02, 0·02 % tannic acid-supplemented diet; total-C, total-cholesterol; LDL-C, LDL cholesterol; HDL-C, HDL-cholesterol; AI, atherogenic index; BW, body weight.
a,b Mean values within a row with unlike superscript letters were significantly different among groups (P < 0·05).
* LDL-C = (Total-C) − (HDL-C) − (TAG/5).
† AI = (Total-C − HDLC)/HDL-C.
Hepatic lipid profiles and morphology
The effects of CF and TA supplementation on the hepatic lipid levels are shown in Table 1(B). The liver weight was significantly increased in the CF group in comparison with the other groups. The hepatic cholesterol and TAG contents were significantly lowered in the TA group compared with the control group. The hepatic apo B concentration was significantly decreased in the TA group, although the apo A-I concentration was not different between the groups. The changes in the hepatic lipid profiles were further confirmed by a histopathological analysis of the hepatic dissections after staining with haematoxylin and eosin (Fig. 1). The accumulation of hepatic lipid droplets was found to be highest in the control group, while no lipid droplets were observed in the TA group. However, the CF group did demonstrate the formation of some lipid droplets, although not as many as in the control group.

Fig. 1 A histological observation of hepatic tissue from apo E− / − mice supplemented with clofibrate (CF) or tannic acid (TA) for 20 weeks. Representative images of haematoxylin and eosin-stained sections of liver tissue (n 3). Fat accumulation in the form of large fat droplets were present in the control group of apo E− / − mice fed a normal diet (arrows: lipid droplet). The proportion of lipid droplets was markedly lowered in the CF and TA supplemented groups (magnification × 200). C, control diet; CF-0·02, 0·02 % clofibrate-supplemented diet; TA-0·02, 0·02 % tannic acid-supplemented diet.
Hepatic lipid regulating enzyme activities
Table 2 lists the effects of CF and TA on the enzymatic activity of hepatic lipid metabolism. The HMGR activity was significantly lowered by 41 and 71 % in the CF and TA groups, respectively, compared with the control group. However, there was no significant difference in the hepatic ACAT activity among the groups. The CPT and β-oxidation activities were significantly higher in the CF and TA groups, although these groups exhibited no significant difference with respect to fatty acid synthase activity in the liver.
Table 2 Effects of tannic acid (TA) supplementation on hepatic lipid metabolic enzyme activities in apo E−/− mice fed a normal diet for 20 weeks
(Mean values with their standard errors, n 10)
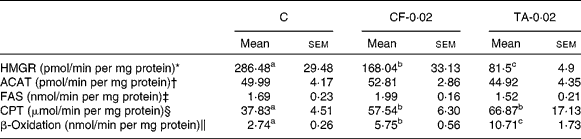
C, control diet; CF-0·02, 0·02 % clofibrate-supplemented diet; TA-0·02, 0·02 % tannic acid-supplemented diet; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; ACAT, acyl-CoA cholesterol acyltransferase; FAS, fatty acid synthase; CPT, carnitine palmitoyl transferase.
a,b,c Mean values within a row with unlike superscript letters were significantly different among groups (P < 0·05).
* pmol mevalonate formed.
† pmol cholesteryl oleate formed.
‡ nmol NADPH oxidised.
§ μmol CoASH oxidised.
∥ nmol NADH oxidised.
Hepatic mRNA expression of cholesterol-regulating enzymes
TA and CF supplementation appeared to alter the gene expression of the cholesterol biosynthesis enzymes. As shown in Fig. 2, the mRNA levels of HMGR were significantly lowered in the apo E− / − mice fed a normal diet along with CF or TA. In particular, the level of HMGR mRNA decreased dramatically in the TA group, which corresponded to a marked decrease in its enzyme activity (Table 2). On the other hand, the hepatic ACAT mRNA level did not differ among the groups.

Fig. 2 Effects of tannic acid (TA) supplement on hepatic mRNA expression of (A) 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) and (B) acyl-coA acyltransferase (ACAT) in apo E− / − mice fed a normal diet for 20 weeks. The effects of dietary TA supplementation on hepatic key lipogenic enzyme mRNA levels were examined by real-time PCR. Values are means, with their standard errors represented (mRNA/GAPDH expression ratio) by vertical bars (n 10). a,b,c Mean values with unlike letters were significantly different among the groups (P < 0·05). C, control diet; CF-0·02, 0·02 % clofibrate-supplemented diet; TA-0·02, 0·02 % tannic acid-supplemented diet; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
PPAR-α protein expression
The changes in the hepatic PPAR-α protein expressions were examined by Western blotting analysis (Fig. 3). TA supplementation markedly increased the hepatic PPAR-α protein level compared with the control group.

Fig. 3 Effects of tannic acid (TA) supplementation for 20 weeks on hepatic PPARα expression in apo E− / − mice fed normal diets. Western blots of hepatic lysate from apo E− / − mice were carried out with antibodies against PPARα and β-tubulin as loading control. Total protein (100 μg) from the liver was electrophoresed on to SDS-phage gel and transferred to polyvinylidene fluoride membranes. C, control diet; CF, 0·02 % clofibrate-supplemented diet; T1, 0·02 % tannic acid-supplemented diet.
Histopathological analysis of atherosclerotic lesions
Histopathological analysis of the atherosclerotic lesions revealed that an advanced atherome containing lipid-rich components was clearly evident in the aortic arch of the control group of apo E− / − mice fed a normal diet (Fig. 4(A) and (B). However, atherome formation was markedly reduced in the CF and TA groups, both of which showed no visible intimal lesions on histopathological examination.

Fig. 4 A histological observation of aortas from apo E− / − mice supplemented with clofibrate (CF) or tannic acid (TA) for 20 weeks. (A) Haematoxylin and eosin-stained transverse-section of an aortic arch. (B) Oil-red O-stained cryosection of an aortic arch (n 3). Advanced fatty plaques containing lipid-rich components were present in the aortic arch, as well as cholesterol crystal deposition in the control group of apo E− / − mice fed a normal diet (arrows: atherome, magnification × 200). C, control diet; CF-0·02, 0·02 % clofibrate-supplemented diet; TA-0·02, 0·02 % tannic acid-supplemented diet.
Discussion
Many tannin-rich medicinal and food plants are appreciated for their antitumour and antiviral activity without any obvious toxicity(Reference Okuda and Yoshida28). These plants are worthy of further extensive investigation, not only in phytochemistry but also with respect to their health-promoting effects. According to the chemical structure, the TA used in the present study is one of the hydrolysable tannins, not condensed tannins. Although available information regarding the absorption of TA is very limited at present, Brave et al. (Reference Bravo, Abia, Eastwood and Saura-Calixto29) reported that only 4–6 % of the ingested TA can be excreted in rat faeces, indicating that absorption and/or degradation of TA occurred and that TA also can affect colonic microflora and/or the fermentation process. The present study reports that dietary TA modulates hepatic lipid metabolism through the elevation of β-oxidation and the inhibition of HMGR in an atherogenic mouse model fed a normal diet. CF was used as a positive control because its biochemical effects are triggered through binding to PPAR-α, causing a pleiotropic response inducing a number of proteins related to β-oxidation(Reference Lapinskas, Corton and Puga30, Reference Lindquist, Svensson and Alexson31).
In the present study, the anti-lipidaemic and anti-atherogenic functions of TA and CF appeared to be partly mediated by an increase in HDL-C as well as by a decrease in plasma total-C or LDL-C concentrations in apo-E-deficient mice. Consequently, the atherogenic index was significantly lower in the TA and CF groups than in the control group. Plasma LDL-C is an important risk factor for the development and progression of atherosclerosis, and the reduction of plasma total-C and LDL-C levels plays a major role in mediating the regression of atherosclerosis. Dietary polyphenol can exert a hypocholesterolaemic effect by enhanced reverse-cholesterol transport and reduced intestinal cholesterol absorption and increased bile acid excretion(Reference Tebib, Besancon and Rouanet32). Fibrates, in general, reduce plasma TAG and LDL and elevate plasma HDL, thereby reducing the cardiovascular risk(Reference Linton and Fazio33). The overexpression of apo A-I, a cholesterol acceptor in ABCA1-mediated transport, increases HDL in apo E− / − mice(Reference Pászty, Maeda and Verstuyft34). The apo A-I and HDL levels are negatively correlated with the risk of atherosclerosis(Reference Tkác, Kimball and Lewis35), and decreasing these levels has been suggested as a possible therapeutic strategy for the treatment of coronary artery disease(Reference Tardif, Heinonen and Noble36). Apo A-I prevents atherosclerosis by inhibiting the formation of foam cells from macrophages(Reference Dansky, Charlton and Barlow37). Thus, an elevation in the plasma HDL-C and apo A-I levels as a consequence of TA and CF supplementation could partly contribute to the prevention of aortic lesion formation in apo E− / − mice.
An increase in cholesterol synthesis in apo E− / − mice represents an adaptive response to the impaired hepatic influx of remnant-derived cholesterol in plasma. However, a TA supplement lowered hepatic HMGR activity significantly, as observed in the CF group, than in the control group. This seemingly implies that the efflux of hepatic lipoprotein cholesterol could be decreased by TA and CF supplementation. Accordingly, the inhibition of HMGR by TA or CF might be considered as one of the cholesterol-lowering approaches, thereby reducing the risk of developing atherosclerosis. Interestingly, there was no significant change in the hepatic ACAT activity with TA or CF supplementation. Inhibitors of HMGR and ACAT are beneficial for treating hypercholesterolaemia and atherosclerosis(Reference Bocan, Mueller and Brown38), and the activity of these enzymes can be regulated independently in the liver(Reference Suckling and Stange39). Supplementation of TA in the present study improved most of the pro-atherogenic variables, as well as the CF supplement, in comparison with the control group, thereby delaying the progression of atherosclerosis. During the advanced stages of atherosclerosis the lesions become mature and are more resistant to dietary interventions(Reference Do, Kwon and Kim40). The present study indicates that TA and CF supplementation can exert a beneficial effect by alleviating or preventing atherosclerosis when provided before the development of an atherome in atherogenic mice. Histological examination of the aortas of the mice in the TA group in particular revealed no visible intimal atherosclerotic lesions. There is increasing evidence that an atherogenic diet induces inflammatory genes potentially through increased oxidative stress. Vascular cell adhesion molecule-1, a member of the Ig superfamily, is known to be expressed by vascular endothelial cells for the recruitment of leucocytes during inflammation. Hypercholesterolaemia may potentiate the response to injury increasing vascular cell adhesion molecule-1 expression, leading to increased macrophage infiltration and subsequent neointimal formation(Reference Oguchi, Dimayuga and Zhu41).
In the present study, TA and CF supplementation markedly lowered the accumulation of hepatic lipid droplets as well as the hepatic cholesterol and TAG levels in apo E− / − mice. The amount of fat stored in the liver is determined by the balance between fatty acid uptake, endogenous fatty acid synthesis, TAG synthesis, fatty acid oxidation and TAG export. Changes in any of these parameters can affect the amount of fat stored in the liver. Green tea consumption has also been shown to lessen the liver damage caused by progressive hepatic steatosis(Reference Imai and Nakachi42). Hepatic steatosis can be caused by a disturbance of the allosteric interaction between malonyl-CoA and CPT1, a key regulatory step in fatty acid oxidation. Inherited CPT1 deficiency in humans is associated with hepatic steatosis during fasting(Reference Gobin, Bonnefont and Prip-Buus43). The disruption of fatty acid oxidation can also account for excess lipid storage in the liver. CPT1 is considered as one of the key regulatory enzymes in fatty acid oxidation(Reference Ohhira, Motomura and Fukuda44). It catalyses the formation of long-chain acylcarnitine, the transportable form of activated NEFA, thus committing NEFA to β-oxidation in the mitochondria. CF, known to induce peroxisome proliferation, is a member of a large class of diverse exogenous and endogenous chemicals known as peroxisome proliferators. Peroxisomes are subcellular organelles that are found in most animal cells and perform diverse metabolic functions, including H2O2-derived respiration, β-oxidation of fatty acids and cholesterol metabolism(Reference Lock, Mitchell and Elcombe45). PPAR-α activation enhances the expression of both CPT1A and ACOX1, leading to mitochondrial and peroxisomal β-oxidation(Reference Gloerich, van Vlies and Jansen46). CF causes a marked increase in the concentration of carnitine in the liver. It leads to an activation of PPAR-α, a ligand-activated transcription factor that acts as an important regulator of lipid metabolism and energy homoeostasis. A hypothesis has been raised that the activation of this nuclear receptor is responsible for the alterations in carnitine homeostasis observed in rodents by either stimulating carnitine uptake or carnitine biosynthesis(Reference Ringseis and Eder47). In the present study, hepatic CPT and β-oxidation activities were significantly elevated in the TA and CF groups. We found that TA and CF enhanced CPT and β-oxidation as well as PPAR-α activity. Excess fat accumulation in the liver may predispose to the development of metabolic abnormalities, including dyslipidaemia. Hepatic fatty acid oxidation seemed to be a contributing factor in the improvement of hyperlipidaemia in apo E− / − mice. The administration of a green tea extract also results in increased energy expenditure and fat oxidation in human subjects(Reference Dulloo, Duret and Rohrer48). CPT1 overexpression in rat hepatocytes increases the fatty acid β-oxidation capacity, leading to the metabolic reorientation towards the oxidation of the exogenous fatty acid taken up by the cells at the expense of esterification into TAG(Reference Stefanovic-Racic, Perdomo and Mantell49). CPT1 is a prime target for elevating hepatic β-oxidation, and its sensitivity to malonyl-CoA may be an important factor in the prevention or correction of hepatic steatosis(Reference Akkaoui, Cohen and Esnous50).
Overall, TA supplementation seemed to play important roles in fatty acid transport and cholesterol homoeostasis that are partly mediated by alteration of enzyme activities of CPT, β-oxidation and HMGR in apo E− / − mice fed a normal diet. CF supplementation also appeared to mediate cholesterol homoeostasis by regulating enzyme activity and mRNA expression of HMGR in apo E− / − mice. However, CF has been reported to exhibit an undesirable hepatotoxic effect, increased liver weight, which is probably due to hepatocyte hyperplasia and hypertrophy(Reference Tosh, Alberti and Agius17). Although we did not measure plasma GOT and GPT activity to determine a toxic effect of TA, information on the physiological effects of tannins is limited and often contradictory regarding their toxicity. Glick & Joslyn(Reference Glick and Joslyn51) reported that food intake depression and subsequent decrease in growth of rats fed TA were observed when supplemented at the levels of 4, 5 and 8 % in the diet. No fatty liver was observed with 5 % TA in the diet. However, dietary TA level in the present study was only 0·02 %. Based on these, 0·02 % dietary TA should be safe and seems to be more desirable than CF for a health-promotion effect by alleviating hepatic lipogenesis and atherogenesis in apo E− / − mice without altering liver weight.
Acknowledgements
The present study was supported by the Bio-Food Research Project from the Korea Science and Engineering Foundation under the Ministry of Science and Technology in Korea and the SRC programme (Center for Food & Nutritional Genomics: grant no. 2009-0063409) of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology. G.-M. D., E.-Y. K., T.-Y. H., Y. B. P., H.-J. K., S.-M. J., M.-K. L. and M.-S. C. contributed to data analyses and manuscript preparation. The authors have declared no conflict of interest.










