The mud crab (Scylla paramamosain), a typical marine omnivorous crab species, is widely distributed in tropical, subtropical and temperate zones of Asia(Reference Li, Ai, Liu, Gui, Tang, Li, Liu and De Silva1), and due to its delicious flavour and nutritional quality, it is increasingly favoured by seafood consumers(Reference Zhao, Wen and Li2). However, wild populations of mud crab have dramatically declined in recent decades due to over-fishing and environmental deterioration(Reference Ye, Tao and Wang3). With the success of larval cultivation, mud crab has become one of the most important aquaculture crustacean species in Asia, especially China(Reference Li, Ai, Liu, Gui, Tang, Li, Liu and De Silva1,Reference Marichamy and Rajapackiam4–Reference Le Vay6) . Mud crab production in China reached 157 712 tons in 2018 and accounted for 66·5 % of total production(7). However, with increasing wild fishery and marine environmental protection, the traditional feeds of trash fish and shellfish are insufficient to meet the requirements of crab culture(Reference Jin, Zhou and Zhang8). Thus, studies on the nutritional requirements of mud crabs are important to develop cost-effective, environmentally friendly and nutritionally balanced formulated feed for mud crab(Reference Li, Ai, Liu, Gui, Tang, Li, Liu and De Silva1). To date, studies have reported the nutritional requirements of mud crab for protein, lipid and phospholipid(Reference Zhao, Wen and Li2,Reference Dong, Tong and Zhang9–Reference Xu, Wang and Han11) ; however, few researchers have focused on trace element requirements of mud crab.
Minerals are essential nutrients for animal life, as cofactors and activators of a variety of enzymes and hormones participating in multiple biochemical processes(12). Adequate supplementation of mineral elements in the diet can enhance growth performance and improve immune function and muscle quality of aquatic organisms(Reference Chitturi, Baddam and Prasad13). Zn is a fundamental microelement that can stabilise cellular membranes and components in organs and tissues(Reference Salgueiro, Zubillaga and Lysionek14). Specifically, it is an essential cofactor for numerous enzymes and proteins involved in many physiological processes including nucleic acid, protein, fatty acid, phospholipid and carbohydrate metabolism that are critical to growth, reproduction and development of aquatic animals(Reference Muralisankar, Bhavan and Radhakrishnan15–Reference Wu, Luo and Hogstrand17). In addition, Zn has key roles in antioxidant defence and immune response partly as an integral constituent of Zn-dependent metalloenzymes such as Cu/Zn superoxide dismutase (Cu/Zn SOD) and alkaline phosphatase (AKP)(Reference Yuan, Luo and Zhu18,Reference Rink19) and so inhibits free-radical-induced oxidative damage to cells and tissues and supports immune functions by interacting with minerals such as Se, Cu and Mg and several other metalloenzymes(Reference Musharraf and Khan20). Therefore, there is an urgent need to understand the nutrition and metabolism of Zn in mud crab.
Although the mechanisms of dietary Zn functions in fish and shrimp have been studied(Reference Davis and Gatlin21,Reference Watanabe, Kiron and Satoh22) , little information is available on the nutritional role of Zn in crabs and, so far, only Zn requirement of Chinese mitten crab (Eriocheir sinensis) has been determined(Reference Li, Gong and Jin23,Reference Sun, Chen and Chen24) . Deficiency of dietary Zn in fish results in decreased growth performance and survival rate, eye cataracts, skin lesions, bone malformations and short body dwarfism(12,Reference Musharraf and Khan20) , while excessive dietary Zn is toxic to fish and reduces antioxidant ability and sperm motility(Reference Luo, Tan and Zheng25–Reference Dekani, Johari and Joo28). In crustaceans, previous studies demonstrated that dietary Zn deficiency reduced the growth of Chinese mitten crab(Reference Li, Gong and Jin23), freshwater prawn (Macrobrachium rosenbergii)(Reference Muralisankar, Bhavan and Radhakrishnan15), grass shrimp (Penaeus monodon)(Reference Shiau and Jiang29) and Pacific white shrimp (Litopenaeus vannamei)(Reference Davis, Lawrence and Gatlin30,Reference Zhang31) , while excessive dietary Zn decreased digestive enzyme activities in freshwater prawn(Reference Muralisankar, Bhavan and Radhakrishnan15). Lin et al. (Reference Lin, Lin and Yang32) compared the effects of different dietary Zn sources (Zn-methionine, Zn-lysine, Zn-glycine and Zn-sulphate) on growth performance and immune function of Pacific white shrimp, showing that Zn-methionine significantly improved survival, percentage weight gain (PWG) and immune-related enzyme activities. Although these studies indicated dietary optimal Zn is vital to crustaceans, comprehensive assessment of dietary Zn level is still relatively limited in crustaceans, especially marine crabs. Therefore, an 8-week nutritional trial was designed to investigate the optimal Zn requirement level for juvenile mud crabs and to evaluate the effects of dietary Zn on growth, tissue Zn bioaccumulation, haemolymph characteristics, antioxidant ability and innate immune response. The outcomes of the study will enhance our understanding of the nutritional metabolism of Zn in mud crab and will also explore strategies to improve disease resistance and antioxidant capacity through nutritional modulation of marine crabs.
Methods
Ethics statement
The study was performed in strict accordance with the Standard Operating Procedures of the Guide for Use of Experimental Animals of Ningbo University. The experimental protocol and procedures were approved by the Institutional Animal Care and Use Committee of Ningbo University.
Diet preparation
Six isonitrogenous (45 % crude protein) and isolipidic (7·5 % crude lipid) experimental diets were formulated to contain different levels of Zn (ZnSO4.7H2O as Zn source), with the analysed Zn concentrations being 44·5, 56·9, 68·5, 97·3, 155·6 and 254·7 mg/kg. Peruvian fishmeal, soyabean meal, krill meal and casein were the main protein sources, with fish oil and soyabean lecithin as the main lipid sources (Table 1). Feeds were manufactured according to the method described in detail previously(Reference Sun, Jin and Jiao33). Briefly, all dry ingredients were ground into fine powder with particle size <177 μm, and micro components including minerals and vitamin premix were added by the progressive enlargement method. Lipid and distilled water (35 %, w/w) were added to the dry ingredients, and the mixture was blended until homogenous in a Hobart-type mixer, and cold-extruded pellets produced (F-26, Machine factory of South China University of Technology) with pellet strands were cut into two uniform sizes (3 and 5 mm diameter pellets) (G-250, Machine factory of South China University of Technology). Pellets were heated at 90°C for 30 min, air-dried to approximately 10% moisture, sealed in vacuum-packed bags and stored at −20°C until use.
Table 1. Formulation and proximate composition of experimental diets (g/kg DM)
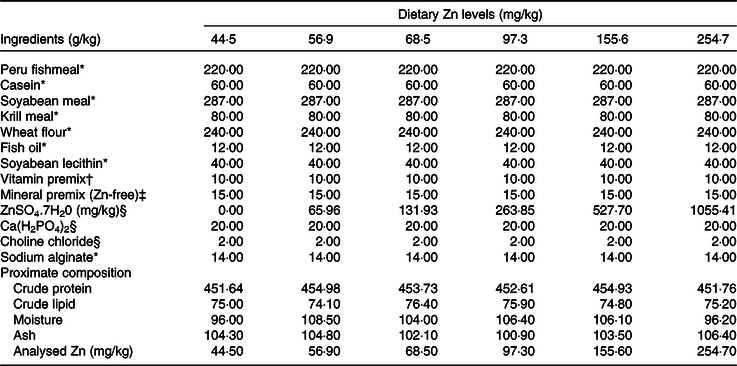
* Ingredients were bought from Ningbo Tech-Bank Corp.
† Vitamin premix was based on Sun et al. (Reference Sun, Jin and Jiao33).
‡ Mineral premix (per kg mineral premix): FeC6H5O7, 4·57 g; CuSO4.5H2O (99 %), 6·61 g; MnSO4.H2O (99 %), 4·14 g; MgSO4.7H2O (99 %), 238·97 g; KH2PO4, 233·2 g; NaH2PO4, 137·03 g; C6H10CaO6.5H2O (98 %), 34·09 g; CoCl2.6H2O (99 %), 1·36 g; K2O3Se, 0·0044 g; KIO3, 0·0013 g.
§ Ingredients were provided by Sinopharm Chemical Reagent Co. Ltd.
Crab rearing and experimental conditions
Juvenile mud crabs were obtained from the breeding base of Ningbo Ocean and Fishery Science and Technology Innovation Center. Prior to the experiment, crabs were acclimated for 2 weeks and fed with a commercial diet (45 % crude protein, 8 % crude lipid and 72 mg/kg Zn; Evergreen Corp.). At the beginning of the experiment, a total of 270 healthy, normally active and similar size juvenile crabs (14·96 (se 1·11) g, in the ninth molting stage) were randomly allocated into 270 single crab units (33 cm × 22·5 cm × 25 cm), to prevent competition and cannibalism. Each feed was randomly assigned to three experimental replicates with fifteen mud crabs in each replicate. Each single crab unit was mutually independent and supplied with continuous flow-through water(Reference Shelley and Lovatelli34). Crabs were fed the allocated experimental diet once daily at 18.00 hours (3–6 % of crab weight), and the daily ration adjusted according to the actual intake (ration – uneaten feed) to ensure feeding to apparent satiation. Faeces and uneaten feed were removed by siphon and spoon-net each morning. During the 10-week experimental period, water temperature in each culture unit was 24·5–29·0°C, salinity approximately 24·1–28·4 g/l, pH 7·3–8·0, ammonia nitrogen was lower than 0·05 mg/l, dissolved O2 was not lower than 6·0 mg/l and Vibrio concentration in seawater was <1·0 × 105 colony-forming units/ml.
Sample collection
At the end of the experiment, most crabs were at molting stage 11 and a few were at stage 12. Crabs were anaesthetised with 0·02 % tricaine methane sulphonate (MS-222), and all crabs in each replicate were counted and weighed to determine the survival and PWG. In each replicate, haemolymph was sampled from six crabs and centrifuged at 3500 rpm for 10 min at 4°C (Eppendorf centrifuge 5810 R). The supernatant was collected and stored at –80°C before analyses of biochemical and enzyme activities. Hepatopancreas from the same six crabs was quickly dissected and weighed to calculate hepatosomatic index, and placed in 1·5 ml centrifuge tubes, frozen in liquid N2 and stored at –80°C prior to the analysis of enzyme activities and gene expression. Muscle, carapace and hepatopancreas from further three crabs were collected and stored at –20°C to determine Zn concentrations and tissue Zn retention rate (ZRR).
Calculations
where W t is the final tissue weight (g), W i is the initial tissue weight (g), Z t is the final Zn concentration (mg/kg), Z i is the initial Zn concentration (mg/kg), W d is the weight of fed diet (g) and Z d is the Zn concentration of the diet (mg/kg). The calculation of these parameters was based on three experimental replicates per diet.
Zinc concentration analysis
All collected tissues (hepatopancreas, muscle and carapace) and experimental diets were weighed and freeze-dried before acid digestion, where samples were digested in 70 % HNO3 solution at 80°C, with the acid solution added dropwise until complete digestion of organic matter. The digested solution was filtered through an aqueous phase syringe filter (SCAA-102, ANPEL Laboratory Technologies Inc.) before tissue Zn concentrations were determined by inductively coupled plasma optical emission spectrometry (PE2100DV, Perkin Elmer).
Haemolymph biochemical analysis
The activities of alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transpeptidase, and the contents of total protein and albumin were analysed in haemolymph supernatant using an automatic biochemistry analyzer (VITALAB SELECTRA Junior Pros), with reagent kits from Biosino Bio-Technology and Science Inc.
Enzyme activity analysis
Hepatopancreas samples were homogenised on ice in nine volumes of normal saline solution and centrifuged at 3500 rpm for 15 min at 4°C, and the supernatant was collected in a PCR tube and stored at –80°C prior to the analysis of enzyme activities. The activities of total SOD, Cu/Zn SOD, catalase (CAT), phenoloxidase (PO), ceruloplasmin (CP), AKP and acid phosphatase (ACP) in haemolymph supernatant, and the activities of CAT, glutathione peroxidase (GPx) and total antioxidation capacity (T-AOC) in hepatopancreas homogenates, as well as the contents of GSH and malondialdehyde (MDA) in both haemolymph and hepatopancreas were assayed by Multiskan spectrum (Thermo) according to the manufacturer’s instructions using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute.
Total RNA extraction, reverse transcription and real-time PCR
RNA extraction, reverse transcription and real-time quantitative PCR were conducted according to methods described in detail previously(Reference Yuan, Jin and Xiong35). Briefly, RNA was extracted from crab hepatopancreas samples of approximately 50 mg by TRIzol reagent following the manufacturer’s protocol with RNA quality and concentration confirmed by 1·2 % agarose gel electrophoresis and ultra-micro spectrophotometer (Nanodrop 2000, Thermo Fisher Scientific), respectively. RNA samples were reverse-converted to complementary DNA (cDNA) using a PrimeScript® RT reagent kit (TaKaRa) according to the manufacturer’s protocol. Elongation factor 1α (ef1a) was chosen as the reference gene (housekeeping gene) after confirmation of its expression stability. Specific primers used for real-time quantitative PCR were designed according to complete cDNA sequences of corresponding genes in the National Center for Biotechnology Information (NCBI) using Primer Premier 5.0 software (Table 2). PCR amplification was conducted by a quantitative thermal cycler (Lightcycler 96, Roche), with reactions containing 2 μl of cDNA, 1·0 μl of each primer, 10 μl of 2× conc SYBR Green I Master (Roche) and 6 μl diethyl pyrocarbonate (DEPC) water. The procedure of quantitative PCR contained an initial activation step at 95°C for 2 min, followed by forty-five cycles of 95°C for 10 s and 58°C for 10 s, and 72°C for 20 s. Standard curves were generated using six different dilutions of one cDNA sample of the 68·5 mg/kg treatment group. Amplification efficiency was measured as: E = 10(–1/Slope) − 1, and the amplification efficiencies of all genes were approximately equal and ranged from 93 to 102 %. Relative expression levels were calculated using the 2−ΔΔCt method, and the 68·5 mg/kg treatment group was used as the control/reference group.
Table 2. Primers for real-time quantitative PCR of mud crab

Cu/Zn sod, copper/zinc superoxide dismutase; F, forward; R, reverse; mitMn sod, mitochondrial manganese superoxide dismutase; gpx, glutathione peroxidase; cat, catalase; trx, thioredoxin; proPO, prophenoloxidase; clr, C-type lectin receptor; toll1, toll-like receptor 1; toll2, toll-like receptor 2; ef1a, elongation factor 1a.
Statistical analysis
For heat map visualisation analysis, all data were homogenised and performed using the online programme Image GP (http://www.ehbio.com/ImageGP/index.php/).
Data are presented as means and standard errors of three replicates (n 3) and analysed by one-way ANOVA followed by Tukey’s multiple-range test. All statistical analyses were conducted using SPSS 16.0 for Windows.
Results
Survival and growth performance
The effects of dietary Zn levels on survival and growth performance of mud crabs are presented in Table 3. Survival ranged from 72·2 to 86·1 %, with no statistical differences among treatments (P > 0·05), although numerically lowest survival was observed in crabs fed the diet containing 254·7 mg/kg Zn. Crabs fed the diet containing 254·7 mg/kg Zn had lower PWG than those fed the 68·5 and 97·3 mg/kg Zn diets, and the highest PWG was observed in crabs fed the diet containing 97·3 mg/kg Zn (P < 0·05). Broken line regression analysis of PWG and dietary Zn level showed y = 0·5232x + 121·08 (R 2 = 0·7261) and y = −0·3406x + 192·76 (R 2 = 0·9208), respectively, and the optimum dietary Zn level was estimated to be 82·9 mg/kg for juvenile mud crabs (Fig. 1). Hepatosomatic index (HSI) did not show any statistical differences among dietary treatments (P > 0·05).
Table 3. Survival, growth performance and morphology index of mud crabs fed with different dietary zinc levels
(Mean values with their standard errors (n 3))

IW, initial weight; PWG, percentage weight gain; HSI, hepatopancreas index.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05) as determined by ANOVA and Tukey’s test.
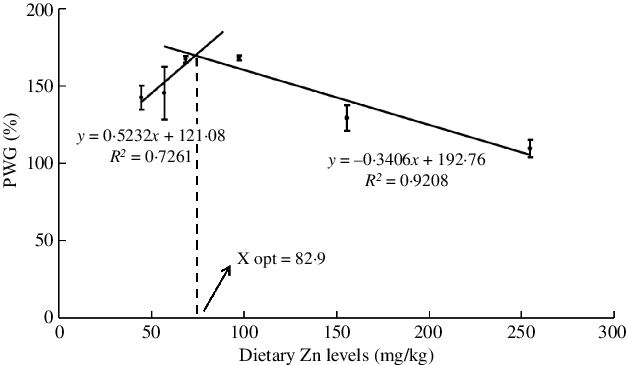
Fig. 1. Relationship between the percentage weight gain (PWG) and dietary zinc level based on two slope broken-line regression analysis, where X opt represents the optimal dietary zinc level for maximum PWG.
Tissue zinc concentration and retention rate
Zn concentrations in hepatopancreas, muscle and carapace significantly increased as dietary Zn level increased from 44·5 to 254·7 mg/kg, with highest tissue Zn concentrations observed in crabs fed the diet containing 254·7 mg/kg Zn (Fig. 2(A)) (P < 0·05).
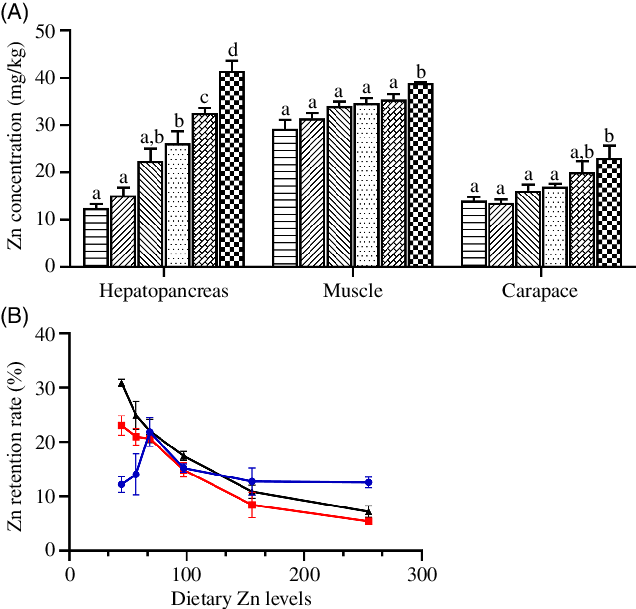
Fig. 2. Zinc concentration (A) and retention rate (B) in hepatopancreas, muscle and carapace of juvenile mud crabs fed diets containing different zinc levels. Values are means (n 3), with standard errors represented by vertical bars. a,b,c,d Mean values for the same tissue with unlike superscript letters were significantly different as determined by ANOVA and Tukey’s test (P < 0·05). (A) ![]() , 44·5;
, 44·5; ![]() , 56·9;
, 56·9; ![]() , 68·5;
, 68·5; ![]() , 97·3;
, 97·3; ![]() , 155·6;
, 155·6; ![]() , 254·7; (B)
, 254·7; (B) ![]() , hepatopancreas;
, hepatopancreas; ![]() , muscle;
, muscle; ![]() , carapace.
, carapace.
The ZRR of crab tissues is presented in Fig. 2(B). The ZRR in hepatopancreas increased as dietary Zn level increased from 44·5 to 68·5 mg/kg reaching a peak value in crabs fed the diet containing 68·5 mg/kg Zn and then decreased as dietary Zn level increased from 68·5 to 254·7 mg/kg. Contrasting results were found in muscle and carapace, where ZRR significantly decreased with increased dietary zinc level, with lowest ZRR in muscle and carapace observed in crabs fed the diet containing 254·7 mg/kg Zn.
Haemolymph biochemical analysis
Biochemical parameters of haemolymph of crabs fed the experimental diets are presented in Table 4. alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, total protein and albumin in haemolymph supernatant were not significantly influenced by dietary Zn level (P > 0·05).
Table 4. Haemolymph characteristics of mud crabs fed with different dietary zinc levels
(Mean values with their standard errors (n 3))
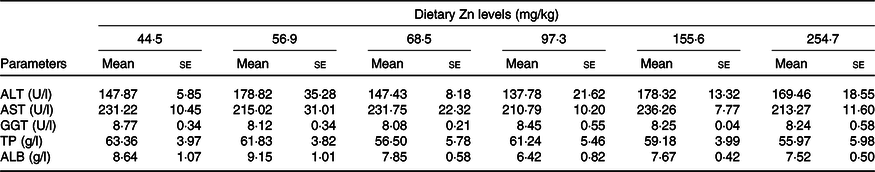
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; TP, total protein; ALB, albumin.
Antioxidant enzyme activities and gene expression in haemolymph and hepatopancreas
The values of antioxidant status parameters in haemolymph and hepatopancreas are shown in Fig. 3. Crabs fed the diets containing 44·5 and 56·9 mg/kg Zn exhibited significantly lower total SOD and Cu/Zn-SOD activities in haemolymph than those fed the other diets (P < 0·05). Compared with crabs fed the 44·5 and 254·7 mg/kg Zn diets, higher CAT activity and GSH content in haemolymph were observed in crabs fed the diet containing 68·5 mg/kg Zn (P < 0·05), while no significant differences were found in haemolymph MDA contents among treatments (P > 0·05). Lowest values of T-AOC, CAT and GSH in hepatopancreas were observed in crabs fed the diet containing 44·5 mg/kg Zn (P < 0·05). Hepatopancreas of crabs fed the diets with 155·6 and 254·7 mg/kg Zn had significantly higher MDA concentrations than those fed the diets with 56·9 and 97·3 mg/kg Zn (P < 0·05).
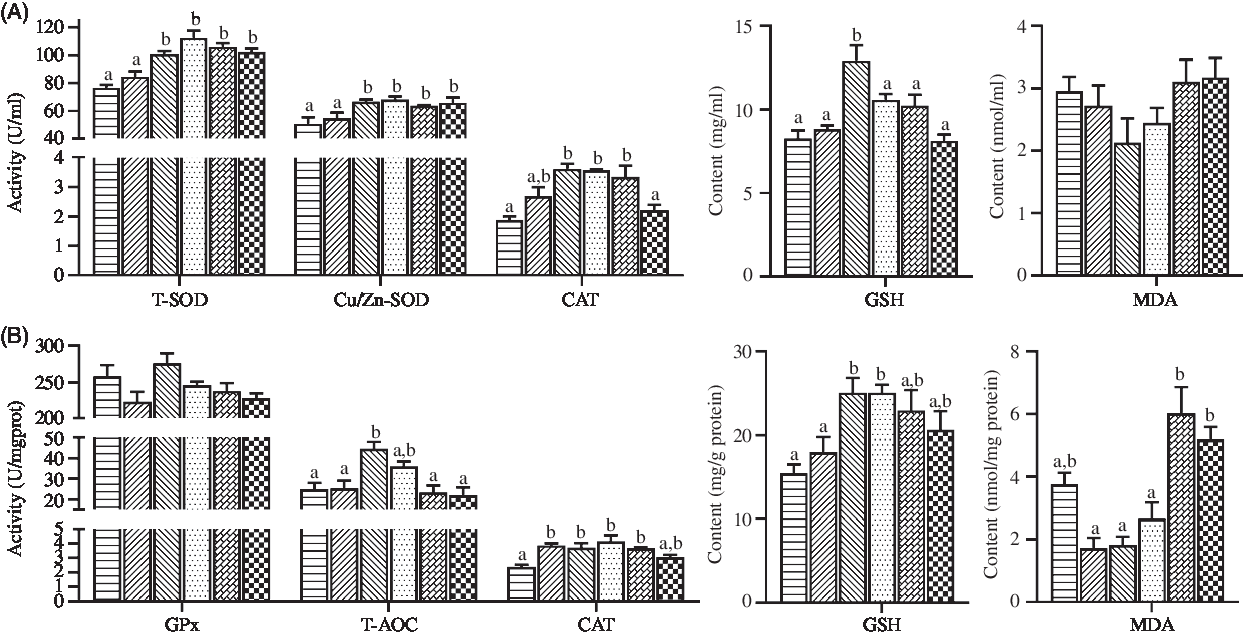
Fig. 3. Antioxidant enzyme activities and antioxidant contents in haemolymph (A) and hepatopancreas (B) of juvenile mud crabs fed diets containing different zinc levels. Values are means (n 3), with standard errors represented by vertical bars. a,b Mean values for the same tissue with unlike superscript letters were significantly different as determined by ANOVA and Tukey’s test (P < 0·05). Cu/Zn-SOD, copper/zinc superoxide dismutase; T-SOD, total superoxide dismutase; CAT, catalase; MDA, malondialdehyde; GPx, glutathione peroxidase; T-AOC, total antioxidation capacity. ![]() , 44·5;
, 44·5; ![]() , 56·9;
, 56·9; ![]() , 68·5;
, 68·5; ![]() , 97·3;
, 97·3; ![]() , 155·6;
, 155·6; ![]() , 254·7.
, 254·7.
The expression levels in hepatopancreas of antioxidant system genes are shown in Fig. 4. The highest relative expression of Cu/Zn sod was observed in crabs fed the diet containing 97·3 mg/kg Zn (P < 0·05), while the expression level of mitMn sod was not affected by dietary Zn level (P > 0·05). The expression level of gpx in hepatopancreas was significantly higher in crabs fed the diet with 97·3 mg/kg Zn than in crabs fed the other diets (P < 0·05). Moreover, crabs fed the 44·5 and 254·7 mg/kg Zn diets had lower relative expression levels of cat and trx than those fed the 56·9 and 97·3 mg/kg Zn diets (P < 0·05).
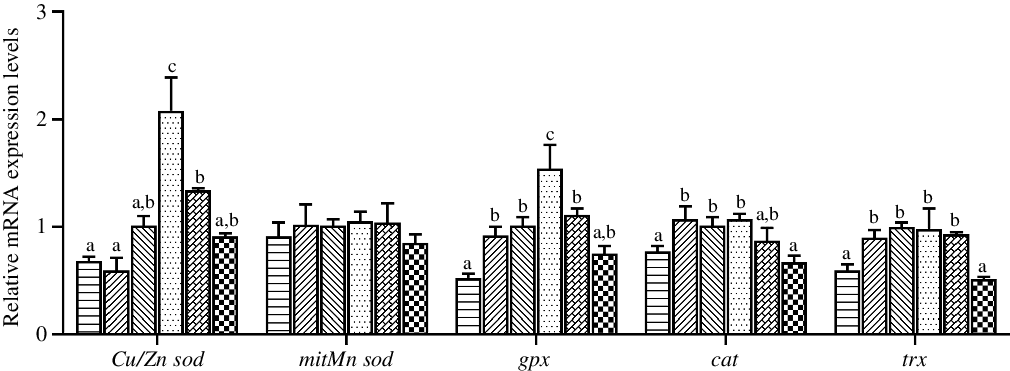
Fig. 4. Effects of dietary zinc level on relative expression of genes involved in oxidation resistance in hepatopancreas of juvenile mud crabs. Values are means (n 3), with standard errors represented by vertical bars. a,b,c Mean values for the same tissue with unlike superscript letters were significantly different as determined by ANOVA and Tukey’s test (P < 0·05). Cu/Zn sod, copper/zinc superoxide dismutase; mitMn sod, mitochondrial manganese superoxide dismutase; gpx, glutathione peroxidase; cat, catalase; trx, thioredoxin. ![]() , 44·5;
, 44·5; ![]() , 56·9;
, 56·9; ![]() , 68·5;
, 68·5; ![]() , 97·3;
, 97·3; ![]() , 155·6;
, 155·6; ![]() , 254·7.
, 254·7.
Innate immunity enzyme activities and gene expression
Activities of enzymes of innate immunity in haemolymph of crabs fed different dietary levels of Zn are presented in Fig. 5. The activity of PO in haemolymph significantly increased as dietary Zn level increased from 44·5 to 97·3 mg/kg and then significantly decreased with further increase of dietary Zn level (P < 0·05). Crabs fed diets containing 97·3–254·7 mg/kg Zn had significantly lower CP activity compared with crabs fed diets containing 44·5–68·5 mg/kg Zn, with lowest CP activity observed in crabs fed the 254·7 mg/kg Zn diet (P < 0·05). The activities of AKP and acid phosphatase in haemolymph were significantly higher in crabs fed the diets containing 68·5 and 97·3 mg/kg Zn compared with activities in crabs fed the other diets (P < 0·05).

Fig. 5. Activities of innate immunity enzymes in haemolymph of juvenile mud crabs fed diets containing different zinc levels. Values are means (n 3), with standard errors represented by vertical bars. a,b,c Mean values for the same tissue with unlike superscript letters were significantly different as determined by ANOVA and Tukey’s test (P < 0·05). PO, phenoloxidase; CP, ceruloplasmin; AKP, alkaline phosphatase; ACP, acid phosphatase. ![]() , 44·5;
, 44·5; ![]() , 56·9;
, 56·9; ![]() , 68·5;
, 68·5; ![]() , 97·3;
, 97·3; ![]() , 155·6;
, 155·6; ![]() , 254·7.
, 254·7.
The expression levels of genes related to innate immunity in hepatopancreas are shown in Fig. 6. The mRNA expression levels of proPO and toll2 in hepatopancreas were significantly up-regulated in crabs fed the 97·3 mg/kg Zn diet compared with crabs fed the other diets (P < 0·05). Dietary Zn level had no significant effect on the mRNA expression levels of clr and toll1 in hepatopancreas (P > 0·05).
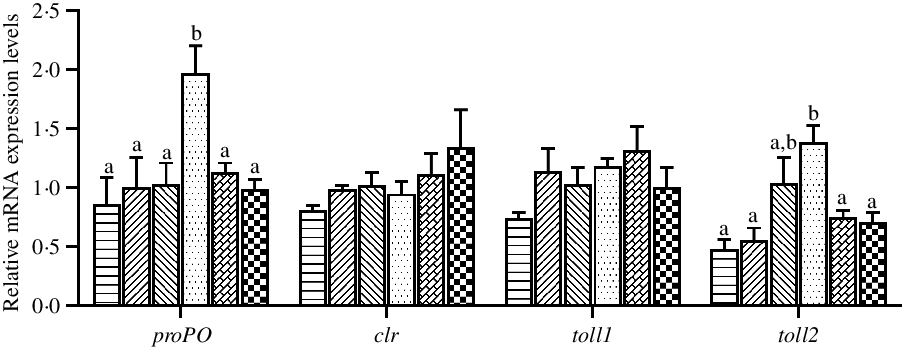
Fig. 6. Effects of dietary zinc level on relative expression of genes involved in innate immunity in hepatopancreas of juvenile mud crabs. Values are means (n 3), with standard errors represented by vertical bars. a,b Mean values for the same tissue with unlike superscript letters were significantly different as determined by ANOVA and Tukey’s test (P < 0·05). proPO, prophenoloxidase; clr, C-type lectin receptor. toll1, toll-like receptor 1; toll2, toll-like receptor 2. ![]() , 44·5;
, 44·5; ![]() , 56·9;
, 56·9; ![]() , 68·5;
, 68·5; ![]() , 97·3;
, 97·3; ![]() , 155·6;
, 155·6; ![]() , 254·7.
, 254·7.
Heat map visualisation analyses of the antioxidative and innate immune parameters
Heat map visualisation was conducted to present the macroscopic effects of dietary Zn level on oxidation resistance and innate immunity parameters of mud crabs (Fig. 7). All data were normalised, with red colour representing higher values and blue colour representing lower values. The results clearly indicated that higher values of activities and gene expression of antioxidation enzymes and parameters, as well innate immunity enzymes and innate immunity-related genes, were observed in crabs fed the diets containing 68·5 and 97·3 mg/kg Zn. The lowest values of peroxidation products were found in crabs fed the diets with 68·5 and 97·3 mg/kg Zn.
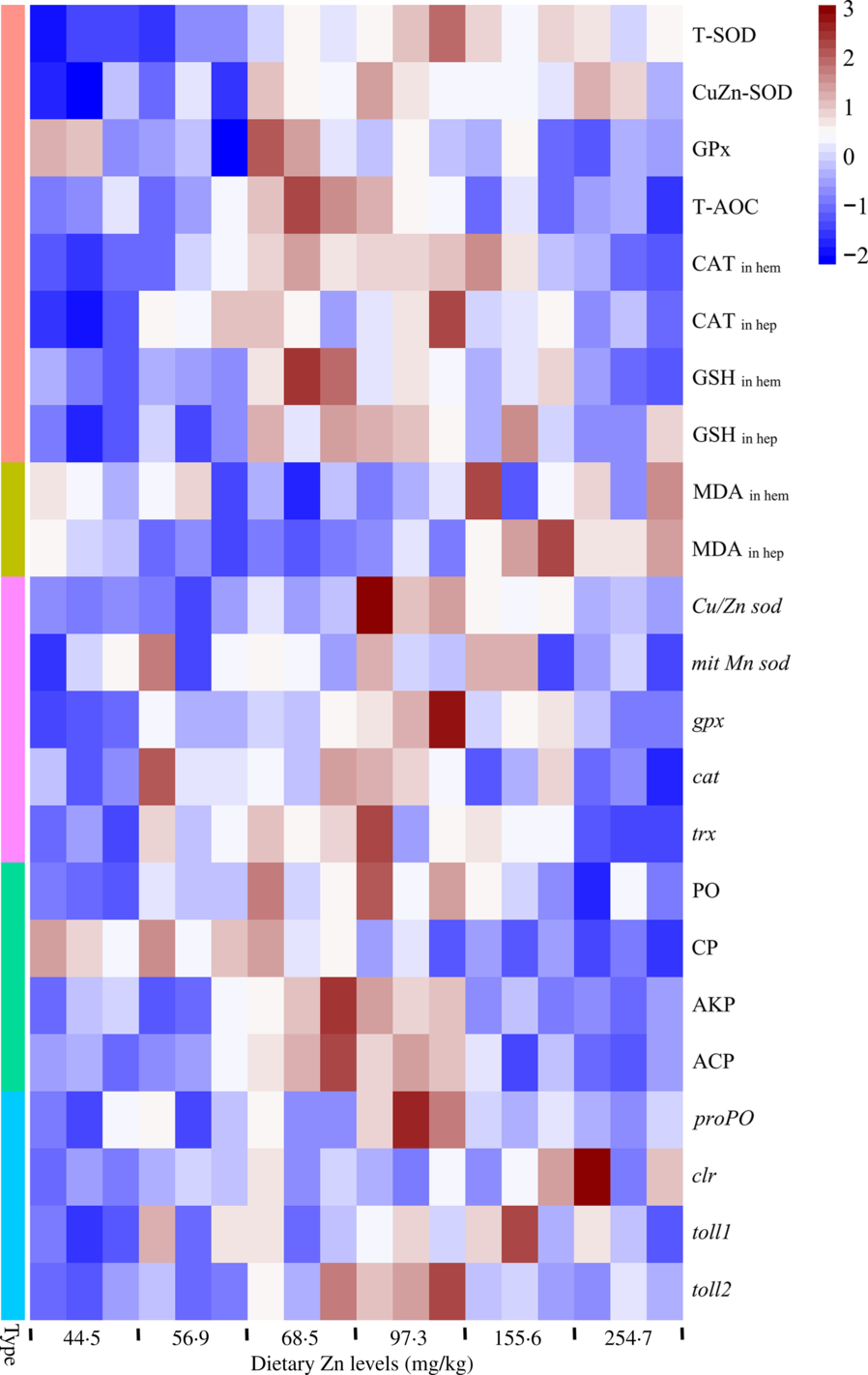
Fig. 7. Heat map visualisation of oxidation resistance and innate immunity parameters in haemolymph and hepatopancreas of mud crabs fed different dietary zinc levels. Before analysis, all data were checked for homogeneity. The colour box for each compound in the heat map indicates the abundance of the compound and represents the fold change according to the scale on the right: red for higher values; blue for lower values. Type: ![]() , antioxidase and antioxidant;
, antioxidase and antioxidant; ![]() , peroxidation products;
, peroxidation products; ![]() , antioxidation-related genes;
, antioxidation-related genes; ![]() , innate immunity enzymes;
, innate immunity enzymes; ![]() , innate immunity-related genes.
, innate immunity-related genes.
Discussion
Due to the limited availability of land culture resources and the high demand of the consumer market, intensive aquaculture has become the main mode of culture in crustaceans(Reference Li, Ai, Liu, Gui, Tang, Li, Liu and De Silva1,Reference Sun, Jin and Jiao33,Reference Sun, Qin and Yu36) . Nevertheless, intensive aquaculture can cause physiological and metabolic disorders and hypoimmunity that can lead to increased disease(Reference Lin, Qiao and Zhang37). Zn is an essential trace element and acts as a cofactor in numerous proteins and enzymes involved in many biological processes(12,Reference Watanabe, Kiron and Satoh22) . Recently, a number of studies have demonstrated that dietary Zn could promote growth, improve anti-stress responses and improve disease resistance in several crustacean species(Reference Muralisankar, Bhavan and Radhakrishnan15,Reference Li, Gong and Jin23,Reference Sun, Chen and Chen24,Reference Shiau and Jiang29,Reference Zhang31,Reference Lin, Lin and Yang32,Reference Guo, Huang and Su38) .
The present study demonstrated that dietary Zn could promote growth performance of juvenile mud crabs, and crabs fed excessive Zn diet had poor PWG, similar to results found in freshwater prawn and Pacific white shrimp(Reference Muralisankar, Bhavan and Radhakrishnan15,Reference Davis, Lawrence and Gatlin30) . Although there were no statistically significant differences in PWG between mud crabs fed the diet without supplementary Zn (44·5 mg/kg Zn) and those with graded Zn, the regression design indicated that dietary Zn supplementation could improve growth performance of mud crab. Based on PWG, the optimal level of dietary Zn for juvenile mud crabs in the present study was 82·9 mg/kg diet. This value was similar to other crustaceans, such as Pacific white shrimp (71·48–95·06 mg/kg)(Reference Zhang31), but different from Chinese mitten crab (105·34 mg/kg)(Reference Sun, Chen and Chen24), freshwater prawn (60 mg/kg)(Reference Muralisankar, Bhavan and Radhakrishnan15) and grass shrimp (34·1 mg/kg)(Reference Shiau and Jiang29). These discrepancies between Zn requirements among crustaceans may be due to differences between the species themselves but may also reflect differences in the studies including growth stage, culture conditions and basal diet. For instance, in the Zn requirement study in Chinese mitten crab, the initial weight of the crab was only 7·16 (se 0·48) mg(Reference Sun, Chen and Chen24), while the initial weight of mud crab in the present study was 14·96 (se 1·11) g.
Zn concentration in different tissues could reflect Zn utilisation(Reference Shiau and Jiang29). In the present study, Zn concentrations in tissues increased with increased dietary Zn level, in agreement with previous studies in Chinese mitten crab(Reference Li, Gong and Jin23), grass shrimp(Reference Shiau and Jiang29), grouper (Epinephelus malabaricus)(Reference Houng-Yung, Yu-Chun and Li-Chi39), hybrid tilapia (Oreochromis niloticus × Oreochromis aureus)(Reference Li and Huang40), Indian major carp (Labeo rohita) and yellow catfish (Pelteobagrus fulvidraco)(Reference Musharraf and Khan20). Furthermore, the main tissue of Zn deposition was hepatopancreas, followed by muscle, and then carapace, which was similar to Chinese mitten crab where Zn concentration in hepatopancreas of crabs fed 85 mg/kg Zn was almost 4-fold higher compared with crab fed 5 mg/kg Zn(Reference Li, Gong and Jin23). In grass shrimp, hepatopancreas Zn concentration was highest in shrimp fed the diet containing the highest Zn level and lowest in shrimp fed the basal, unsupplemented diet(Reference Shiau and Jiang29). These data reflect the role of the hepatopancreas as the main site of nutrient metabolism and mineral bioaccumulation in crustacean species(Reference Dall and Moriarty41). In the present study, ZRR of muscle and carapace of mud crab reduced with increased dietary Zn level, similar to Zn retention in whole body of tilapia fed graded Zn in a soya bean meal-based diet(Reference Li and Huang40). However, ZRR in hepatopancreas of mud crab showed a different trend to the other tissues and reached a peak value in crabs fed 68·5 mg/kg Zn diet and then decreased as dietary Zn level increased further. This indicated that when dietary Zn level was higher than 68·5 mg/kg, Zn deposition rate in hepatopancreas was lower, possibly reflecting negative feedback regulation.
In general, defence systems against lipid peroxidation consist of antioxidant enzymes, such as SOD, CAT and GPx, as well the nonenzymatic antioxidant GSH(Reference Fang, Yang and Wu42–Reference Tang, Feng and Jiang44). SOD catalyses the dismutation of superoxide anion to hydrogen peroxide and oxygen, and Zn is required at the active centre of Cu/Zn-SOD(Reference Nozik-Grayck, Suliman and Piantadosi45). CAT and GPx are responsible for scavenging radicals and are involved in protective mechanisms within tissues following oxidative process and phagocytosis(Reference Feng, Tan and Liu46). GSH is a scavenger in the body that can remove free radicals such as superoxide ions (O2 −) and hydroxyl groups (OH−)(Reference Rodríguez-Ariza, Peinado and Pueyo47). In the present study, lowest total SOD, Cu/Zn-SOD and CAT activities, and total antioxidation capacity and GSH levels were observed in crabs fed the diets containing 44·5 and 56·9 mg/kg Zn compared with those fed the diets containing 68·5 and 97·3 mg/kg Zn. Deficiency or excessive dietary Zn, decreasing biological efficiency of antioxidation enzymes and antioxidants, has been reported previously in other aquatic animals(Reference Muralisankar, Bhavan and Radhakrishnan15,Reference Musharraf and Khan20,Reference Luo, Tan and Zheng25,Reference Wu, Feng and Jiang26,Reference Feng, Tan and Liu46,Reference Huang, Jiang and Wen48) . Production of MDA results from the peroxidation of PUFA, influencing cell membrane fluidity as well as the integrity of biomolecules, and is an important indicator of peroxidation(Reference Devasena, Lalitha and Padma49) reflecting the antioxidant status of aquatic animals(Reference Jiang, Wu and Huang27). In the present study, the lowest MDA concentrations in haemolymph and hepatopancreas were observed in crabs fed the diets containing 68·5 and 97·3 mg/kg Zn, which indicated that appropriate levels of dietary Zn contribute to the reduction of peroxidation products largely due to the actions of the antioxidant enzymes(Reference Feng, Tan and Liu46). Similar results were obtained in grass carp (Ctenopharyngodon idella)(Reference Wu, Feng and Jiang26), Indian major carp(Reference Musharraf and Khan20), Nile tilapia (O. niloticus)(Reference Huang, Jiang and Wen48), Jian carp (Cyprinus carpio)(Reference Feng, Tan and Liu46) and yellow catfish(Reference Luo, Tan and Zheng25).
In the present study, expression levels of genes involved in antioxidation such as Cu/Zn sod, gpx, cat and trx were increased in crabs fed diets containing 68·5 and 97·3 mg/kg Zn compared with those fed the other diets. The expression data were consistent with the higher activities of Cu/Zn-SOD and CAT at these dietary Zn levels, which demonstrated a positive correlation between gene expression and enzymatic activity. Dietary Zn could directly interact with intracellular transcription factors to regulate gene transcription and expression(Reference Bonaventura, Benedetti and Albarède50) or indirectly regulate gene expression by stimulating various signalling pathways, hormones, cytokines and other intermediate regulatory substances(Reference Murakami and Hirano51). Overall, the results indicated that optimum dietary Zn level could enhance oxidation resistance capabilities of mud crab, partly due to its role in maintaining activities of Cu/Zn-SOD and other antioxidant enzymes(Reference Bagchi, Carryl and Tran52). Meanwhile, Zn is also a free-radical scavenger and can inhibit the synthesis of free radicals(Reference Bray and Bettger53), and it is thought that Zn shields membrane from Fe-initiated peroxidation of lipids by blocking negatively charged Fe binding sites(Reference Zago and Oteiza54). Thus, the synergistic actions of Zn with water-soluble or lipid-soluble antioxidants can prevent lipid peroxidation(Reference Musharraf and Khan20).
Immunological responses are important indicators of health status in organisms(Reference Muralisankar, Bhavan and Radhakrishnan15). Since crustaceans have no adaptive immunity memory cells to produce immunoglobulins, they mainly depend on innate immune systems for host defence(Reference Yuan, Jin and Xiong35). The prophenoloxidase (proPO) system is considered as a constituent of the immune system and probably responsible, at least in part, for the non-self recognition process of the defence mechanism in crustaceans, when transformed to the active form (PO) by metal ions(Reference Jiravanichpaisal, Lee and Söderhäll55), with PO being one of the most important enzymes involved in the innate immune system of invertebrates(Reference Wang, Jiang and Zhang56). In the present study, crabs fed the diet with 44·5 mg/kg Zn had significantly lower PO activity in haemolymph than those fed the 97·3 mg/kg Zn diet, indicating that the appropriate dietary Zn level could activate PO activity and promote immune responses. Furthermore, excessive dietary Zn (254·7 mg/kg) supplementation in mud crab decreased PO and CP activities in haemolymph. Cu is a key component of PO and CP(Reference Yuan, Jin and Xiong35,Reference Cao, Miao and Xu57) , and there is interaction between Cu and Zn(Reference Cousins58). Thus, excessive dietary Zn may disrupt normal Cu metabolism and thus restrain the activities of PO and CP in mud crab. There often exists interaction between Zn and other cations, including competitive inhibition during gastrointestinal absorption, due to similarities in the physiochemical attributes of these cations(Reference Musharraf and Khan20). Another Zn-dependent metalloenzyme whose activity could be regulated by dietary Zn level is AKP(12). All highly purified AKP have been shown to be Zn (II) metalloenzymes, whose role was related to the saturation of Zn (II) binding sites(Reference Coleman59). Thus, AKP can be used as an important indicator to evaluate Zn status in aquatic animals(Reference Jiang, Wu and Huang27). In the present study, AKP activity in haemolymph was higher in crabs fed the diets containing 68·5 and 97·3 mg/kg Zn compared with crabs fed the other diets, and similar results were found in blunt snout bream (Megalobrama amblycephala)(Reference Jiang, Wu and Huang27), grass carp(Reference Tang, Feng and Jiang44), Indian major carp(Reference Musharraf and Khan20), Nile tilapia(Reference do Carmo e Sá, Pezzato and Ferreira Lima60) and Siberian sturgeon (Acipenser baerii)(Reference Moazenzadeh, Rajabi Islami and Zamini61).
The ProPO and Toll pathways are two major signalling pathways of crustaceans that are essential for inducing immune-related genes during innate immune response(Reference Li and Xiang62). In the present study, expression of proPO and toll2 in crabs fed the diet containing 97·3 mg/kg Zn was up-regulated compared with those fed the other diets. Higher expression levels of toll were found in hepatopancreas of Pacific white shrimp when the level of Zn in feed was appropriate(Reference Guo, Huang and Su38). Inhibitory Zn finger protein (A20) is an important protein involved in the Toll receptor, and so up-regulation of toll in the optimal dietary Zn group might be related to the mediation of A20(Reference Blander and Medzhitov63). Overall, this may indicate that optimal dietary Zn levels could enhance the innate immunity response of crustaceans via impacts on AKP, ProPO and Toll systems. However, other than the consistent effects on AKP, dietary Zn affects the immune regulation of fish in different ways. In basa catfish (Pangasius hypophthalmus), dietary Zn supplement resulted in higher contents of total protein and globulin in serum(Reference Kumar, Krishnani and Kumar64) and, in rainbow trout (Oncorhynchus mykiss), dietary supplement with Zn-enriched yeast significantly increased serum lysozyme activity, complement activity and total immunoglobulin(Reference Gharekhani, Azari Takami and Tukmechi65). A study on grass carp also found that dietary Zn deficiency decreased fish intestinal immune barrier function through regulation of NF-κB, TOR, Nrf2, c-Jun N-terminal kinase (JNK) and myosin light-chain kinase (MLCK) signalling pathway(Reference Song, Jiang and Liu66).
The bioinformatic statistical tool ‘heat map’ has been used to analyse multi-level and complex data and provide integrative analysis of large data sets in multiple treatment groups and data sets in different measure units, analytical levels and tissues(Reference Auman, Boorman and Wilson67). Heat map is thus a useful way to visualise complex and diversified data with colour-coded arrays indicating the intensity (or amount) of the dependent variable(Reference Pleil, Stiegel and Madden68). Using heat map and multivariate correlation analysis, Yuan et al. (Reference Yuan, Luo and Zhu18) clearly demonstrated that different dietary Zn sources (zinc sulphate, Zn amino acid complex, mixed Zn source) showed inconsistent biological effects in Pacific white shrimp, and shrimp fed the mixed Zn source diet showed better growth response and meat quality. In the present study, the heat map clearly showed that crabs fed the diets with 68·5 and 97·3 mg/kg Zn up-regulated the expression of genes and enhanced the activities of antioxidant and innate immunity enzymes and decreased the contents of peroxidation products.
Conclusion
In summary, based on the broken-line regression analysis between PWG and dietary Zn level, the optimal dietary Zn requirement of juvenile mud crabs was estimated to be 82·9 mg/kg. Moreover, the results of the present study demonstrated a positive correlation between tissue Zn bioaccumulation and dietary Zn levels in mud crabs, particularly in hepatopancreas. Furthermore, the expression levels and activities of antioxidant and innate immune enzymes were increased in crabs fed the diets containing 68·5 and 97·3 mg/kg Zn and, therefore, enhanced the antioxidant defence and innate immune responses of mud crabs.
Acknowledgements
The authors were thankful to all the members in the FSN lab (Laboratory of Fish and Shellfish Nutrition, Ningbo University) for their assistance. The authors also thank Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences (NIMTE, CAS) for use of the ICP-OES and Zhu L.H. for valuable help during the determination of mineral concentration.
This study was supported by National Key R & D Program of China (2018YFD0900400), China Agriculture Research System-48 (CARS-48), the National Natural Science Foundation of China (41476125), the Nature Science Foundation of Zhejiang Province (LY17C190002), Key Research Program of Zhejiang Province of China (2018C02037) and Major Agriculture Program of Ningbo (2017C110007). This research was also sponsored by K. C. Wong Magna Fund in Ningbo University.
J. X. L., M. J. and Q. C. Z. conceived and designed the experiments. J. X. L., T. T. Z., X. X. W., Y. Y., X. C. and J. J. L. performed the experiments. J. X. L. analysed the data. J. X. L. and T. T. Z. contributed reagents/materials/analysis tools. J. X. L., T. T. Z., X. X. W., Y. Y. and X. C. prepared the crab diets. J. X. L. conducted the crab feeding trial. J. X. L., T. T. Z., X. X. W., Y. Y. and X. C. collected the samples. J. X. L., D. R. T. and Q. C. Z. wrote the paper. All authors contributed to and approved the manuscript.
The authors declared that there were no conflicts of interest.
















