INTRODUCTION
The global incidence of typhoid fever was estimated to be about 21 650 000 illnesses in 2000 and varies greatly between regions. Typhoid fever incidence is highest among infants and children living in South Central and South East Asia [Reference Crump, Luby and Mintz1]. Typhoid fever is a severe systemic illness that is characterized by sustained fever, systemic toxicity and abdominal pain [Reference Mandell, Bennett and Dolin2]. S. Typhi colonizes only humans. The disease is acquired by consuming water or food contaminated by the faeces of a person who has typhoid fever or who is a carrier [Reference Heymann3]. While transmission via sexual relations between men has also been reported [Reference Reller4], true person-to-person transmission is rare. The ID50 of S. Typhi is about 106 organisms by ingestion, but varies considerably with gastric acidity. The risk of infection increases as the size of the inocula increases [Reference Hornick and Woodward5].
Salmonella enterica subspecies enterica serotype Typhi (Salmonella Typhi) belong to the family of Enterobacteriaceae and are Gram-negative, motile, non-lactose-fermenting bacilli. Although most S. enterica serotypes cannot be distinguished by biochemical reactions, S. Typhi can be provisionally identified by its production of only trace amounts of hydrogen sulphide and being less active biochemically than other serotypes within the subspecies. Confirmation depends on the detection of surface antigens using specific antisera. S. Typhi belongs to Salmonella serogroup D and possesses somatic antigen O9, a single flagellar antigen Hd, and the polysaccharide virulence antigen Vi. Resistance to traditional first-line antimicrobial agents, such as ampicillin, chloramphenicol, and trimethoprim–sulfonamide combinations has emerged worldwide. More recently isolates with reduced susceptibility to fluoroquinolones and third-generation cephalosporins (i.e. ceftriaxone) have been increasingly recovered [Reference Murray6].
Building on our previously published work on typhoid fever epidemiology [Reference Crump, Luby and Mintz1], we conducted a review of the recent scientific literature to identify contemporary gaps in the understanding of the global epidemiology of typhoid fever. An improved understanding of typhoid fever epidemiology would allow policy-makers to decide how to allocate resources to typhoid fever vs. other health priorities and would allow health officials to select and monitor the effectiveness of public health interventions. We summarize below recent available data on the morbidity and mortality burden, the age, and the geographic and temporal distribution of typhoid fever. To provide context to the global epidemiology of typhoid fever, we also describe typhoid diagnostics and pathogen-specific preventive measures. Finally, data gaps and existing research needs are discussed.
METHODS
We systematically searched the English-language scientific literature published between 1984 and 2005 using the Medline database, restricting the search to low and medium human development countries according to the United Nations Development Programme's Human Development Index (HDI) (http://hdr.undp.org/2004; accessed 21 March 2005). A set of articles including the relevant epidemiological terms were cross-linked with a set of articles including the relevant pathogen-specific terms (Table 1). The resulting cross-linked set was reviewed for publications addressing typhoid fever morbidity, mortality, age distribution, geographic distribution, temporal distribution, pathogen-specific preventive measures, and diagnostics. Particularly for morbidity and mortality burden, population-based studies with culture confirmation of cases were considered primary data sources. When these were limited, hospital- and clinic-based studies were included. Publications were then evaluated for their contribution to an understanding of the global epidemiology of typhoid fever, and gaps in the data were identified.
Table 1. Terms used in literature search to identify gaps in data on enteric disease burden

Crude incidence figures are given, without correction for sensitivity of blood culture, or for the proportion of febrile episodes for which blood-culture specimens were not collected. Publications were sought with information on the incidence of the infection by region according to the 21 regions of the United Nations Department of Social and Economic Affairs, Population Division (http://esa.un.org/unpp/index.asp?panel=5; 2004 revision, accessed 4 October 2005). We examined the correlation between the incidence of typhoid fever and the national per capita gross domestic product (GDP), adjusted for purchasing power parity (PPP) in year 2000 dollars, at the time that the studies were conducted (http://unstats.un.org/unsd/cdb/cdb_series_xrxx.asp?series_code=29922; accessed 28 July 2006). We searched for publications with information on the age-specific incidence, morbidity, and mortality.
RESULTS
Morbidity
Incidence
The most recent published estimate of the global burden of typhoid fever determined that 21 650 000 illnesses occurred worldwide in 2000 [Reference Crump, Luby and Mintz1] and that the incidence was highest in South and South East Asia and among infants and young children (Figs 1 and 2).
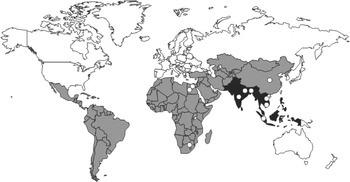
Fig. 1. Geographic distribution of population-based studies of typhoid
fever incidence. (Adapted from Crump et al. [Reference Crump, Luby and Mintz1].) ■, High incidence
(>100 episodes/100 000 per
year); ![]() , Medium incidence
(10–100 episodes/100 000 per
year);
, Medium incidence
(10–100 episodes/100 000 per
year); ![]() , Low incidence
(<10 episodes/100 000 per
year); □, region with human
development index (HDI)
countries; ○, site of contributing disease
incidence study.
, Low incidence
(<10 episodes/100 000 per
year); □, region with human
development index (HDI)
countries; ○, site of contributing disease
incidence study.
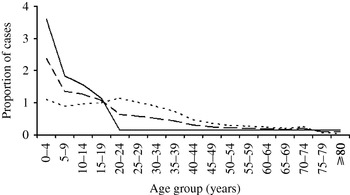
Fig. 2. Age-specific incidence of typhoid fever (from Crump et al. [1]). —, High (>100/100 000 per year); – – –, medium (10–100/100 000 per year); - - - -, Low (<10/100 000 per year).
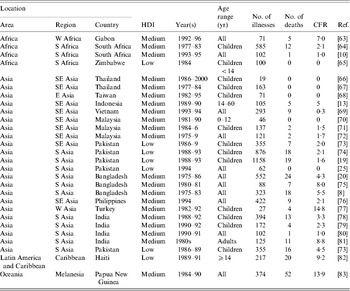
A computer search of the English scientific literature published between 1984 and 2005 from low- and medium-HDI countries for population-based typhoid fever incidence studies using blood culture confirmation of cases yielded 10 publications (Table 2); three studies from Africa (South Africa and Egypt) and seven from Asia (Bangladesh, China, India, Indonesia, Nepal, and Vietnam). Five (50%) of these publications were reports of vaccine studies and all 10 were from medium-HDI countries. Crude typhoid fever incidence in these studies ranged from 13 to 976 illnesses/100 000 persons per year. Five studies included subjects of all ages; the remainder were restricted to persons within specific age ranges. Because of limitations of blood culture sensitivity for typhoid fever (Table 3) and variation of typhoid fever incidence with age (Fig. 2), it would be appropriate to adjust crude incidence for these factors when attempting to extrapolate typhoid fever incidence to the global population [Reference Crump, Luby and Mintz1].
Table 2. Population-based studies of typhoid fever incidence 1984–2005
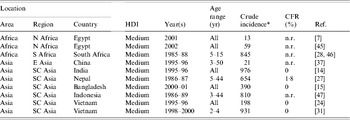
HDI, Human development index; CFR, case-fatality rate; n.r., not reported.
* Per 100 000 persons per year.
Studies excluded for high human development index country: 2 from Chile [Reference Levine48, Reference Levine49].
Studies excluded for pre-1984: 1 from Chile [Reference Black50], 2 from Egypt [Reference Wahdan51, Reference Wahdan52]; 1 from India [Reference Chuttani53], 1 from Poland [54], 4 from the USSR [Reference Hejfec55–Reference Hejfec58], 2 from Yugoslavia [59, 60], 1 from Guyana [Reference Ashcroft61], and 1 from Tonga [Reference Tapa and Cvjetanovic62].
Among the 10 population-based studies, there was a non-significant relationship between typhoid fever incidence and per capita GDP (Fig. 3) (R=−0·04, P=0·9115). One study that showed high typhoid incidence was done in South Africa, a country with a relatively high GDP but with considerable maldistribution of wealth. When this outlier study was excluded, a non-significant trend towards an inverse relationship between typhoid fever incidence and per capita GDP is seen (R=−0·63, P=0·0717).

Fig. 3. Incidence of typhoid fever by per capita gross domestic product (adjusted for purchasing power) of low- and medium-HDI countries, 1984–2005 (n=10 studies).
Complications
Intestinal perforation is an important complication of typhoid fever. Unfortunately, no population-based studies that use blood culture confirmation of S. Typhi infection report the rates of this or other complications. Hospital-based studies capture information on the most severe illnesses among persons who have access to health-care services and are likely to overestimate the true complication rate substantially [Reference Crump7]. A review of intestinal perforation rates in typhoid fever series from all countries from the pre-antibiotic era before 1950 indicate median (range) rates of 1·9% (0·8–6·3%). The same review shows a rate of intestinal perforation in developed countries of 0% (0–0%) in the post-antibiotic era after 1950 and in developing countries in the post-antibiotic era after 1950 of 3·6% (0·1–41%) [Reference Butler8]. Case-series published between 1984 and 2005 from low- and medium-HDI countries report median (range) intestinal perforation rates of 2·8% (0·6–4·9%) (Table 4). Risk factors for intestinal perforation in typhoid fever include male gender, older age, inadequate antimicrobial therapy, and short duration of symptoms [Reference Butler8–Reference Khan10].
Other complications occur, but their rates are not well quantified in either population- or hospital-based studies. A recent review of typhoid fever complications involving specific organ systems indicated that they occur with the following frequencies in hospital series: central nervous system (encephalopathy, cerebral oedema, subdural empyema, cerebral abscess, meningitis, ventriculitis, transient Parkinsonism, motor neuron disorders, ataxia, seizures, Guillain–Barré syndrome, psychosis) 3–35%; cardiovascular system (endocarditis, myocarditis, pericarditis, arteritis, congestive cardiac failure) 1–5%; pulmonary system (pneumonia, empyema, bronchpulmonary fistula) 1–6%; bone and joint (osteomyelitis, septic arthritis) <1%; hepatobiliary system (cholecystitis, hepatic abscess, splenic abscess, peritonitis, paralytic ileus); 1–26%, and the genitourinary system (urinary tract infection, renal abscess, pelvic infection, testicular abscess, prostatitis, epididymitis) <1%. Soft tissue infections (psoas abscess, gluteal abscess, cutaneous vasculitis) and haematological complications (haemophagocytic syndrome) are rare [Reference Huang and DuPont11].
While the geographic distribution of typhoid fever and HIV overlap, no studies have comprehensively evaluated the effect of HIV on typhoid fever incidence or complications.
Epidemic vs. endemic disease
Large typhoid fever outbreaks make substantial contributions to the local disease burden. For example, during a large waterborne outbreak of typhoid fever in Tajiskistan during 1997 more than 1200 cases of typhoid fever were detected each week during the epidemic's peak in the city of Dushanbe which had a population of about 600 000 people [Reference Mermin12]. While large outbreaks occur, endemic typhoid fever is thought to be responsible for most typhoid fever illnesses worldwide. However, we were unable to find population-based typhoid fever surveillance studies of sufficient duration to capture incidence before, during, and after typhoid fever epidemics. Therefore, the relative contribution of epidemics to disease burden is not known.
Mortality
Five (50%) of 10 population-based studies conducted between 1984 and 2005 and representing 64 338 person-years of follow-up report mortality data. These five studies showed median (range) case-fatality rates (CFRs) of 0% (0–1·8%) (Table 2). There are several limitations to generalizing CFRs for typhoid fever from these studies. In all studies ethical requirements governing human subject research would have optimized the clinical management of patients found to have typhoid fever probably leading to lower CFRs than may be seen in the source population. Second, these studies were not designed to assess mortality as a primary outcome.
Hospital-based series provide another way to estimate typhoid fever mortality, but only capture information on the most severe illnesses among persons who have access to health-care services [Reference Crump7]. The literature search identified 28 hospital-based studies reporting CFRs (Table 5): four studies from Africa, 22 from Asia, and one each from Latin America/Caribbean and Oceania. The median (range) CFR in these studies was 2·0% (0–14·8%).
Table 3. Comparison of diagnostic tests for typhoid fever

* Varies according to sample volume.
† Varies according to carrier prevalence in study population.
A review of published typhoid fever series from the pre-antibiotic era before 1950 showed median (range) CFRs of 11% (7·5–19%). The same review shows a median (range) CFR for typhoid fever in developing countries in the post-antibiotic era after 1950 of 6·1% (0·1–41%) [Reference Butler8].
Appropriate antimicrobial therapy is known to reduce mortality from typhoid fever [Reference van den Bergh13]. A consequence of the emergence of antimicrobial resistance in S. Typhi is that empiric treatment choices for typhoid fever often include antimicrobial agents to which the infecting organism is resistant. This is especially challenging in settings where laboratory capacity to isolate S. Typhi from blood is absent or inadequate and the prevalence of resistant S. Typhi at both the individual and population level is unknown.
Table 4. Hospital-based studies of typhoid fever intestinal perforation, 1984–2005
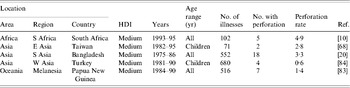
HDI, Human development index.
Age distribution
Two contemporary population-based studies address the age distribution of typhoid fever and suggest that, in areas highly endemic for typhoid fever, infection is more common in infants and pre-school children than in older persons [Reference Sinha14, Reference Brooks15]. The age distribution of typhoid fever has been modelled for typhoid fever at high (>100/100 000 per year), medium (10–100/100 000 per year), and low (<10/100 000 per year) incidence countries using data from studies evaluating the age distribution of the infection [Reference Sinha14, Reference Brooks15] and using surveillance data [Reference Crump7]. The resulting age distribution curves are reproduced in Figure 2 [Reference Crump, Luby and Mintz1].
No population-based study has evaluated age-specific mortality for typhoid fever. Although by no means benign [Reference Brooks15], typhoid fever is thought to be less severe in children [Reference Ferreccio16–Reference Arora18] than in adults. Hospital in-patient series show that infants are at greater risk of dying from typhoid fever than older children [Reference Bhutta19, Reference Butler20].
Geographic distribution
The best summary of the geographic distribution of typhoid fever has been modelled from population-based incidence studies (Table 2 and earlier studies), adjusted for age-specific incidence curves (Fig. 2), and diagnostic test sensitivity (Table 3). The resulting data on geographic distribution of typhoid fever are summarized in Figure 1 [Reference Crump, Luby and Mintz1]. The very few contemporary studies from which this figure was developed are illustrated with open symbols (○). Based on these data sources, most typhoid fever illnesses occur among infants and young children in South and South East Asia.
Table 5. Hospital-based studies of typhoid fever mortality, 1984–2005
HDI, Human development index; CFR, case-fatality rate.
Temporal distribution
The incidence of typhoid fever is reputed to follow seasonal patterns [Reference Hunter, Strickland and Magill21]. In some locations a peak occurs in the hot, dry months of the year. This is often attributed to a concentration of organisms in water when supply is inadequate due to lack of rain. However, in other places, a peak is reported during the rainy season. This is often attributed to a breakdown in the systems that separate sewage from drinking water. A review of typhoid fever seasonality publications between 1984 and 2005 confirms that both seasonal patterns are reported [Reference Battikhi22–Reference Mirza, Beeching and Hart25].
Pathogen-specific preventive measures
Three licensed vaccines are available to prevent typhoid fever; parenteral inactivated whole-cell vaccines, oral attenuated S. Typhi Ty21a vaccine, and parenteral Vi capsular polysaccharide vaccine. These vaccines confer about 70% protection in older children and adults and do not protect young children or infants [Reference Ivanoff, Levine and Lambert26–30]. More recently a conjugate vaccine has been developed that binds Vi to a non-toxic recombinant protein that is antigenically identical to Pseudomonas aeruginosa exotoxin A (Vi-rEPA). In a clinical trial in Vietnam, the vaccine showed 90% efficacy in children aged 2–5 years [Reference Lin31] and this level of protection was sustained for more than 3 years after vaccination [Reference Lanh32]. To date, routine typhoid fever vaccination has rarely been adopted in typhoid-endemic countries [Reference Bodhidatta33]. Reasons include cost, lack of protection for young children, difficulties integrating currently available typhoid vaccines with current expanded programme on immunization (EPI) schedules, and the need for repeated booster doses.
Non-vaccine measures for typhoid fever prevention can be divided into community measures and individual measures [Reference Heymann3]. Community measures for typhoid fever prevention include promotion of hand washing; sanitary disposal of human faeces; provision of safe drinking water; sanitary food preparation; pasteurization or boiling of milk and dairy products; quality control procedures for the food industry; and shellfish sanitation. Individual measures for typhoid fever prevention include education of patients, convalescents, and carriers in personal hygiene; breastfeeding throughout infancy; exclusion of typhoid carriers from food handling and from patient care provision; and provision of typhoid fever vaccine to persons at high risk due to occupation or travel.
Commonly recommended measures for typhoid fever control include reporting illnesses to health authorities, use of enteric precautions for ill patients until no fewer than three stool samples have proved negative for S. Typhi, safe disposal of faeces, urine, and soiled articles, identification and management of the source of infection, identification and screening of exposed individuals, and specific treatment of ill persons and carriers with an effective antimicrobial agent, most often a fluoroquinolone. Identification of chronic asymptomatic carriers through serological screening of the general population in an endemic area appears not to be useful [Reference Gupta34]. Typhoid fever epidemics are managed by epidemiological identification of the source, disinfection of drinking water, followed by control measures directed to the epidemiologically identified source, e.g. intensive search for the case or carrier, selective elimination of suspected contaminated food, pasteurization or boiling of milk [Reference Heymann3]. In a large waterborne epidemic of typhoid fever in Dushanbe, Tajikistan, implementation of chlorination of the city water supply lead to a dramatic reduction in typhoid fever incidence [Reference Mermin12]. The introduction of widespread sewage treatment in Santiago, Chile, in 1992 was associated with a 90% reduction of typhoid fever and hepatitis A [Reference Cabello and Springer35, Reference Wolff36]. Although currently not routinely recommended, one study shows that Vi vaccine deployed in a typhoid fever epidemic provided 71% protection against infection [Reference Yang37].
Case-control studies indicate that the predominant route of transmission of typhoid fever varies both in time and location. For example, in Tajikistan after independence from the Soviet Union deterioration of the municipal water treatment and distribution system led to lack of chlorination, equipment failure, and back-siphonage. This was associated with widespread consumption of faecally contaminated water and a massive typhoid fever outbreak in 1997 [Reference Mermin12]. In Pakistan, eating ice cream, eating food from a roadside cabin during the summer months, taking antimicrobials during the 2 weeks before illness onset, and drinking water at the worksite were independently associated with typhoid fever [Reference Luby38]. In Indonesia, recent typhoid fever in the household, not using soap for hand washing, sharing food from the same plate, and no toilet in the household were independent risk factors for typhoid fever [Reference Vollaard39]. The fraction of typhoid fever illness attributable to water, specific foods, or to typhoid fever carriers is poorly characterized both globally and at the local and regional levels. The lack of data on attribution limits the ability of policy-makers and public health officials to plan for transmission-based interventions to interrupt typhoid fever.
Diagnostics
The gold standard test for diagnosis of typhoid fever remains the culture of bone marrow. The sensitivity of this test exceeds 90% and the specificity is 100%, assuming that bacterial identification methods are performed correctly [Reference Gilman40]. Because obtaining samples for culture of bone marrow is invasive and requires technical expertise and equipment, blood culture is more often used for diagnosis even though the sensitivity of a single culture is about 40% [Reference Gilman40–Reference Wain42]. Blood culture is more effected by prior antimicrobial use than bone marrow culture [Reference Wain42]. The sensitivity of blood culture for typhoid fever is improved by collection of larger volumes of blood [Reference Wain42].
The surface antigens of S. Typhi are shared by many other salmonellae and with other bacterial pathogens. This hampers specific serological diagnosis. Commercially available serological assays such as Multi-Test Dip-S-Ticks (PANBIO INDX Inc., Baltimore, MD, USA), TyphiDot (Malaysian Biodiagnostic Research SDN BHD, Singapore, Malaysia), and TUBEX (IDL Biotech, Sollentuna, Sweden) all have shortcomings of both sensitivity and specificity, with the Widal test performing the worst of all (Table 3).
DISCUSSION
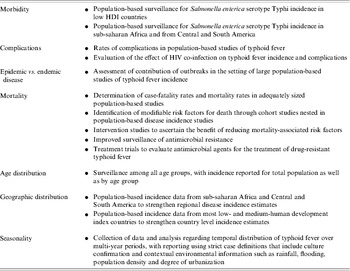
This review highlights the large gaps in data on the burden of typhoid fever infections for low- and medium-HDI countries (Table 6). It also identifies additional research needs with respect to the burden of disease among infants and population-based rates of typhoid fever complications and mortality. Comprehensive microbiological, clinical, and population-based epidemiological studies using blood culture confirmation of cases or using novel study designs with alternative diagnostic methods could help to address these data gaps and further inform prevention. However, the implementation of such research would lead to and would require improvements in patient management that could be expected to reduce case fatality and complication rates.
Table 6. Data gaps and research needs for typhoid fever in low- and medium-HDI countries
HDI, Human development index.
Contemporary population-based studies using blood culture confirmation of cases are lacking or are extremely limited in all socioeconomic groups and geographic regions. Data are particularly limited for populations living in low-HDI countries and those in sub-Saharan Africa and Central and South America, where laboratory diagnostic capacity for typhoid fever may not be widely available. Of the 10 contemporary, population-based studies of typhoid fever that evaluate disease incidence using blood culture for confirmation of cases the reported incidence ranges from 13 to 976/100 000 persons per year. These studies are likely to have been done preferentially in high-incidence sites that make generalization of results from a single location to a whole country or region difficult. Only two studies report typhoid fever incidence simultaneously in different age groups making the burden among infants and young children difficult to estimate [Reference Sinha14, Reference Brooks15]. Preliminary evidence that the age distribution of typhoid fever varies according to disease incidence needs to be confirmed by population-based studies conducted in both low and medium typhoid fever incidence settings. A sound understanding of both incidence and mortality in different age groups is needed to make decisions about typhoid fever vaccine policy. Although large typhoid fever epidemics are recognized to occur occasionally [Reference Mermin12], the relative contribution of epidemic vs. endemic diseases to the overall burden of typhoid fever has not been established.
Little is known about long-term temporal trends in typhoid fever incidence in low- and medium-HDI countries and what underlies such trends. To improve understanding of long-term trends, collection of data and analysis is required over multi-year periods, with reporting using strict case definitions that include culture confirmation and contextual environmental information such as rainfall, flooding, population density and degree of urbanization.
No population-based typhoid fever study in our series reported rates of typhoid fever complications. Consequently, we calculated rates from hospital-based studies. Hospital-based typhoid fever studies showed median (range) intestinal perforation rates of 2·8% (0·6–4·9%). Rates of complications other than intestinal perforation are not consistently reported in contemporary hospital-based studies. Therefore, the total morbidity attributable to typhoid fever cannot currently be calculated. It is probable that hospital-based studies capture only the most severe illness among persons who have access to health care and therefore such studies may overestimate the rates of complications [Reference Crump7]. No study has systematically evaluated the effect of HIV co-infection on typhoid fever incidence and complications. Data are needed on the rates of complications from population-based studies. Such tracking might be done in areas where a discrete number of health facilities provide care for the majority of the population.
With the continued emergence of antimicrobial resistance in S. Typhi [Reference Crump43], improved surveillance for antimicrobial resistance is needed as well as continued efforts to evaluate antimicrobial agents for the treatment of drug-resistant S. Typhi infection.
Only five population-based studies in our series reported data that allowed calculation of the typhoid fever CFR. In these five studies, the median (range) typhoid fever CFR was 0% (0–1·8%). When using case studies to examine rates of typhoid fever complications, it is likely that the ethics of population-based typhoid fever incidence or vaccine studies will require enhanced clinical management of patients which could alter the measured CFR leading to underestimation of the CFR in such studies.
Policy-makers lack data to make decisions about pathogen-specific prevention measures for typhoid fever. The effect of non-vaccine prevention and control measures, such as implementation of safe drinking water, has not been systematically evaluated. The fraction of typhoid fever attributable to various routes of transmission (i.e. water, food) has not been established. Furthermore, cost–benefit assessments, such as a comparison of the rapid implementation of water disinfection with the introduction of typhoid vaccines in the epidemic setting, have not been done. Finally, studies confirming the beneficial effect and feasibility of scaling up both vaccine interventions and non-vaccine measures in intervention studies would be useful to inform decisions on the distribution of resources to specific prevention programmes.
The diagnosis of typhoid fever provides an ongoing challenge both for patient care and for surveillance. The diagnosis of typhoid fever can rarely be made using clinical history and physical examination alone [Reference Crump7]. Bone marrow aspiration and culture is the gold-standard diagnostic test [Reference Gilman40], but it is not practical for use in the field. Consequently, blood culture has become the practical gold standard, yet it lacks sensitivity and is also challenging to implement in the field in resource-poor settings. While a number of rapid diagnostic tests have been evaluated, none achieves the sensitivity of bone marrow culture and none has the specificity of the culture-based methods [Reference Olsen44]. Continued efforts are needed to identify a simple, rapid diagnostic test for typhoid fever. In parallel, efforts should be made to expand access to and to improve blood culture technologies in low- and medium-HDI countries.
There are substantial gaps in the current understanding of typhoid fever epidemiology. These gaps are so large that policy-makers may have insufficient information to select the most appropriate interventions to reduce typhoid fever illnesses and death and to monitor the effects of such interventions. To address gaps in the current understanding of typhoid fever incidence, complications, and CFR, large population-based studies using blood culture confirmation of cases are needed in representative sites, especially in low-HDI countries outside Asia. Although such information is expensive to acquire, it would be invaluable for the prioritization of precious public health resources for pathogen-specific interventions, such as vaccination programmes targeting typhoid fever. Additional data regarding complications, modifiable risk factors for mortality, the burden of disease among infants, effectiveness of non-vaccine prevention measures, and improved diagnostics would add greatly to our knowledge of typhoid fever.
ACKNOWLEDGEMENTS
This work was supported in part by the U.S. National Institutes of Health Fogarty International Center and by grant number 32143 from the Bill and Melinda Gates Foundation ‘Assessment of diarrhea disease burden and public health programs to control diarrhea in Asian subcontinent and Africa’.
DECLARATION OF INTEREST
None.











