Introduction
Delimiting the distribution of a species is a complex task as many determining factors (abiotic and biotic conditions, dispersal capability and adaptability to new conditions, among others) are difficult to assess in the field (Soberón and Peterson Reference Soberón and Peterson2005). In addition, there are marked differences between the methods available for determining such boundaries (e.g. Mota-Vargas and Rojas-Soto 2012). However, it is very important to delimit the distribution of a species as accurately as possible, as this has fundamental implications for the systematics, biogeography and particularly the conservation of the species (Mota-Vargas and Rojas-Soto 2012). Such is the case of the Bearded Wood Partridge Dendrortyx barbatus, a species endemic to the temperate forests of the Sierra Madre Oriental (SMO) mountain range in Mexico. Although the species is currently listed as “Vulnerable” (IUCN 2000), the understanding of this species’s distribution has changed frequently and this has had important implications for assessing its risk category (Collar et al. Reference Collar, Gonzoga, Krabbe, Madroño, Naranjo, Parker and Wege1992, Howell and Webb Reference Howell and Webb1995, IUCN 2000, Eitniear et al. Reference Eitniear, Aguilar and Cornejo2008).
Since its description by Gould in 1844, the limited cumulative knowledge of the natural history and distribution of the Bearded Wood Partridge has led to its classification as ecologically and geographically rare (Collar et al. Reference Collar, Gonzoga, Krabbe, Madroño, Naranjo, Parker and Wege1992) and it was therefore assessed as “Critically Endangered” in 1994 (IUCN 2008, SEMARNAT 2011). However, when new records of the species provided more information about its environmental requirements, it was suggested that its known range be expanded (Eitniear et al. Reference Eitniear, Aguilar, Gonzáles, Pedraza and Baccus2000, Aguilar Reference Aguilar2000). Its global threat category was changed to “Vulnerable”, two levels of risk lower than before (IUCN 2008), although SEMARNAT (2011) in its latest version of the Official Mexican Legislation (the NOM-ECOL-059), continued to list it as “Endangered”.
In addition to the distribution size, other criteria are necessary to assign categories of risk; for example, population size and threats to a species such as the transformation of disturbed natural environments (Aldama et al. Reference Aldama, Goettsch, Soberón, Tambutti, Sánchez and Medellín2007). In the case of the Bearded Wood Partridge, not many aspects of its biology are known, mainly because it is difficult to observe in the field, which has hindered research. For example, little is known about the habitat requirements of this species. It was originally regarded as almost exclusively associated with cloud forest. However, there have been recent records of the species in pine-oak forests, pine forests, tropical medium forest and even shaded coffee crops (Eitniear et al. Reference Eitniear, Aguilar, Baccus and Carroll2001, Aguilar Reference Aguilar, Gómez de Silva and Oliveras de Ita2003). These records indicate that its ecological requirements are less specific than previously thought.
Traditionally the analysis of species’ distributions has used locality records to delimit their range; however, in most cases, there are few records and even when there are a sufficient number of them, they are biased by site accessibility (Peterson et al. Reference Peterson, Navarro and Benítez-Díaz1998). Recently an increasing number of algorithms based on ecological niche modelling (ENM; Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettmann, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, McC Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberon, Williams, Wisz and Zimmermann2006) are being used to improve our knowledge of species distribution by filling in gaps in geographic data (Godown and Peterson Reference Godown and Peterson2000, Anderson et al. Reference Anderson, Gómez-Laverde and Peterson2002, Rojas-Soto et al. Reference Rojas-Soto, Alcántara-Ayala and Navarro-Sigüenza2003, Mota-Vargas and Rojas-Soto Reference Mota-Vargas and Rojas-Soto2012). ENM require the use of the locality records available for a species in combination with environmental data (Pulliam and Dunning Reference Pulliam, Dunning, Meffe and Carroll1997, Carroll et al. Reference Carroll, Zielinski and Noss1999, Manel et al. Reference Manel, Dias, Buckton and Omerod1999, Cowley et al. Reference Cowley, Wilson, León-Cortés, Gutierrez, Bulman and Thomas2000).
The present study was carried out with the aims of compiling updated records of Bearded Wood Partridge in the field, modelling its ecological niche, and analysing its ecological and geographical distribution to refine our understanding of its current area of distribution.
Methods
Collection of historical records
We compiled a database of all available records from two sources: available digital information, including the Global Biodiversity Information Facility database (GBIF, www.gbif.org; 83 records), the World Information Network on Biodiversity (REMIB 2010; 22 records), and the Atlas of the Birds of Mexico (Navarro-Sigüenza et al. Reference Navarro-Sigüenza, Peterson and Gordillo-Martínez2003; 47 records). Records from literature were also used (Lowery and Newman Reference Lowery and Newman1951, Davis Reference Davis1952, Collar et al. Reference Collar, Gonzoga, Krabbe, Madroño, Naranjo, Parker and Wege1992, Eitniear et al. Reference Eitniear, Aguilar, Gonzáles, Pedraza and Baccus2000, Reference Eitniear, Aguilar, Baccus and Carroll2001, Rojas-Soto et al. Reference Rojas-Soto, Según–Sanchez and Navarro-Sigüenza2001, Aguilar Reference Aguilar2000, Reference Aguilar, Gómez de Silva and Oliveras de Ita2003, Martínez-Morales Reference Martínez-Morales2007, Villa-Bonilla et al. Reference Villa-Bonilla, Rojas-Soto, Colodner-Chamudis and Tejeda-Cruz2008, Mota-Vargas Reference Mota-Vargas2008; 47 records). Records repeated in multiple sources were removed and only information from the original source was used. We omitted all doubtful and ambiguous information (i.e. information that did not have precise data locality and records with dubious coordinates that could not be verified). We georeferenced each locality with the help of gazetteers (INEGI 1992) and in some cases with the use of Google Earth (http://earth.google.es/). After removing duplicate information and verifying the coordinates, we had a total of 41 historical records, which were used to run the first ENM using the GARP algorithm (Figure 1; details below) which formed the basis for the selection of certain regions and also for conducting intensive field work to search for and verify historical localities (Mota-Vargas and Rojas-Soto Reference Mota-Vargas and Rojas-Soto2012).

Figure 1. Geographical distribution of the Bearded Wood Partridge obtained using GARP (dark grey) from 41 historical records (∼21,971 km2, including the Sierra Norte de Oaxaca). The triangles correspond to historical records, the white dots correspond to field work records. The two southernmost known localities where the species was recorded are shown with arrows (Puerto Soledad and Chilchotla). The thin black lines are the state lines.
Field work
Based on the first ENM (Figure 1) we selected 22 regions from a large part of the potential distribution area of the species (also identifying some of the historically associated localities) to carry out field surveys and to obtain new localities for the species. The field work was carried out over a period of eight months (April–November 2010) with a total of 20 days in the field. Due to the cryptic nature of the species and the difficulties in observing these birds directly, we applied the playback technique proposed by Carroll and Hoogesteijn (Reference Carroll, Hoogesteijn and Jenkins1995). This consists of transmitting the song of the species for 30 seconds, waiting for 30 seconds to listen for a response and playing the song again; this is done three times in a row, for a total time of three minutes. It has been widely demonstrated that the species responds to such auditory stimuli (Aguilar Reference Aguilar2000, Eitniear et al. Reference Eitniear, Aguilar, Baccus and Carroll2001). Digital recordings of the Bearded Wood Partridge (territorial song of a male) were used, as recorded in 1996 by Eitniear in Coatepec, Veracruz, and played on a CD player connected to an amplifier.
Sampling was carried out between 06h30 and 10h00, and again from 18h30 to 20h00, because these are times of highly vocal activity (Eitniear et al. Reference Eitniear, Aguilar, Baccus and Carroll2001, Aguilar Reference Aguilar, Gómez de Silva and Oliveras de Ita2003). At each sampling point, a high, clear location was chosen to provide an observation point and a good auditory environment, although in some cases playback was carried out inside the forest. The amplifier was aimed in different directions to obtain a response from the species, data were collected on the presence or absence of the species, and the geographical coordinates were obtained using a Garmin XL12 GPS device. Occasionally, upon arrival at the sampling site, the bird sang spontaneously (i.e. without the auditory stimulus). The bird’s presence at such sites was corroborated immediately using the playback method and the corresponding data were recorded. If a response was obtained the first time the bird’s song was played, the information was recorded and then another locality was visited. When the species did not respond at the first monitoring point, a new point was sought (approximately 100 m away), and so forth until we got a response or until we had attempted to get a response at five different points. The geographic coordinates of the location where each response was obtained were noted (compass direction and estimated distance) and points were plotted on a digital map of the study area with the assistance of the Arc View 3.2 Geographic Information System (GIS) (ESRI 1999) and Google Earth (2010). Based on the new locality records obtained during the field surveys (95 records) we ran a second ENM (41 historical records + 95 field surveys records = 136 records) using two algorithms (see below for details).
Since we did not record the species as present at any of the sites we first visited in the southern region, we increased the number of sites to search along two geographic routes. The first route was approximately 25 km long and characterised mainly by pine and cloud forest. The second route covered 20 km and was characterised mainly by pristine cloud forest. In both cases, a comprehensive search was conducted in localities along these two routes and that were an average of 2 km away from one another. These efforts were fruitless, in spite of adequate weather conditions and only a day’s difference from monitoring the other locations where the species was recorded frequently.
Ecological niche modelling
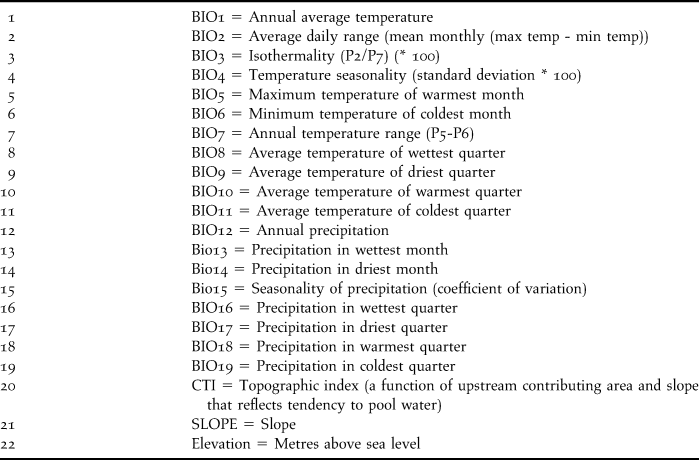
In both ENM exercises, we used 22 environmental variables to characterise the species’s niche: 19 bioclimatic variables obtained from the WorldClim project (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005) and three topographic variables from the Hydro 1K project (USGS 2001), both with a spatial resolution of 0.0083 ° (∼ 1 km2; Table 1).
Table 1. Environmental variables used for ecological niche modeling.
We used the Genetic Algorithm for Rule-set Production (GARP). In order to compare the results obtained during the second ENM, we used MaxEnt, another widely used algorithm. The application of GARP has been well documented in extrapolation exercises (Peterson, Reference Peterson2003a,b, Raxworthy et al. Reference Peterson2003, Peterson et al. Reference Raxworthy, Martínez-Meyer, Horning, Nussbaum, Schneider, Ortega-Huerta and Peterson2005), although some authors have suggested that MaxEnt has better predictive success rates, particularly for small sample sizes (Pearson et al. Reference Pearson, Raxworthy, Nakamura and Peterson2006, Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettmann, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, McC Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberon, Williams, Wisz and Zimmermann2006). The differences between these widely used algorithms are not a concern since they do not differ significantly (Tsoar et al. Reference Tsoar, Allouche, Steinitz, Rotem and Kadmon2007; Warren et al. Reference Warren, Glor and Turelli2008). Furthermore, both algorithms have been used widely in the delimitation of bird distributions (e.g. Godown and Peterson Reference Godown and Peterson2000, Peterson Reference Peterson2001, Phillips et al. Reference Phillips, Dudík and Schapire2004, Rojas-Soto et al. Reference Rojas-Soto, Martínez-Meyer, Navarro, Oliveras de Ita, Gómez de Silva and Townsend Peterson2008).
GARP is an artificial intelligence algorithm that uses points of presence in combination with digital environmental data, and works in an iterative way based on rules (atomic, logistic regression and ranges; see Stockwell and Peters Reference Stockwell and Peters1999). The resulting model is a product of the relationship between localities and environmental variables. These rules are evaluated and tested and then they are incorporated into or rejected by the model based on their behaviour. This process continues successively until a set of rules are produced that describe the ecological niche which can later be projected into geographic space, producing a prediction of potential presence (Stockwell and Noble Reference Stockwell and Noble1991, Stockwell and Peters Reference Stockwell and Peters1999). We ran 100 models for Bearded Wood Partridge; for each model we used 80% of the locality records and the other 20% were used for evaluation. The interface of the program allowed the best subset to be obtained, so we chose the 10 best models with a default value of 10% or less of the omission error; we then selected the models whose values were closest to the median of the commission error value (Anderson et al. Reference Anderson, Lew and Peterson2003). The final consensus map included 98% of the records.
MaxEnt (Phillips et al. Reference Phillips, Anderson and Schapire2006) uses the principle of maximum entropy to calculate the most likely distribution for each species. Eighty percent of the localities were used as training points and 20% were validation points; the maximum number of iterations was 1,000 with a convergence limit of 0.00001 and a regularisation value of 1. The prediction obtained (in terms of probability between 0 and 1) was transformed to a binary map (where 0 is absence and 1 is presence) with a threshold value of 0.229 that included 98% of the records. The final maps (from GARP and MaxEnt) were edited to remove the areas predicted but that are far from the known range of the species. The maps were edited using GIS.
Analysis of the ecological distribution patterns
The ecological distribution was first analysed with a description of the value ranges for each of the 22 variables for each locality where the species was recorded. With these values, we conducted a principal component analysis (PCA) using the program PAST (http://norges.uio.no/past/download.html) to analyse the most important environmental variables. We graphically compared the interaction between the values of the two most important variables for the first principal component. These values were obtained and compared for: a) each of the locality records, b) each of the pixels of potential presence (GARP only) combined with the primary vegetation of Mexico, which corresponds to " vegetation that would develop in an area under environmental conditions similar to today, without human influence" (INEGI 2003), and c) for the pixels representing the SMO; this with the aim of viewing the environmental requirements of the species in a geographic context.
Results
The first modelling exercise predicted an area of 21,971 km2 and includes 40 (98%) of the historical records (Fig. 1). From the 22 regions visited during the field work, we obtained 95 new locality records, of which 77 (81%) were in areas predicted by the first model, and the other 18 (19%) were in non-predicted sites (Appendix 1). In addition, based on the map, an intensive search was also conducted along two routes in the Sierra Norte of Oaxaca (SNO), but the species was not detected there.
The two final versions of the potential geographic distribution maps (Figure 2) generated by GARP and MaxEnt, delimit the species’s distribution using 136 records (41 historical + 95 from field work). Both maps have the same distribution patterns, are based on good prediction accuracies (for GARP χ2 333–369 P < 0.05 and for MaxEnt AUC = 9.73), overlap by 11,914 km2 (i.e. 92% overlap of GARP in relation to MaxEnt), and in both cases include the potential presence of its ecological niche in the SNO. However, based on historical surveys and the field work carried out in this study, Bearded Wood Partridge appears to be absent south of the Santo Domingo River, suggesting that the river could represent a climatic barrier limiting the geographical distribution of the species and reducing its distribution to an area of approximately 17,956 km2 according to GARP, and 12,974 km2 according to MaxEnt.

Figure 2. Geographical distribution of the Bearded Wood Partridge derived from the final exercise of ecological niche modeling. The geographical distribution obtained using MaxEnt is in black, the geographical distribution obtained using GARP is in dark grey. The white dots indicate the sites where the species was not detected. The arrow and wide grey line highlight the location of the Santo Domingo River. The thin black lines are the state lines.
With respect to the 22 environmental variables (Figure 3 and Table 1), Bearded Wood Partridge occurs in temperature and precipitation ranges that do, in fact, correspond to temperate forests; however, the narrow range of isothermality is noteworthy as is the relatively wide range of seasonality.

Figure 3. Climatic description of the species based on its potential presence. Displayed is the interval of values for each of the variables used for modeling as follows: Bio1-Bio11 are temperature and Bio12-Bio19 are precipitation values. *CTI units are explained in HYDRO 1K (http://edc.usgs.gov).
From the results of the PCA (Figure 4, Table 2), the first principal component explained close to half of the variance (42%) and the two variables with the most weight were annual precipitation and elevation; for component two (31%) the most important variables were slope and seasonal temperature. Figure 3 shows the values for principal components 1 and 2, and highlights the localities across four altitudinal intervals for the purpose of demonstration. Component one describes a clear altitudinal pattern, segregating highland (over 2,000 m a.s.l.) from lowland (1,200 m) records, and showing that most of the records occur between 1,200 and 2,000 m.

Figure 4. Analysis of the first two principal components. Symbols indicate the locality records according at four elevation intervals.
Table 2. Coefficients for each of the variables of the first three principal components. Numbers in bold indicate the most important variables.

From the comparison of average annual rainfall and elevation among the locality records, the pixels that represent the potential geographical distribution (ENM) and the pixels that correspond to the SMO (Figure 5), it is apparent that there are environmental restrictions on the values of the localities and potential presence with respect to the values of the SMO. Based on comparison of the different types of vegetation that make up the potential distribution obtained, cloud forest coincided with the species’s main ranges of elevation and precipitation, while the elevation range of the medium-sized deciduous forest was more restrictive for Bearded Wood Partridge. The elevation range of pine-oak forests was favourable although the range of precipitation they receive partially restricts the distribution of this species. Based on the geographical location of the 136 records of this species, 89 localities were situated in cloud forest, 37 in pine-oak forest and 10 in medium deciduous forest.

Figure 5. Comparison of the two main variables values (annual precipitation and altitude) in the potential distribution of the Bearded Wood Partridge defined by vegetation types (remarked by diferent lines), the locality records (white dots) and the Sierra Madre Oriental (thin solid line).
Discussion
Due to disagreement about the geographical and ecological distribution of the Bearded Wood Partridge, it was first necessary to carefully compile the historical and currently known locality records for the species. Considering the importance of the species (because of its risk category) and its known range extension, the total of 41 historical records is low, especially considering that the avifauna in the SMO region is relatively well known (Navarro-Sigüenza et al. Reference Navarro-Sigüenza, Garza-Torres, López de Aquino, Rojas-Soto, Sánchez-González, Luna-Vega, Morrone and Espinoza2004). This may result from the evasive behaviour that is characteristic of the species.
The collection of 95 new records in just half a year of field work, with an average of four days of monitoring per month was very high in comparison with the number of historical records; this was perhaps the result of the new methods used, both in the selection of potential areas to search (from the first modelling exercise), and more intensive search effort using the auditory playback method. However, this greater success also suggests that the species is more common than was previously thought, on the basis of observations.
On the other hand, due to the fact that the field trips were not exclusively restricted to sites predicted by the first modelling exercise, there were some localities where the species was not predicted by the first modelling exercise (e.g. Villa and Nauzontla, in Puebla; Atzalán and Tepetlán, in Veracruz; and Santa María Chilchotla, in Oaxaca; Appendix 1). However, these localities did correspond to areas close to those predicted by the first modelling exercise (i.e. at an average distance of less than 3 km), so in general the first model allowed for the optimisation of sampling effort in the field. This also helped us validate the presence of the species in localities with very old records, for example Miramar in Xilitla, SLP, where it was recorded in 1950 (Davis Reference Davis1952) or in Huachinango, Puebla, in 1947 (Collar et al. Reference Collar, Gonzoga, Krabbe, Madroño, Naranjo, Parker and Wege1992).
The final modelling exercise (based on 136 localities) for both algorithms (GARP and MaxEnt), produced maps whose predictions of geographical potential suggested the presence of Bearded Wood Partridge in areas located in the middle of the historic localities, thereby giving geographical continuity to the locality records throughout the known distribution area. Based on the extent, location, and shape of the predicted area, which also included a high percentage of locality records used to validate the models (more than 95%), we believe that the predictions made by GARP and MaxEnt are both reliable representations of the actual distribution of the species. The first tended to over-predict and the second tended to over-fit with respect to the localities used; however, the areas predicted by both algorithms are similar in shape and extent.
Aguilar (Reference Aguilar2000) suggested that the species was present in the SNO and even further south, based on the continuous of vegetation and well preserved cloud forest. However, since we did not detect it during our intensive search in the Sierra Norte of Oaxaca, we suggest that the Bearded Wood Partridge is absent from this region even though it offers pristine cloud forest and environmental conditions favourable for the species. While the possibility of local extinction cannot be ruled out, there is no historical evidence of this species’s presence in the SNO (Binford Reference Binford1988). Furthermore, although Aguilar (Reference Aguilar2000) recorded the species in Puerto Soledad, Oaxaca (the southernmost known locality record), in our study the presence of the species was only verified for Chilchotla, a town near Puerto de la Soledad, Oaxaca. No localities were recorded further south (Figure 1). An absence of records of a species is dependent on several factors, including the methods employed in searching for it, the season of the year, the climatic conditions and does not on its own indicate true biological absence. Although our results suggest that the species is absent from this region, they are not conclusive and future surveys are needed to confirm this.
Based on information gathered in the field and an analysis of the geography of the region, we suggest that the Santo Domingo River in Oaxaca, located approximately 10 km south of the towns of Puerto Soledad and Chilchotla, represents the southernmost limit of Bearded Wood Partridge’s distribution, and is a geographical and environmental barrier to the species (accessibility sensu Soberón and Peterson Reference Soberón and Peterson2005 and Soberón Reference Soberón2007). We make this assertion because the river has an average altitude of 200 m in the SNO, and is characterised by a warm climate with evergreen and semi-deciduous forests and, coincidentally, it is known that this river represents a distribution limit for other species (e.g. mammals such as Jalapan pine vole Microtus quasiater and Big small-eared shrew Cryptotis magna; and amphibians such as Pseudoeurycea bellii; Paniagua and Morrone Reference Paniagua and Morrone2009).
With regard to the limits of the distribution to the east, west and north, it is most likely environmental conditions that shape the geographical pattern observed, since according to the ENM these environmental requirements are not met beyond the known recorded locations, unlike the southern region of the SNO. The species’s geographical distribution is represented by the environmental conditions found over 17,956 km2 according to GARP and 12,974 km2 based on MaxEnt. In spite of the differences between the two algorithms, in both cases, the shape and location of the distribution area, as well its approximate extent, are twice the 6,900 km2 listed by IUCN (2000) and still used today. This indicates that there are historical localities of the species that apparently were not considered by IUCN (2000): south-east of Hidalgo, north of Puebla and northern Oaxaca around its border with Puebla (Figure 1). During our field surveys we identified the species at 91% of the places we visited, and on average we obtained 4.3 responses from different groups of individuals per locality. Although the abundance data are not presented per locality, the response data are of great value considering both the evasive behaviour of the species and the values previously reported by Eitniear et al. (Reference Eitniear, Aguilar, Baccus and Carroll2001).
In relation to the environmental description, we know that the SMO is a continuous geographical feature with a broad range of environmental conditions (two of which are represented in Figure 4: annual precipitation and elevation), and where the restriction of the potential distribution of the species is observed. In addition, considering the environmental conditions provided by the various types of vegetation (Figure 4), the ranges of altitude and precipitation in cloud forest had the greatest overlap with the potential distribution, unlike the tropical medium forest, which had more restricted ranges for these two variables. While pine-oak forests occurred in a favourable elevation range, the range of precipitation they receive would be restrictive for this species. The wide range of environmental conditions is reflected by the various types of vegetation in which the species is found, with the highest percentage of records in cloud forest (65.4 %), followed by pine-oak forests (27.2 %) and finally, tropical medium forest with 10 (7.3 %).
Analysis of the variables associated with the potential presence of the species (Figure 2) shows that Bearded Wood Partridge requires specific combinations of ranges which, identified by the analysis of PCA (Figure 3, Table 2), facilitate the recognition of its patterns of environmental distribution (Figure 4). Although the SMO is a continuous geographical feature, the species is restricted geographically and ecologically to certain ranges of annual precipitation and elevation. IUCN (2000) suggests the species is present across an elevational band of 900–3,100 m; however, in this study we did not record it above 2,500 m, and even the prediction of the final ENM (that allows for extrapolations) did not predict it at these elevations; though it is possible that the species occurs there.
This study has expanded our knowledge of the known environmental distribution of Bearded Wood Partridge. In spite its known preference for the environmental conditions of the cloud forest, we also found it in other types of vegetation such as pine-oak forest and tropical medium forest, showing that the species is not restricted to the cloud forest as was previously thought (Leopold Reference Leopold1977, Collar et al. Reference Collar, Gonzoga, Krabbe, Madroño, Naranjo, Parker and Wege1992, Howell and Webb Reference Howell and Webb1995). On the other hand, during the field trips, the majority of the localities where we recorded the species were in landscapes with partially (and sometimes highly) disturbed forests, confirming that the species is tolerant of different degrees of habitat alteration and disturbance, as suggested earlier (Eitniear et al. Reference Eitniear, Aguilar, Baccus and Carroll2001, Aguilar Reference Aguilar, Gómez de Silva and Oliveras de Ita2003), although the degree of tolerance is not known. Considering this, in combination with its geographical distribution, ecological requirements and abundance, we would suggest that the species not be regarded as “Endangered” as it is currently listed according to Mexican Legislation (SEMARNAT 2011). We would also suggest that while the species is well placed in the “Vulnerable” category as defined by IUCN (2000), its geographical range should be redefined. Finally, we hope that the results of this study will stimulate further research on the natural history, abundance and distribution of Bearded Wood Partridge at finer scales, in order to contribute to the long-term survival of this enigmatic partridge.
Acknowledgements
Adriana Sandoval, Antonio Maruri, Claudia Gallardo and Pedro Mota helped with the field work. Adolfo Navarro and Sergio Aguilar contributed historical locality records. Rosario Landgrave provided assistance with the GIS. Antonio Guillén and Eduardo Pineda provided logistical support during the field work. This study benefited from the support of the Centro Tlaxcala de Biología de la Conducta de la Universidad Autónoma de Tlaxcala and the Instituto de Ecología, A. C. The Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Programa de Movilidad Estudiantil (ECOES) provided the first author with financial support in the form of a scholarship and a field work grant, respectively.
Appendix 1. Localities visited during the field work showing the presence and absence of Dendrortyx barbatus. The first column lists the Mexican states (SLP = San Luis Potosí, Hgo = Hidalgo, Ver = Veracruz, Pue = Puebla, Oax = Oaxaca). The second column lists the Municipality, and the third and fourth columns are the consecutive numbers of the localities visited and their names. The last two columns give the number of records by locality as well the date recorded.













