Obesity/overweight is a major global health problem that leads to increased mortality. This condition in early life may be related to postnatal nutrition and can evoke metabolic disorders and several co-morbidities, increasing cardiovascular risk and favouring CVD in adulthood. Estimates of deaths related to CVD increased about 14 % between 2006 and 2016(Reference Mello, Luft and Meyer1–4).
Studies investigating the relationship between events in early life, as nutritional insults, and functional status in the future belong to a new research field named ‘developmental origin of health and disease’ (DOHaD). The history of DOHaD as a research field reached a milestone with David Barker's theory encompassing the programming of diseases with fetal origins(Reference Barker, Eriksson and Forsén5). The understanding that the environment and individual lifestyle directly interact with the genome to influence epigenetic changes is growing fast(Reference Penkler, Hanson and Biesma6). These changes alter homeostasis through the remodelling of organs and tissues(Reference Langley-Evans7). As the heart is not entirely developed soon after birth, nutritional insults in early life may contribute to the occurrence of cardiac diseases in adulthood also through direct effects(Reference Pelouch, Kolář and Milerová8).
Animal models comprise an interesting strategy to evaluate future outcomes related to nutritional insults in early life and developmental plasticity. Studies with male animals (mice and rats) report that overnourishment during lactation induces metabolic and haemodynamic heart impairment during adulthood. In general, experimental models of neonatal overfeeding encompass litter size reduction that allows milk supply increase to the offspring. This experimental model is cheap and effective to investigate short- and long-term consequences of neonatal overweight(Reference Kennedy9–Reference Vieira, Soares and Bernardo12). However, such evidence is not available for female animals.
Despite the accumulating evidence that sex leads to differences in biology, for several reasons, the variable sex has been largely ignored in biomedical research(Reference Docherty, Stanford and Panattieri13). In humans, there are sex differences regarding CVD. The literature points out sex differences in cardiometabolic disorders and differences between men and women with hypertrophic cardiomyopathy(Reference Gerdts and Regitz-Zagrosek14,Reference Siontis, Ommen and Geske15) . Individualised medicine must consider sex and gender to initiate personalising care, allowing the improvement of the outcomes. For this, evidence supporting sex-specific decisions also needs to be provided by basic scientists(Reference Miller16). Thus, the present study aimed to evaluate biometric, nutritional and cardiovascular outcomes related to neonatal overweight/overnourishment in young and adult Wistar rats of both sexes.
Materials and methods
Animals and experimental model
The Ethics Committee of Fluminense Federal University (Niteroi, Brazil) approved the use of animals (Comissão De Ética No Uso De Animais (CEUA) UFF812/2016) following the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (NIH) publication no. 8023, revised 1978). All rats received standard chow (Nuvilab®) and water ad libitum at controlled conditions (22°C, 55–65 % humidity, 12 h light–12 h dark cycle). The breeding laboratory of the University provided Wistar rats used for mating (F0 generation). Male (n 10) and female rats (n 20) about 3 months of age and no kinship were mated (two females for one male) for 5 d. Pregnant rats placed in individual cages gave birth to ten to twelve pups after 21 d of gestation. The offspring (F1 generation) were divided into two groups at postnatal day 1 to minimise stress by simple randomisation(Reference Bailoo, Reichlin and Würbel17):
Control – eight pups per mother (four males and four females);
Overweight – four pups per mother (two males and four females).
There was a total of seventy-two rats from the F1 generation:
Control – thirty-two animals (sixteen males and sixteen females) – four litters;
Overweight – forty animals (twenty males and twenty females) – ten litters.
Offspring analysis occurred at postnatal days 30 and 150, being considered young and adult animals(Reference Schneider18). Whenever possible, data were collected precisely from the same rats at both ages. Euthanasia happened at the end of the experimental period after administrating a lethal dose of thiopental intraperitoneally.
Biometric and nutritional analyses
Body mass was monitored from birth to postnatal day 150, while food intake monitoring began upon weaning at postnatal day 21, allowing biometric and nutritional analysis(Reference Bernadis and Patterson19,Reference Novelli, Diniz and Galhardi20) .
Feed efficiency was estimated between postnatal days 21–30, 30–150 and 21–150, using the formula: (final body mass – initial body mass)/Σfood intake.
It was possible to record other biometric parameters of anaesthetised rats using a tape measure: nose-to-anus length (NAL), abdominal circumference (AC) and thoracic circumference (TC) (cm).
BMI was calculated through the formula: body mass/NAL2.
It was possible to achieve complete biometric and nutritional data from eight animals/group at both ages.
Echocardiography studies
The analyses of cardiac structure and function were performed through transthoracic echocardiography using a portable ultrasound system equipped with a 10 MHz transducer (Siemens Accusion Cypress). Previously the animals were anaesthetised with ketamine plus xylazine (50 mg + 5 mg/kg intraperitoneally). The assays were performed according to the American Society of Echocardiography(Reference Lang, Bierig and Devereux21) and all parameters were measured at least three times per animal. The parameters recorded to address cardiac structure were left ventricular internal diameter (LVID), interventricular septum thickness (IVS) and left ventricular posterior wall thickness (LVPW), measured in systole and diastole, as well as relative wall thickness, left ventricle mass, and left atrium:aorta ratio. Systolic volume, ejection fraction and fractional shortening, related to functional parameters, were calculated through algorithms of the equipment software. The parameter recorded to evaluate diastolic function was mitral deceleration time. It was possible to achieve complete echocardiographic data from at least ten animals per group at both ages.
Haemodynamic evaluation
Haemodynamic evaluation was performed by indirect measurement of systolic blood pressure and heart rate through the tail-cuff method(Reference Johns, Gavras and Handy22,Reference Fritz and Rinaldi23) . The assays occurred in the morning after 3 d of acclimatisation using the ADInstruments ML125 NIBP (Non-Invasive Blood Pressure) system connected to the ADInstruments PowerLab/400 digital–analogue converter. The signal was analysed using LabChart 6 Pro software (ADInstruments). Final systolic blood pressure and heart rate values of each animal were calculated by taking the average of six successful separate measurements obtained in the absence of spontaneous tail movement in awake rats.
Thus, because of the assay's stress bias, it was not possible to record haemodynamic parameters of all animals submitted to echocardiography. It was possible to achieve complete haemodynamic data from eight animals/group, preferably at both ages.
Maximal effort ergometer test
After 3 d of acclimatisation, responsive animals (non-sedentary) were also submitted to a maximum effort ergometer test (day 4). Non-responsive animals (sedentary) were discarded from this test. Thus, it was not possible to evaluate all rats submitted to previous assays. Data were achievable from at least five animals per group, preferably at both ages.
The protocol comprised a treadmill (Imbrasport®), without inclination and initial speed of 0⋅9 km/h, followed by progressive increments of 0⋅3 km/h every 3 min until animals were considered to be exhausted. The end of the test was determined when the animals remained still for at least 10 s. The parameters recorded were distance travelled, time spent and maximum speed developed in the test(Reference Molnar, Servais and Guichardant24,Reference Wonders, Hydock and Hayward25) .
Statistical analysis
The Kolmogorov−Smirnov test was applied to verify normality and data were expressed as mean vales and standard deviations. Body mass recorded throughout lactation was analysed using a two-way ANOVA. The tested factors were litter size v. time. As the interaction was significant, the simple effects were analysed by Bonferroni's post hoc test for multiple comparisons between control and overweight groups within the same sex. The unpaired t test was used to compare data obtained from these groups after weaning at the same age as well as weight gain during lactation. Statistical analyses were performed using Prism Software (Graph Pad Prism 7.0). A value of P < 0⋅05 was considered statistically significant.
Results
Body and nutritional analysis
Figs 1(a) and 1(b) show the body mass of male and female offspring throughout lactation. Reduced litters presented higher body mass during lactation and increased weight gain (Fig. 1(c) and 1(d)). Similar values of body mass, NAL, TC and BMI were seen between groups within the same sex at postnatal days 30 and 150. Nevertheless, adult males from reduced litters presented higher AC and AC:TC ratio than those from normal ones (Tables 1 and 2).

Fig. 1. Body mass (a, b) and weight gain (c, d) in grams throughout lactation. (a, c) Male offspring. (b, d) Female offspring. -○-, □, Control group (n 16); -■-, ■, overweight group (n 20). Values are means, with standard deviations represented by vertical bars. Body mass data were analysed using two-way ANOVA followed by Bonferroni's post hoc test. Weight gain was analysed using the unpaired t test. * P < 0⋅05 v. respective control group.
Table 1. Biometric parameters of male offspring
(Mean values and standard deviations)

BM, body mass; NAL, nose-to-anus length; AC, abdominal circumference; TC, thoracic circumference.
* P < 0⋅05 v. respective control group. Data were analysed using the unpaired t test.
Table 2. Biometric parameters of female offspring
(Mean values and standard deviations)

BM, body mass; NAL, nose-to-anus length; AC, abdominal circumference; TC, thoracic circumference.
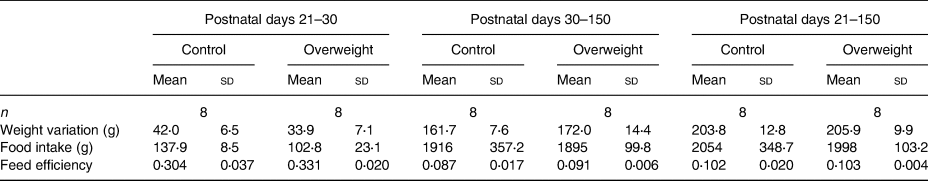
Despite no differences in feed efficiency, food intake was found lower in rats from reduced litters compared with those from regular litters soon after weaning. In the same period, females from reduced litters presented lower weight gain than their respective controls (Tables 3 and 4).
Table 3. Nutritional parameters of male offspring
(Mean values and standard deviations)

* P < 0⋅05 v. respective control group. Data were analysed using the unpaired t test.
Table 4. Nutritional parameters of female offspring
(Mean values and standard deviations)
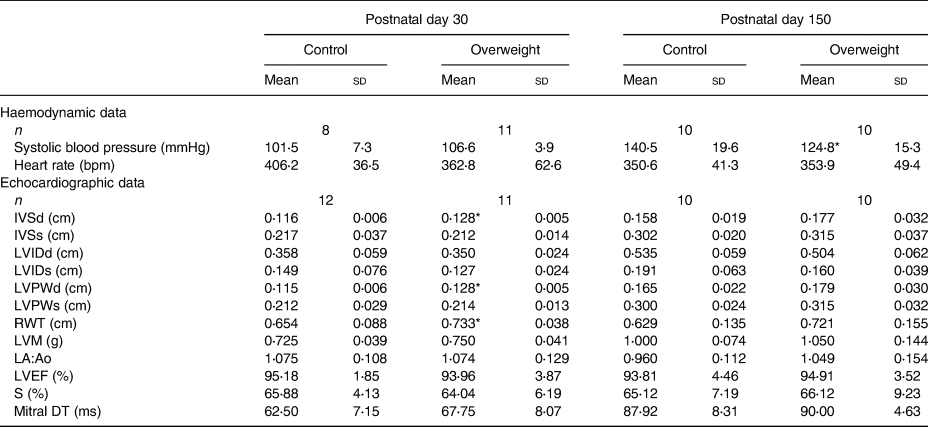
Haemodynamic and echocardiographic parameters
Tables 5 and 6 show haemodynamic and echocardiographic parameters from male and female animals, respectively. Male rats from reduced litters presented higher systolic blood pressure and structural changes in youth (as higher IVSd, IVSs, LVPWd, LVPWs and LMV) and adulthood (higher IVSd and relative wall thickness, lower LVIDd) than from regular ones (Table 5). Curiously, adult female rats from reduced litters presented lower systolic blood pressure compared with their respective controls. They also presented structural changes characterised by an increased IVSd, LVPWd and relative wall thickness in youth without functional alterations (Table 6). All animals presented ejection fraction superior to 80 % and similar values of mitral deceleration time.
Table 5. Haemodynamic and echocardiographic data of male offspring
(Mean values and standard deviations)

IVSd, interventricular septum thickness diastole; IVSs, interventricular septum thickness systole; LVIDd, left ventricle internal diameter diastole; LVIDs, left ventricle internal diameter systole; LVPWd, left ventricle posterior wall thickness diastole; LVPWs, left ventricle posterior wall thickness systole; RWT, relative wall thickness; LVM, left ventricle mass; LA:Ao, left atrium:aorta ratio; LVEF, left ventricle ejection fraction; FS, fractional shortening; mitral DT, mitral deceleration time.
* P < 0⋅05 v. respective control group. Data were analysed using the unpaired t test.
Table 6. Haemodynamic and echocardiographic data of female offspring
(Mean values and standard deviations)
IVSd, interventricular septum thickness diastole; IVSs, interventricular septum thickness systole; LVIDd, left ventricle internal diameter diastole; LVIDs, left ventricle internal diameter systole; LVPWd, left ventricle posterior wall thickness diastole; LVPWs, left ventricle posterior wall thickness systole; RWT, relative wall thickness; LVM, left ventricle mass; LA:Ao, left atrium:aorta ratio; LVEF, left ventricle ejection fraction; FS, fractional shortening; mitral DT, mitral deceleration time.
* P < 0⋅05 v. respective control group. Data were analysed using the unpaired t test.
Performance on maximal effort ergometer test
Overweight and control groups of male and female offspring presented similar performance on the maximal effort ergometer test (Fig. 2).

Fig. 2. Data from the maximal effort ergometer test (a−f) at postnatal days (PND) 30 and 150. (a–c) Male offspring: control (□; n 5); overweight (■; n 8). (d–f) Female offspring: control (□; n 8); overweight (■; n 7). (a, d) Time spent (h). (b, e) Distance travelled (km). (c, f) Maximum speed developed (km/h). Values are means, with standard deviations represented by vertical bars. Data from the maximal effort ergometer tests were analysed using the unpaired t test. * P < 0⋅05 v. respective control group.
Discussion
Litter size reduction soon after birth and throughout lactation has led to overweight in the neonatal period but not in youth or adulthood. Despite this, the early nutritional insult has favoured differences in haemodynamic and echocardiographic parameters later in life. The literature has previously reported related findings in adult male rats submitted to neonatal overfeeding. However, none of the studies investigated the outcomes of the same insult in female rats. According to the results here achieved, distinct outcomes may be seen in male and female rats.
As expected, the reduction of litter size leads to neonatal overweight, according to the literature, and could be addressed by the higher weight gain. Thus, this useful experimental model was validated in the present study, allowing the investigation of short- and long-term consequences of overfeeding(Reference Plagemann, Harder and Rake26–Reference Habbout, Li and Rochette28). Studies have reported that litter size reduction may increase maternal milk availability to the offspring, leading to higher body weight(Reference Vieira, Soares and Bernardo12,Reference Cunha, Pereira and Pereira29–Reference Thole, Rodrigues-Cunha and Carvalho34) . As the hypothalamic area related to food intake and satiety is not entirely structured at the beginning of the lactation period, milk intake seems to be limited only by gastrointestinal tract capacity(Reference McMillen, Adam and Muhlhausler35,Reference Rinaldi, Ribeiro and Marques36) .
Litter size may modulate milk content. The literature has reported that the TAG content of the milk from dams submitted to litter reduction increases between the 10th and 21st days of lactation. Thus, neonatal overweight may also be induced by the higher energy content of maternal milk(Reference Cunha, Pereira and Pereira29,Reference Assumpção, dos Santos and Andrade37) .
Differences regarding food intake are also in agreement with the literature that describes hypophagia in young animals submitted to overfeeding during lactation(Reference Castro, Cesiana and Oliveira38). Although the consequence over body mass is controversial, the similarity about feed efficiency and body weight here observed suggests the occurrence of catch-down growth, a phenomenon also reported by other studies encompassing similar animal models(Reference Nery, Pinheiro and Muniz39–Reference Velkoska, Cole and Morris43).
The literature has correlated anthropometric markers of adiposity, systolic blood pressure and cardiovascular risk, not only in humans but also in rats(Reference Novelli, Diniz and Galhardi20,Reference Radovanovic, Santos and Carvalho44,Reference Abdul, Ur and Yousaf45) . According to the relationship ascribed, data indicate that adult male rats from reduced litters presented increased cardiovascular risk compared with regular ones. Abdominal fat deposition is related to pathological conditions and may favour atherosclerosis and acute myocardial infarction(Reference Moura and Monteiro46). Although the literature has already reported the increase of blood pressure in adult male rats due to neonatal overfeeding(Reference Plagemann, Harder and Rake26,Reference Habbout, Delemasure and Goirand47–Reference Boubred, Daniel and Buffat50) , the same analysis has not included female rats. Thus, data from the present study suggest that the reduction in litter size does not affect the cardiovascular risk of female animals as described for males.
Higher levels of systolic blood pressure, as seen in young and adult male rats submitted to litter size reduction, predispose to diastolic dysfunction and structural remodelling of the left ventricle, a central change in the pathogenesis of cardiac dysfunction. Indeed, echocardiographic data of the present study suggest the occurrence of myocardial hypertrophy and concentric remodelling of the left ventricle in these animals. These structural alterations may eventually lead to ventricular dilation and systolic dysfunction in heart failure progression(Reference Nadruz51–Reference Zile, Gaasch and Carroll58). Although changes regarding echocardiographic parameters in this animal model have not been described previously, the literature reports that overnourishment during lactation may increase cardiac sensitivity to insulin and leptin. The consequent improvement of glucose uptake and energy supply would favour cardiac hypertrophy in male rats(Reference Pereira, Moreira and de Carvalho59).
Despite no preliminary signs of cardiovascular risk increase in female rats from reduced litters, there were differences regarding echocardiographic parameters. Data suggest the occurrence of cardiac structural changes in young females. The lack of cardiac hypertrophy inferences in adulthood may be discussed, taking sexual maturation into account. Female rats reach puberty around postnatal day 30(Reference Leibowitz, Akabayashi and Alexander60) and reproductive senescence occurs between 15 and 20 months of age(Reference Sengupta61). An oestrogen-cardioprotective effect throughout the reproductive phase is widely ascribed in many studies. This hormone can act directly on cardiac myocytes. Its negative modulatory effect on gene expression of plasma membrane Ca2+ channels reduces the risk of arrhythmias and other cardiovascular events. Oestrogen may also mitigate cardiac hypertrophy by increasing the expression of atrial natriuretic peptide and decreasing apoptosis/necrosis of cardiac/endothelial cells(Reference Kuller, Meilahn and Cauley62–Reference Liu, Gao and Kang66).
Echocardiography data suggest that the reported structural changes are without functional impairment(Reference Lang, Bierig and Devereux21). These data may explain the similar performance noticed for the animals on the maximum effort ergometer test. Exercise intolerance, the main symptom of diastolic heart failure, can be assessed by cardiopulmonary exercise tests that constitute an accurate, reliable and reproducible method that yields important outcomes(Reference Kitzman and Groban67). Maximal effort ergometer tests have already been applied to assess cardiorespiratory capacity in rats(Reference Marques, Rocha and dos Santos68). The literature provides a linear relationship between maximum speed and O2 consumption(Reference Rodrigues, Figueroa and Mostarda69).
The present study presents a few limitations that do not allow mechanicist discussion but do not compromise data interpretation and the main findings. There was no monitoring of milk consumption, secretion and content during lactation. Thus, it is not possible to precisely explain why the reduction in litter size generated neonatal overweight. Besides, the lack of hormonal dosage makes a more detailed discussion about the cardioprotective effects of oestrogen in this experimental model somewhat speculative.
In conclusion, the present study corroborates the literature that reports an increase in cardiovascular risk in male rats due to neonatal overfeeding. It also shows that the rise of anthropometric markers of adiposity and blood pressure programme cardiac hypertrophy and concentric remodelling without functional impairment. Likewise, contributing to personalised/gender medicine, this study has shown for the first time that similar early insult in female rats promotes cardiac hypertrophy in youth without changes in biometric and haemodynamic parameters. More studies are warranted to investigate sex differences better and the underlying mechanism involved in cardiac structure preservation in adult female rats submitted to neonatal overnourishment, as well as reproductive senescence impact.
Acknowledgements
The present study was financially supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (grant no. E-26/200⋅964/2017 and E-26/203⋅400/2015), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (fellowships received by G. A. de A. and R. da S. F.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (fellowship received by S. de S. P.).
G. A. de A., R. da S. F., S. de S. P. and N. N. R. performed data collection. F. C. F. B. contributed to data analysis and interpretation by the leading author (C. B. V. S.). C. B. V. S. also designed the study, supervised all aspects of its implementation, and wrote the paper along with G. A. de A.
There are no conflicts of interest.












