INTRODUCTION
Rickettsiae are Gram-negative intracellular bacteria, with more than 20 validated species in the genus Rickettsia, of which 14 are confirmed human pathogens [Reference Raoult and Roux1]. Tick-borne Rickettsia spp. are associated with several human diseases in Europe including Rickettsia conorii conorii [agent of Mediterranean spotted fever (MSF)] and R. conorii israelensis (Israeli spotted fever) both transmitted primarily by Rhipicephalus sanguineus [Reference Parola, Paddock and Raoult2]; R. slovaca and R. raoultii [agents of tick-borne lymphadenopathy (TIBOLA), also called Dermacentor-borne necrosis erythema lymphadenopathy (DEBONEL)] transmitted primarily by Dermacentor marginatus and D. reticulatus [Reference Punda-Polic3–Reference Parola5], as well as other pathogenic rickettsiae (e.g. R. helvetica) transmitted by Ixodes ricinus [Reference Parola6].
Species in the genus Rickettsia are separated into three groups: first, an ancestral group containing R. bellii; second, the typhus group (TG) which includes the agent of louse-borne epidemic typhus, R. prowazekii, and the agent of flea-borne murine typhus, R. typhi, and third, the spotted fever group (SFG), whose members are associated mainly with ticks, but also fleas and mites [Reference Brouqui4]. Ixodid ticks serve as the main vectors and reservoirs of SFG rickettsiae [Reference Stanczak7, Reference Sprong8]. Ticks sustain rickettsial transmission cycles trans-ovarially and trans-stadially as well as passing on the rickettsiae to vertebrate hosts during feeding when their salivary glands are infected [Reference Sréter-Lancz9].
Previously unrecognized species of Rickettsia are continuously being isolated from an ever-increasing number of tick species around the world; however, for the majority their pathogenicity remains to be determined. Some rickettsiae previously considered to be non-pathogenic have later been associated with human disease, such as R. slovaca, R. helvetica and R. aeschlimannii and therefore investigations into the presence of rickettsiae in ticks are warranted [Reference Parola, Paddock and Raoult2].
Recent reports from the UK suggest that two potential tick vector species are increasing their geographical range. Recent evidence from the Health Protection Agency's UK tick surveillance scheme provides evidence suggesting an expansion in the range of I. ricinus when compared with historical data [Reference Jameson and Medlock10] thus confirming previous anecdotal evidence [Reference Scharlemann11]. Similarly there is evidence of D. reticulatus being reported in new, geographically distinct foci in England facilitated by the movement of animals and subsequently being responsible for human and animal biting issues [Reference Jameson and Medlock12]. Owing to the increasing evidence supporting the pathogenic status of rickettsiae in Europe and given that some of the tick species implicated in transmission are expanding their range in the UK it would seem prudent to ascertain the existence of tick-borne rickettsiae in British ticks, and this is the rationale for the present study.
Prior to this study there had been no reports of Rickettsia spp. in British ticks of public or veterinary health concern. A recent study [Reference Graham, Mainwaring and Du Feu13] has described the detection of wildlife-associated Rickettsia spp. in populations of I. lividus ticks in northwest England which mainly parasitize migratory Riparia riparia (sand martin). It is also worthy of note that cat fleas in the UK transmit R. felis [Reference Kenny14]. I. ricinus is the main vector of Borrelia burgdorferi s.l. (the causative agent of Lyme borreliosis) and is also a vector of Anaplasma phagocytophilum [Reference Guy, Tasker and Joynson15], Babesia spp. [Reference Zintl16] and louping ill virus [Reference Laurenson17] in the UK. The incidence of humans being bitten by I. ricinus is therefore well established, and it is conceivable that this species might also pose a vector risk from rickettsiae.
I. ricinus is the most ubiquitous tick in the UK [Reference Jameson and Medlock10, Reference Pietzsch18] found in a variety of habitats from woodland, grassland, upland moor and heathland [Reference Medlock19] where it acquires blood from a variety of hosts including rodents, birds, hares, rabbits, squirrels, livestock and deer, and is the most important species associated with dogs and humans in the UK. Much less is known about the distribution and ecology of D. reticulatus in the UK except that it has been reported historically [Reference Tharme20, Reference Hillyard21] and more recently from sand dune systems in west Wales and north Devon, associated with cattle and dogs (Medlock et al. unpublished data). Recently it has been reported in parts of Essex where it is now established [Reference Jameson and Medlock12].
METHODS
In total 401 British ticks [338 I. ricinus (144 nymph, 38 male, 156 female) and 63 D. reticulatus (22 male, 41 female)] from various sites throughout England, Wales and Scotland were tested for the presence of Rickettsia spp. Ticks were collected from various animal hosts and from vegetation in a range of ecologically and geographically distinct areas [Reference Jameson and Medlock10]. All D. reticulatus ticks were collected from vegetation by blanket dragging in Essex (England) and Gwynedd (Wales) during spring 2009 and 2010. I. ricinus ticks were collected from hosts (dogs, deer, humans) and from vegetation by dragging in Devon, Dorset, Essex, Gloucestershire, Hampshire, Herefordshire, Northumberland, Wiltshire (all England), Ross & Cromarty and Inverness-shire (both Scotland) between 2006 and 2009.
Total DNA was extracted from each of the ticks separately by alkaline lysis as described previously [Reference Schouls22]. DNA extracts were stored at −20°C. The primers and probes that were used for polymerase chain reaction (PCR) and reverse line blotting (RLB) analysis were as described previously [Reference Tijsse-Klasen23]. The 16S rRNA gene (~360 bp) of Rickettsia spp. was amplified with the HotStarTaq master mix (Qiagen, Germany) with the following conditions: 15 min at 94°C, then cycles of 20 s at 94°C, 30 s at 72°C, 30 s at 72°C, lowering the annealing temperature by 1°C each cycle until reaching 62°C, then 40 cycles at this annealing temperature followed by a final elongation step for 7 min at 72°C. All samples were analysed on agarose gels. R. felis, a flea-borne Rickettsia was used as a positive control, with water used as a negative control. All samples were tested once. Some positive samples were subjected to PCR of two independent markers. The citrate synthase gene (gltA; ~850 bp) was amplified using primers CS409d and Rp1258n [Reference Roux24] under following conditions: 15 min at 95°C, then 40 cycles of 30 s at 94°C, 30 s at 54°C, 55 s at 72°C followed by a final elongation step for 7 min at 72°C. The rrl-rrf intergenic spacer (ITS; ~530 bp) was amplified using primers ITS-F and ITS-R [Reference Vitorino25] under the following conditions: 15 min at 95°C, then cycles of 60 s at 94°C, 60 s at 66°C, 60 s at 72°C, lowering the annealing temperature by 1°C each cycle until reaching 56°C, then 35 cycles at this annealing temperature followed by a final elongation step for 7 min at 72°C. All PCRs were carried out using HotStarTaq master mix and 5 μl DNA extract. PCR products were sequenced by dideoxy-dye termination sequencing of both strands, and compared with sequences in GenBank (http://www.ncbi.nlm.nih.gov/) using BLAST. The sequences were aligned and analysed using BioNumerics 5.1 (Applied Maths, Belgium). In The Netherlands, R. helvetica was found in some habitats at a prevalence of up to 67% [Reference Sprong8] in I. ricinus and therefore (double) infections with other Rickettsia spp. might be missed. Two RLB probes were able to hybridize to DNA of most Rickettsia spp. except for R. helvetica and closely related species. None of the R. helvetica-positive samples reacted with these two probes, minimizing the chance of a possible double infection in these ticks. To minimize cross-contamination and false-positive results, positive and negative controls were included in each batch tested by the PCR and RLB assays. Furthermore, DNA extraction, PCR mix preparation, sample addition, and PCR analysis were performed in assigned separate laboratories.
RESULTS
The 16s rRNA gene of Rickettsia spp. was detected in 39/401 (9·7%) of ticks tested (Tables 1 and 2, Fig. 1), including 22/338 (6·5%) I. ricinus (8/143 nymph, 8/38 male, 6/156 female) and 17/63 (27%) D. reticulatus (6/21 male, 11/39 female). All negative controls remained negative. 16S rRNA sequences of the different positive I. ricinus samples showed 100% homology with R. helvetica (10/22), 98–99% homology with R. limoniae (2/22), 97% with R. massiliae (6/22), 97% with R. canadensis (1/22) and 96–98% with R. bellii (3/22). Infection of three of the R. helvetica-positive I. ricinus ticks was confirmed with the gltA gene (100% homology with U59723.1) and one with rrl-rrf ITS (99% homology with AY125017.1). Additionally, two of the ticks with closest 16S rRNA sequence matches to R. massiliae were also positive for Rickettsiae on the gltA and rrl-rrf ITS markers [87% to R. helvetica (EU359285.1) and 89% to R. felis (DQ139799.1), respectively]. Further information on the specific gene and tick stage is given in Table 1. 16S rRNA sequences of the positive D. reticulatus samples showed 100% homology with R. raoultii (13/17), 99% with R. limoniae (1/17), 98% with R. bellii (1/17), 97% with R. typhi (1/17) and 96% with R. bellii and 96% with R. massiliae (1/17). Rickettsia infection of seven of the 13 R. raoultii-positive samples was confirmed by the amplification and sequencing of gltA [100% R. raoultii (DQ365804.1)] and/or rrl-rrf ITS [99% R. massiliae (CP000683.1); no rrl-rrf ITS R. raoultii sequence deposited in GenBank as at July 2010].
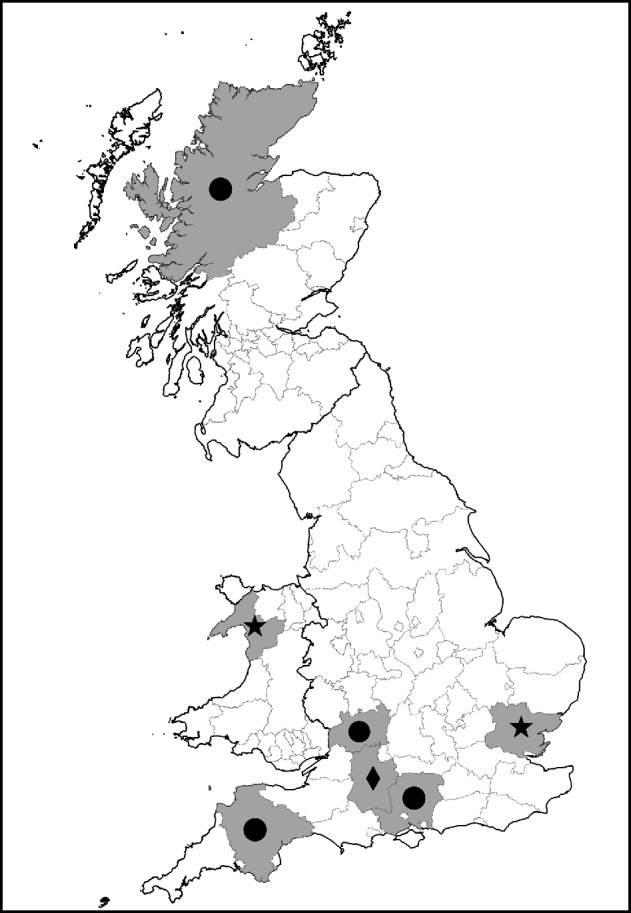
Fig. 1. Map showing counties with ticks positive for Rickettsia spp. of public health concern. •, R. helvetica; ⋆, R. raoultii; ◆, R. massiliae.
Table 1. Number and host association of ticks screened for presence of Rickettsia spp.
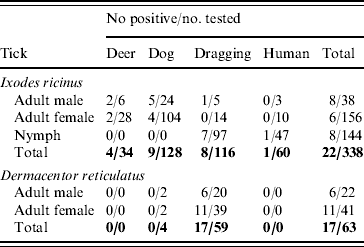
Table 2. GenBank accession numbers and results of Rickettsia spp. detected in ticks
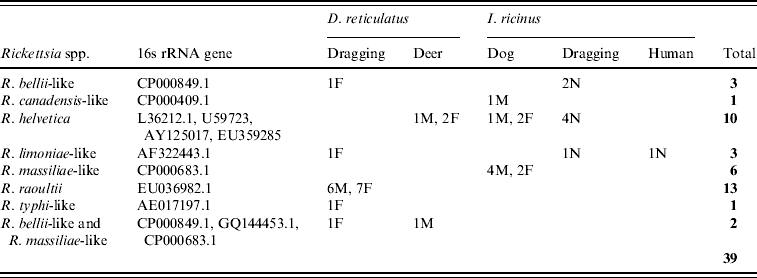
F, Female; M, male; N, nymph.
I. ricinus ticks positive for R. helvetica included engorged female and unengorged male ticks removed from deer in Hampshire and Ross & Cromarty; engorged female and unengorged male ticksFootnote † removed from dogs in Gloucestershire and Devon and unfed questing nymphs also from Devon. All positive ticks that could be confirmed with gltA or rrl-rrf ITS were found in Devon. This might indicate that ticks in this region have a higher rickettsial load and are therefore also positive in less sensitive PCR assays. The geographical spread of these ticks and the occurrence of R. helvetica in various stages and questing ticks suggest that this rickettsial species is widespread.
Regarding the other rickettsial isolates in I. ricinus, R. bellii-like Rickettsia were detected in unfed questing nymphs from Devon and an unengorged male from Dorset, R. canadensis-like Rickettsia in an unfed questing male from Devon, R. limoniae-like Rickettsia from an unfed questing nymph and unengorged nymph on a human from Devon and Gloucestershire, respectively, and R. massiliae-like Rickettsia detected from two engorged female and four un-engorged male ticks all from the same dog in Wiltshire.
D. reticulatus ticks positive for R. raoultii were questing unfed male and female ticks from Gwynedd (12/13) and Essex (1/13). Evidence of R. bellii-, R. limoniae-, R. typhi- and R. massiliae-like Rickettsia was from unfed questing female ticks from Essex. The high prevalence of R. raoultii (12/25, 48%) in D. reticulatus in the field site in Gwynedd is worthy of note and further investigations of this and neighbouring sites for rickettsiae-infected ticks is required. It is also interesting that one of the Essex D. reticulatus was positive for R. raoultii as this tick population was considered to have been imported on animals from Wales [Reference Jameson and Medlock12].
DISCUSSION
This preliminary study provides the first evidence by PCR and sequencing of possibly 11 different species of Rickettsia in ticks in the UK. Several, but not all, species were confirmed by sequencing of the gltA gene or rrl-rrf ITS, including R. helvetica in I. ricinus and R. raoultii in D. reticulatus. The former tick species appears to have a widespread distribution across south-west England and parts of Scotland, and the latter is present in well-established tick populations in Wales and recently imported populations in Essex. Further testing of tick samples from the HPA tick surveillance scheme and ongoing field projects are continuing.
The occurrence of these rickettsiae in ticks in the UK does not confirm that they are transmitted to humans or indeed are the cause of clinical or subclinical infections. Further studies of human sera are required. Nevertheless, tick-borne diseases are not uncommon in either the UK or Europe and increasing evidence of the pathogenic nature of rickettsiae in Europe suggests that we should not be complacent. R. helvetica, R. raoultii and R. massiliae have been implicated as pathogenic for humans. In humans, the pathogenicity of R. helvetica as a self-limiting illness associated with headache, myalgias, rash or eschar has been confirmed [Reference Nilsson, Lindquist and Pahlson26, Reference Chmielewski27]. It has also been linked with aneruptive fever [Reference Fournier28], sarcoidosis [Reference Nilsson29], meningitis in Sweden [Reference Nilsson, Elfving and Pahlson30] and fatal cases of acute perimyocarditis [Reference Nilsson, Lindquist and Pahlson26]. R. raoultii has recently been associated with TIBOLA/DEBONEL, along with R. slovaca [Reference Parola5]. The former appears to be associated with fever, painful eschar, painful adenopathies, headache, asthenia, and occasionally face oedema and rash. These studies suggest that although R. raoultii is perhaps less pathogenic than R. slovaca, the exposure to R. raoultii through a tick bite is probably more frequent than exposure to R. slovaca. High prevalence rates of R. raoultii in Dermacentor ticks in the UK appear to conform to findings in Europe [Reference Parola5, Reference Marquez31].
The R. massiliae-like 16S rRNA sequences found in I. ricinus only shared 97% homology with R. massiliae from Genbank. As other sequences of R. massiliae are unavailable in Genbank, it was not possible to compare the ITS and gltA from British ticks with R. massiliae.
The origin of all other rickettsial DNA sequences found in our tick lysates, including the R. typhi-like sequences is unknown and remains to be investigated. The sequences can also be derived from other sources than viable, pathogenic rickettsiae, e.g. from endosymbionts or environmental contamination [Reference Parola5, Reference Tijsse-Klasen23].
This study is preliminary, but these findings suggest that UK ticks could be harbouring a number of rickettsiae. Further studies are required to fully assess the UK distribution and prevalence of these rickettsiae in ticks and to ascertain the importance of these findings to UK public health.
DECLARATION OF INTEREST
None.







