Introduction
Varroa destructor Anderson and Trueman is the most serious ecto-parasitic mite that has significantly contributed to the decline of the Western honeybees (Apis mellifera L.), both wild and managed, particularly in Europe and North America (Neumann and Carreck, Reference Neumann and Carreck2010; Francis et al. Reference Francis, Nielsen and Kryger2013; Smith et al. Reference Smith2014; Kielmanowicz et al. Reference Kielmanowicz2015). The mite invaded A. mellifera colonies outside its native host range in Southeast Asia where it was originally restricted only to its natural host Apis cerana (reviewed in Nazzi and Le Conte, Reference Nazzi and Le Conte2016). The infestations by the mites can have significant negative effects on susceptible A. mellifera populations, especially the ones of European origin, mainly because they lack or poorly express the behavioural mechanisms displayed by the mite's original host to counter infestation (Ritter, Reference Ritter1981; Fries et al. Reference Fries1996). These behavioural mechanisms include: efficient hygienic behaviour (the ability of nurse honeybees to detect, uncap and remove dead or diseased/parasitized brood) and grooming behaviour (the ability of individual honeybees to remove mites off their bodies or from those of their nest mates thereby sometimes inflicting physical injuries to the mites during the removal process) as well as entombing of drone broods (Peng et al. Reference Peng1987; Boecking and Spivak, Reference Boecking and Spivak1999; Rath, Reference Rath1999). Additionally, the mite reproduces only in the less abundant and seasonally occurring drone brood in colonies of A. cerana, whereas its reproduction takes place in both drone brood and the more abundant worker brood which occurs throughout the breeding season in A. mellifera colonies (Rath, Reference Rath1999). As a result, beekeepers in the affected countries practice periodic miticide treatment to prevent the collapse of honeybee colonies within 1 or 2 years (Lee et al. Reference Lee2010; Neumann and Carreck, Reference Neumann and Carreck2010; Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010).
The reproductive cycle of Varroa mite takes place entirely in sealed brood cells and synchronizes with the sealed brood development time of the host larvae (Martin, Reference Martin1994). A foundress mite invades a worker brood cell shortly before it is capped and lays her first unfertilized egg, ~60–70 h following cell capping (Ifantidis, Reference Ifantidis1983; Martin, Reference Martin1994). This unfertilized egg develops into a male while the subsequent three to four fertilized eggs which are laid at approximately 30 h interval each develop into females (Ifantidis, Reference Ifantidis1983; Martin, Reference Martin1994). A mite can lay up to five eggs in worker brood and up to six eggs in drone brood (Martin, Reference Martin1994). It takes about 6 and 7 days for female and male mites, respectively, to develop into adults (Martin, Reference Martin1994). Mating between the mite's offspring occurs within the sealed brood cells once they reach adulthood with the male Varroa mite dying shortly afterwards. The foundress mites together with one or two viable, mature and mated daughter mites attach themselves to the honeybee that emerges from the cell leaving behind all immature mites which ultimately die inside the cells. Therefore, a foundress mite is considered to reproduce successfully when one or two viable, mature and mated daughter mites emerge from the cell during each reproductive cycle (Ifantidis, Reference Ifantidis1983; Martin, Reference Martin1994). Thus, the duration of the post-capping stage of worker brood and the mite offspring mortality in these cells are factors which can potentially influence the reproductive success of foundress mites (Martin, Reference Martin1994; Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010; Ardestani, Reference Ardestani2015). Alternatively, mites could be considered non-reproductive because they die in the cell without reproducing, produce no offspring, produce only male offspring or produce offspring that fail to reach maturity before the developing honeybee pupa hatches as an adult (Harbo and Harris, Reference Harbo and Harris1999). While reproducing inside the brood cells, the mite and her offspring feed on the fat body of the developing pupae and the foundress together with the mature female offspring continue to feed on the adult honeybee after emergence from the cells (Ramsey and VanEngelsdorp, Reference Ramsey, VanEngelsdorp and Simone-Finstrom2017). In the course of feeding, the mites can/often transmit lethal pathogens to the individual honeybee (Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010), which affects the individual honeybee physically and physiologically (Aronstein et al. Reference Aronstein2012; VanDooremalen et al. Reference VanDooremalen2012; Annoscia et al. Reference Annoscia2015).
However, some A. mellifera populations are reported to display behavioural mechanisms including hygienic and grooming behaviours and suppression of mite reproductive success which allow these honeybee populations to coexist with the mite for longer periods without requiring any in-hive miticide treatment (Peng et al. Reference Peng1987; Fries et al. Reference Fries1996; Calderón et al. Reference Calderón2010; Calderón et al. Reference Calderón, Urena and van Veen2012; Locke et al. Reference Locke2012; Strauss et al. Reference Strauss2013; Strauss et al. Reference Strauss2016). For example, previously we had shown that, the surviving African savannah honeybee, Apis mellifera scutellata (Lepeletier) in Kenya maintains a lower mite colony infestation (~3-fold lower) than their susceptible A. mellifera hybrids of European origin found in the USA (Nganso et al. Reference Nganso2017). Furthermore, they also express a higher grooming behaviour towards the mite than their European counterparts, although both honeybee subspecies express similar levels of hygienic behaviour. However, both hygienic and grooming behaviours could not explain the lower mite infestation levels recorded in A. m. scutellata colonies. Grooming behaviour was identified as a potential tolerant mechanism displayed by the African savannah honeybee towards infestation by the mite, suggesting that other resistant mechanisms such as suppression of mite reproduction might explain the lower mite population growth observed in colonies of the savannah honeybee. The suppression of the reproductive success of Varroa mite in the worker brood cells by A. mellifera populations is considered a crucial adaptive resistant mechanism (Fries et al. Reference Fries, Camazine and Sneyd1994; Harris et al. Reference Harris2003; Martin and Medina, Reference Martin and Medina2004; Mondragón et al. Reference Mondragón, Martin and Vandame2006). It explains the slow rate of mite population growth within their colonies and slight variations in this trait could underline resistance development towards the mite. The suppression of the mite reproductive output which translates into lower mite fertility, fecundity and reproductive success in worker brood cells has been found to explain honeybee resistance towards the mites in various populations. These populations include A. m. scutellata in South Africa (Strauss et al. Reference Strauss2016), Africanized honeybees in Brazil (Calderón et al. Reference Calderón, Urena and van Veen2012), the oldest Varroa tolerant European honeybee populations, A. m. ligustica in the island of Fernando de Noronha in North-eastern Brazil (Brettell and Martin, Reference Brettell and Martin2017), Avignon and Gotland honeybee populations in France and Sweden, respectively (Locke and Fries, Reference Locke and Fries2011; Locke et al. Reference Locke2012), the Russian honeybee population in the USA (de Guzman et al. Reference de Guzman, Rinderer and Frake2008) and the Norwegian honeybee population (Oddie et al. Reference Oddie, Dahle and Neumann2017). In the present study, we aimed to investigate mite reproduction in worker brood cells of A. m. scutellata to explain the low mite numbers recorded in their colonies.
Materials and methods
Study sites
The study was conducted in Nairobi, Kenya in November 2015 (the short rainy season), January 2016 and February 2018 (the hot dry season). The hot dry season is characterized by a drastic reduction or cessation in brood rearing while the short rainy season is characterized by increased brood rearing in savannah honeybee colonies (Raina and Kimbu, Reference Raina and Kimbu2005). All the colonies were housed in standard Langstroth hives containing 3–4 brood combs and were not treated with acaricides to reduce mite infestations.
Four and 14 (14 = 7 colonies used in each hot dry season) queen right colonies of A. m. scutellata were selected at an apiary in Kithimani (1°8′S, 37°25E) during the short rainy and hot dry season, respectively, while three colonies were selected at an apiary in Kilimanbogo (1°8′S, 37°21′E) during the short rainy season. Both apiaries are located within the county of Machakos and hosted A. m. scutellata colonies that originated from locally captured swarms (Hepburn and Radloff, Reference Hepburn and Radloff1988; Raina and Kimbu, Reference Raina and Kimbu2005; Muli et al. Reference Muli2014).
Assessment of Varroa mite reproduction in worker brood cells
To quantify Varroa mite reproductive output, we used the method described by Strauss et al. (Reference Strauss2016) with slight modifications. Briefly, 200 worker brood cells containing pupae at the molting stage were inspected in each colony (Martin, Reference Martin1994). All the colonies in each of the apiary were screened for brood at this stage and only positive colonies were used. These were four colonies in November 2015, seven colonies in January 2016, seven colonies in February 2018 at the apiary in Kithimani and three colonies in November 2015 at the apiary in Kilimanbogo. We used this stage because at the time of emergence of the young honeybees from the worker cells, the foundress mites have already completed their reproduction and it becomes easy to estimate their reproductive output. To determine Varroa mite reproduction, we initially generated count data on the number of foundresses, mature daughter mites, immature daughter mite and males in each infested cell. We used only singly infested cells to determine the reproductive success of the mites in worker brood cells of A. m. scutellata (Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010). For each infested cell, we further collected data on infertility (alive and dead foundresses with no offspring), fertility (production of offspring), fecundity (number of offspring produced), number of viable, mated and mature daughters and presence (alive and dead) or absence of adult males. The mating status of the daughter mites was determined by the simultaneous presence of one live mature daughter and one live adult male in a worker brood cell during an inspection of infested cells (Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010; Locke et al. Reference Locke2012; Strauss et al. Reference Strauss2016; Brettell and Martin, Reference Brettell and Martin2017). We also determined the fecundity and number of mature mated female offspring produced in cells infested by two or more foundress mites.
Assessment of the post-capping duration of worker brood
The duration of the post-capping stage of worker brood was determined in three colonies at the apiary in Kithimani. Two frames containing approximately 300 mature worker larvae prior to capping were removed from the central region of each colony and marked. Snap shots were taken to record the position of all sealed and unsealed worker broods after which the marked frames were returned to their colonies. The frames were then inspected twice a day (morning and evening) to record worker cells that were capped and monitored those until the honeybees emerged from the cells. A total of 657 worker brood cells were recorded in the savannah honeybee colonies. During each inspection period, photographs were taken. The number of brood that emerged from the worker cells and the number of days they took to emerge were recorded to determine the average duration of the sealed worker brood stage of A. m. scutellata through a thorough analysis of the photographs.
Statistical analysis
Statistical analyses were performed using R-Software version 3.2.5 (R Development Core Team, 2015) and the alpha level was set at 0.05 (Pirk et al. Reference Pirk2013). The generalized linear model (GLM) with logit link and binomial distribution error was used to examine the differences in the percentage of fertile and infertile foundress mites, and the percentage of foundress mites with viable mated daughter mites, unmated daughter mites and only male produced per cell and per foundress among the short rainy (November 2015) and hot dry seasons (January 2016 and February 2018) at the apiary in Kithimani. To compare the average number of offspring and mated daughter produced per cell and per foundress among the short rainy and hot dry seasons at the apiary in Kithimani, we used the GLM with log link and binomial distribution error. We also used the GLM with log link and binomial distribution error to compare the average number of offspring and mated daughter produced per cell and per foundress in worker cells infested by 1 or 2–4 foundresses in each season in the colonies of the African savannah honeybee.
Results
Assessment of Varroa mite reproduction in worker brood cells
Reproduction in singly infested cells
The patterns of Varroa mite reproduction during the different seasons of assessment in colonies of A. m. scutellata are presented in Tables 1 and 2.
Table 1. Comparison of the reproductive parameters of Varroa foundress mites produced per cell and per fertile foundress in singly infested worker brood cells in A. m. scutellata during the hot dry and short rainy seasons at the apiary in Kithimani, Kenya
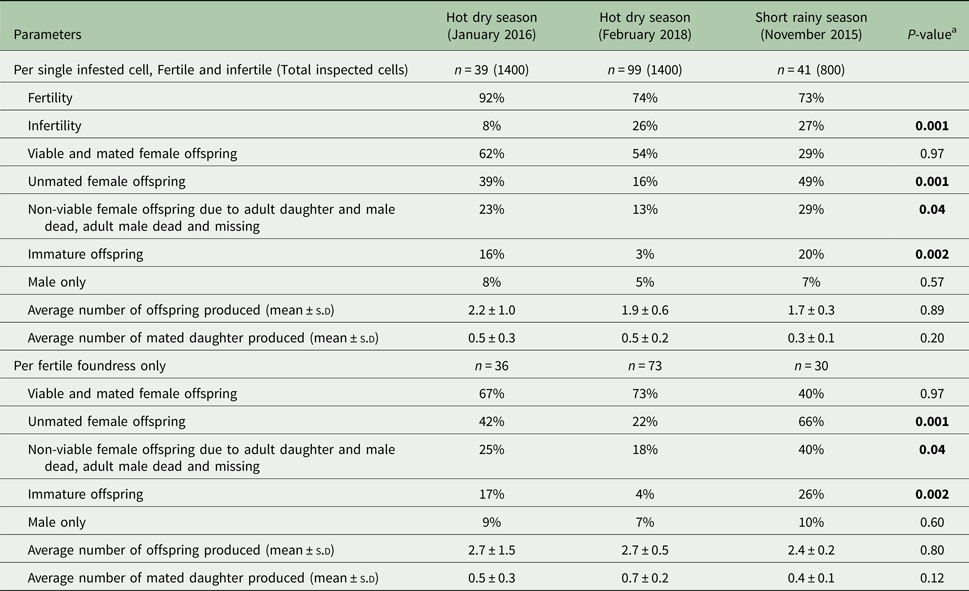
a p values were calculated by generalized linear model (GLM) with log and logit links.
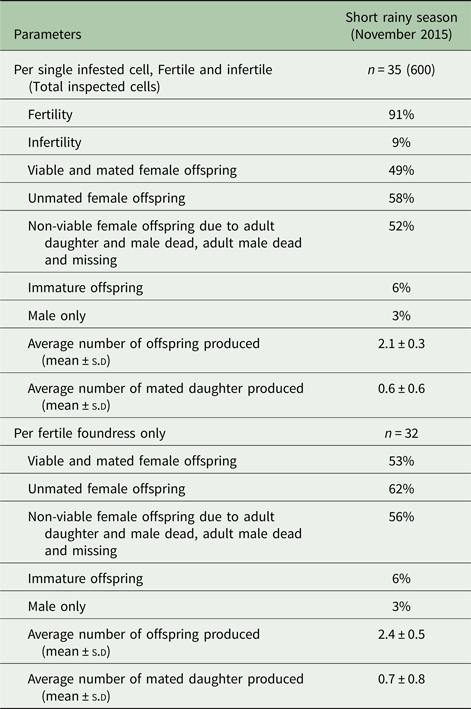
Table 2. Reproductive parameters of Varroa foundress mites produced per cell and per fertile foundress in singly infested worker brood cells in A. m. scutellata during the short rainy season at the apiary in Kilimanbogo, Kenya
The percentage of infertile mites was significantly lower during the hot dry season (January 2016) than the short rainy (November 2015) and hot dry (February 2018) seasons at the apiary in Kithimani (df = 16: χ2 = 0.64; P = 0.001, Table 1). However, there were no significant differences in the average number of offspring produced per cell (df = 16: χ2 = 0.02; P = 0.89, Table 1) and foundress (df = 16: χ2 = 0.07; P = 0.80, Table 1) and the average number of mated daughter mites produced per cell (df = 16: χ2 = 1.63; P = 0.20, Table 1) and foundress (df = 16: χ2 = 2.45; P = 0.12, Table 1) among these seasons at the same apiary. Likewise, there were no significant differences in the percentage of viable mated daughter mites produced per cell (df = 16: F = 0.002; P = 0.97, Table 1) and foundress (df = 16: F = 0.002; P = 0.97, Table 1) and the percentage of only male produced per cell (df = 4: χ2 = 0.33; P = 0.57, Table 1) and foundress (df = 4: χ2 = 0.28; P = 0.60, Table 1) among these seasons at the apiary in Kithimani. Furthermore, the percentage of unmated daughter mites produced per cell (df = 13: χ2 = 12.13; P = 0.001, Table 1) and foundress (df = 13: χ2 = 12.11; P = 0.001, Table 1) was significantly lower during the hot dry season (February 2018) than the short rainy (November 2015) and hot dry (January 2016) seasons at the apiary in Kithimani.
Reproduction in multiply infested cells
During the hot dry season (January 2016) at the apiary in Kithimani, the mites reproduced in all the 9 cells infested with 2 live foundresses and a total of 34 offspring were produced, with 3.8 ± 0.3 (mean ± s.d) offspring produced per cell (Fig. 1A). There was no significant difference in the average number of offspring produced per cell (df = 10: χ2 = 1.46; P = 0.23) and per foundress (df = 10: χ2 = 2.45; P = 0.12) as well as, the average number of mated daughter produced per foundress (df = 10: χ2 = 0.70; P = 0.40) between multiply and singly infested worker cells (Fig. 1A). However, the average number of mated daughter produced per cell was significantly higher in multiply infested worker cells than in singly ones (df = 10: χ2 = 5.07; P = 0.02) (Fig. 1A).
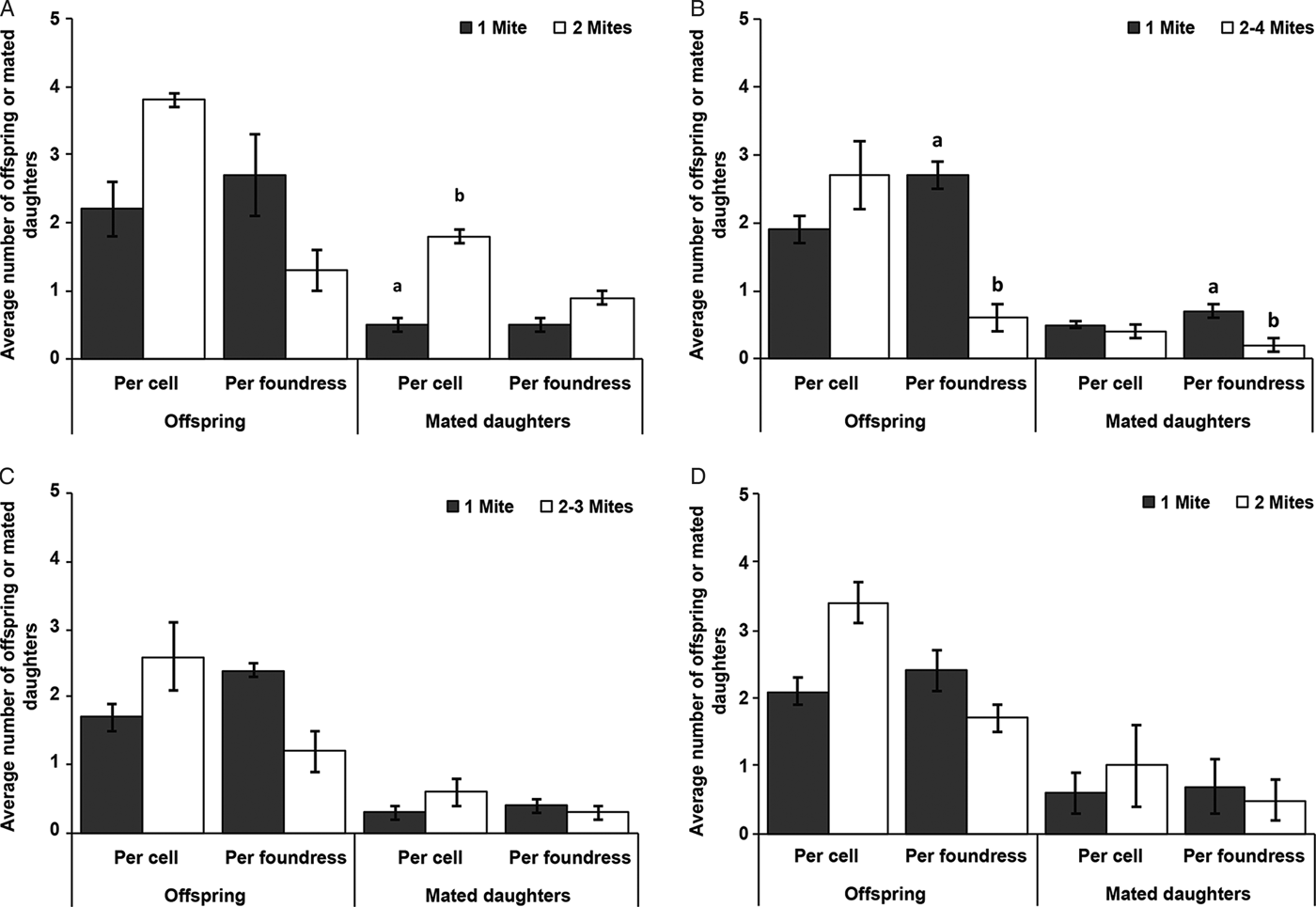
Fig. 1. The average number of offspring and mated daughters (mean ± s.e.) produced per cell and per foundress in singly and multiply infested worker brood cells in A. m. scutellata during the hot dry seasons (January 2016 and February 2018) at the apiary in Kithimani (A) and (B) respectively, short rainy season (November 2015) at the apiary in Kithimani (C) and short rainy season (November 2015) at the apiary in Kilimanbogo (D). Only fertile foundresses were considered. Pair of bars with letters indicates significant effects for each category.
During the hot dry season (February 2018) at the apiary in Kithimani, the mites reproduced in 62 out of the 64 cells infested with 2–4 live foundresses and a total of 170 offspring were produced, with 2.7 ± 1.4 (mean ± s.d) offspring produced per cell (Fig. 1B). There was no significant difference in the average number of offspring (df = 12: χ2 = 0.36; P = 0.55) and the average number of mated daughter (df = 12: χ2 = 0.0; P = 1) produced per cell between multiply and singly infested worker cells (Fig. 1B). However, the average number of offspring (df = 12: χ2 = 9.64; P = 0.002) and the average number of mated daughter (df = 12: χ2 = 9.70; P = 0.002) produced per foundress were significantly lower in multiply than singly infested worker cells (Fig. 1B).
During the short rainy season (November 2015) at the apiary in Kithimani, there was reproduction in 10 out of the 11 worker cells infested with 2–3 live foundresses and a total number of 26 offspring were produced, with 2.6 ± 1.0 (mean ± s.d) offspring produced per cell (Fig. 1C). There was no significant difference in the average number of offspring produced per cell (df = 6: χ2 = 1.33; P = 0.25) and per foundress (df = 6: χ2 = 1.97; P = 0.16) as well as, the average number of mated daughter produced per cell (df = 6: χ2 = 1.05; P = 0.31) and per foundress (df = 6: χ2 = 0.0; P = 1) between multiply and singly infested worker cells (Fig. 1C).
During the short rainy season (November 2015) at the apiary in Kilimanbogo, the mites reproduced in all the 8 worker cells infested with two live foundresses and a total of 27 offspring were produced, with 3.4 ± 0.5 (mean ± s.d) offspring produced per cell (Fig. 1D). There was no significant difference in the average number of offspring produced per cell (df = 4: χ2 = 0.53; P = 0.47) and per foundress (df = 4: χ2 = 0.08; P = 0.78) as well as, the average number of mated daughter produced per cell (df = 4: χ2 = 0; P = 1) and per foundress (df = 4: χ2 = 0.2; P = 0.65) between multiply and singly infested worker cells (Fig. 1D).
Assessment of the post-capping duration of worker brood
The average duration of the post-capping developmental time of A. m. scutellata worker brood was 265.2 ± 0.04 h.
Discussion
Mite reproduction in singly infested worker cells
In colonies of the African savannah honeybee, we recorded a higher infertility rate for the mites during the short rainy (November 2015) and the hot dry (February 2018) seasons which are characterized by increased and reduced brood rearing, respectively, at the apiary in Kithimani (26–27%). In contrast, a lower infertility rate of the mites was recorded during the hot dry season (January 2016) at the same apiary (8%) which was similar to the infertility rate recorded during the short rainy season at the apiary in Kilimanbogo (9%). The amount of brood present in honeybee colonies is a host feature that is known to significantly influence the fertility and the population dynamic of the mites (Lodesani et al. Reference Lodesani, Crailsheim and Moritz2002). It appears that when brood is available in the colonies, features of the mites such as the reproductive capacity during their lifetime and lifespan might also influence their reproductive rate and population dynamics in honeybee colonies (Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010). Despite the variability in the fertility rates of the mites observed in worker brood cells of A. m. scutellata, the reproductive success of foundress mites remained similar to those reported in other surviving honeybee populations (Medina and Martin, Reference Medina and Martin1999; Locke and Fries, Reference Locke and Fries2011; Calderón et al. Reference Calderón, Urena and van Veen2012; Locke et al. Reference Locke2012; Strauss et al. Reference Strauss2016; Brettell and Martin, Reference Brettell and Martin2017; Oddie et al. Reference Oddie, Dahle and Neumann2017). Thus, these results suggest a strong suppression of mite reproduction in worker brood cells of A. m. scutellata in Kenya and this could be a plausible explanation for the low mite numbers recorded previously in colonies of this honeybee subspecies (Nganso et al. Reference Nganso2017).
In this study, we found that the post-capping duration of worker brood of A. m. scutellata could not explain the lower reproductive success of the mites recorded in their colonies. Up to 3–5 eggs were laid and 1–2 viable, mature and mated daughter mites emerged in worker brood cells of this honeybee subspecies. This finding suggests that when oviposition is initiated, up to five eggs are laid and there is sufficient time for one and sometimes two daughter mites to emerge from the worker cells of A. m. scutellata according to Varroa developmental charts (Martin, Reference Martin1994). Interestingly, we identified high infertility rates (26–27%) and percentage of unmated female offspring (39–58%) as well as low fecundity (1.7–2.2, mean number of eggs laid) as exciting parameters that appears to explain the lower mite reproductive success in colonies of the savannah honeybee studied herein (Tables 1 and 2). These parameters seem to interact with one another during different seasons to reduce the number of viable female offspring produced in worker brood cells of the African savannah honeybee. The low mite fecundity recorded in this study was similar to those reported in worker brood cells of the surviving A. m. scutellata population in South Africa (1.7 ± 0.3, mean ± s.d) (Strauss et al. Reference Strauss2016); though it is much lower than those reported in other surviving or susceptible honeybee populations (3.1–4.9, mean number of eggs laid) (Medina and Martin, Reference Medina and Martin1999; Martin, Reference Martin2001; Alattal et al. Reference Alattal, Rosenkranz and Zebitz2006; Locke and Fries, Reference Locke and Fries2011; Calderón et al. Reference Calderón, Urena and van Veen2012; Locke et al. Reference Locke2012; Brettell and Martin, Reference Brettell and Martin2017). Also, an increase in the percentage of infertile mites over time (from 13 to 30%) has been reported as a parameter that suppresses the mite reproduction in worker brood cells of the surviving A. m. scutellata population in South Africa (Martin and Kryger, Reference Martin and Kryger2002; Strauss et al. Reference Strauss2016). Furthermore, we identified offspring mortality for both sexes and absence (missing) of male offspring as key factors that appear to be responsible for the high number of unmated daughters produced in the African savannah honeybee colonies (23–52%). Mite offspring mortality has also been reported as a major factor that accounts for the lower mite reproductive output and population growth in the surviving Africanized honeybee colonies in Brazil; despite the fact that the fertility of the mites is currently reported to be at the same level as in European honeybee colonies (Mondragón et al. Reference Mondragón, Martin and Vandame2006; Calderón et al. Reference Calderón2010; Calderón et al. Reference Calderón, Urena and van Veen2012). Offspring mortality or absence (missing) within the worker brood cells has been reported to be due to failure to locate the single feeding site established by the foundress mite on the developing honeybee brood and the disturbance or damage of the first egg which is usually male when the pre-pupae molts into pupae, respectively (Donzé and Guerin, Reference Donzé and Guerin1994; Donze et al. Reference Donze1996; Calderón et al. Reference Calderón2010; Calderón et al. Reference Calderón, Urena and van Veen2012).
Mite reproduction in multiply infested cells
The reproduction of mites in multiply infested cells can also influence their reproductive success and population growth in honeybee colonies (Rosenkranz et al. Reference Rosenkranz, Aumeier and Ziegelmann2010). In this study, we observed that the number of offspring produced per individual mite in multiply infested cells was generally lower than those produced in singly infested cells in A. m. scutellata colonies though the difference was only significant during the hot dry season (February 2018) (Fig. 1). Additionally, there was a general reduction in the number of female offspring produced per foundress in multiply than singly infested cells in colonies of this honeybee subspecies though the difference was only significant during the hot dry season (February 2018) (Fig. 1). However, the number of female offspring produced per cell was generally higher in multiply than singly infested cells in the savannah honeybee colonies though the difference was only significant during the hot dry season (January 2016) (Fig. 1). In multiply infested cells where competition for food resources is expected, the fecundity and reproductive success of individual mites is generally reduced compared with those of singly infested cells (Fuchs and Langenbach, Reference Fuchs and Langenbach1989; Martin, Reference Martin1995; Martin and Medina, Reference Martin and Medina2004; Mondragón et al. Reference Mondragón, Martin and Vandame2006). The higher reproductive success of the mites recorded in multiply infested cells in this study might be due to the lower incidence of offspring mortality and absence recorded in multiply infested cells than those of singly infested cells (Strauss et al. Reference Strauss2016). Moreover, daughter mites have a greater chance to mate successfully before emerging from multiply infested cells because more than one adult male can be produced (Martin, Reference Martin1995). In this study, however, only a single male offspring was produced in all multiply infested cells of A. scutellata. Therefore, the probability that all the daughter mites produced in these cells will receive sufficient sperms before emerging from the cell is questionable. Hence, though the reproductive success of mites remains high in these cells, there could be a chance that not all the daughter mites will receive sufficient sperm from the male before emerging from the cell (Donze et al. Reference Donze1996; Wendling et al. Reference Wendling2014). Our findings corroborate results of a previous study which also reported a significant reduction in the number of offspring produced per individual mite in multiply infested worker cells compared to singly infested ones; though the number of mated daughters produced per cell was higher in multiply infested cells compared to singly infested cells in A. m. scutellata colonies in South Africa (Strauss et al. Reference Strauss2016).
In conclusion, the A. m. scutellata population studied herein showed evidence of resistance towards mite attack. This translates into the strong suppression of the mite reproductive success recorded in worker brood cells. This lower reproductive output was mainly due to the high mite infertility rates and percentage of unmated daughter mites as well as low mite fecundity recorded in infested cells of A. m. scutellata. The mortality of adult male and female offspring and the absence (missing) of male offspring in a considerable number of worker brood cells were identified as major factors responsible for the lower production of mated daughters in the savannah honeybee colonies. The consistency of results regarding mite reproduction in two geographically distinct A. m. scutellata populations (South Africa, Strauss et al. Reference Strauss2016 and Kenya, this study) suggests general adaptations towards V. destructor within African honeybees, most likely due to the higher number of wild colonies and lack of miticide use in their colonies (Pirk et al. Reference Pirk, Crewe and Moritz2017). Nonetheless, because the number of multiply infested cells recorded in this study was low, we recommend that the data should be treated with caution. We recommend further verification of the reproductive values of the mites obtained herein in other A. m. scutellata populations distributed in other climatic zones in Africa to help shed more light on the evolution of tolerance and resistance mechanisms towards Varroa mites.
Acknowledgments
We would like to thank C. Nzuki, and S. Mulaeh in Kenya and Dr James Ellis at the University of Florida, USA for availing their apiaries for this study. We are also grateful to Muema Wilson and Munyao Mutemwa, Onyimbo Nixon, Kenya and Bryan Smith, USDA/ARS- CMAVE for their assistance in the field work.
Financial support
We gratefully acknowledge the financial support for this research by the following organizations and agencies: US Department of Agriculture (USDA)/ARS- Grant # 58-6615-3-011-F; UK's Department for International Development (DFID); Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); and the Kenyan Government. Immense gratitude to the German Academic Exchange Service (DAAD) In-Region Scholarship for funding the research work through a PhD fellowship at the International Centre of Insect Physiology and Ecology (icipe) and the Office of International Research Programs at USDA-ARS for providing the financial support needed for the research conducted in the USA.
Conflicts of interest
None
Ethical standards
Not applicable








