Longitudinal studies have shown that poor growth during the fetal, infant and childhood periods is associated with increased risk of obesity and related chronic diseases in adulthood( Reference Law, Shiell and Newsome 1 , Reference Schroeder, Martorell and Flores 2 ). Additionally, prospective studies of adult cohorts have shown that leg length, as a marker of childhood growth, is inversely associated with the risk of obesity( Reference Smith, Bogin and Varela-Silva 3 , Reference Velasquez-Melendez, Silveira and Allencastro-Souza 4 ), CVD( Reference Lawlor, Davey Smith and Ebrahim 5 – Reference Langenberg, Hardy and Kuh 7 ) and the metabolic syndrome( Reference Bogin and Varela-Silva 8 – Reference Varela-Silva, Frisancho and Bogin 10 ). The use of the leg length as a marker of poor growth status is related to the cephalo-caudal growth gradient, the pattern of growth common to all mammals. A special feature of human growth is that between birth and puberty the legs grow relatively faster than other post-cranial body segments. Because legs grow faster than the trunk, their growth is also more susceptible to environmental stress during the postnatal period. Short stature due to relatively short legs is generally a marker of an adverse environment during infancy and childhood( Reference Wu, Moore and Dube 11 ).
Although height and leg length are associated with risk of chronic diseases, the sitting height ratio (SHR) allows individuals with different heights to be compared in terms of the percentage of the body that is composed by the relative length of the legs. Because the SHR is a better descriptor of body shape and body shape changes due to environmental stress than just height or leg length( Reference Kawachi and Subramanian 12 ), it is likely to show a stronger association with obesity and other risk factors. The SHR expresses the percentage of total stature that is due to the length of the head, neck and trunk( Reference Bogin and Varela-Silva 13 ). As a marker of early growth, the SHR of adults is, in part, indicative of net nutritional status, as it results from the synergetic relationship between nutrition, infection and physical activity during the most susceptible period of growth when the legs are growing faster( Reference Varela-Silva, Frisancho and Bogin 10 , Reference Bogin and Varela-Silva 14 ).
Several anthropometric measures have been used as proxies for total or abdominal fat to assess risk for several chronic diseases, the most widely recognized of which is BMI( Reference Soto Gonzalez, Bellido and Buno 15 ). One of the measures proposed for abdominal obesity is waist-to-height ratio (WHtR), correcting the waist circumference (WC) for the height of the individual, and it is also correlated with abdominal fat measured using imaging techniques( Reference Ashwell, Cole and Dixon 16 ). Additionally, the correction of WC for height offers the advantage that it is possible that a single WHtR boundary value may be useful in different ethnic, age and sex groups( Reference Ashwell and Hsieh 17 ).
An integrative approach to the effects of poor growth on adiposity has been lacking. Human growth is a complex influence of genomic, epigenetic, endocrine, environmental and socio-economic factors; therefore, as much as possible, it is important to bring together and tease out the multitude of possible factors affecting growth that can impact the risk of obesity later in life. Our aim was to investigate, retrospectively in a population-based cohort, the association between stunting as measured by short stature and relatively short legs (a high SHR) and adiposity in adults.
Methods
Study design and participants
The current research is based on the EPIPorto cohort study, comprised of 2485 Portuguese adults residing permanently in the city of Porto, Portugal, described in detail elsewhere( Reference Ramos, Lopes and Barros 18 ). Participants were recruited between 1999 and 2003 by random digit dialling using households as the sampling frame, followed by simple random sampling to select one eligible person among permanent residents in each household. After being selected and informed about the study details, each participant was invited to visit our department to complete a demographic, social, behavioural and medical questionnaire, as well as an anthropometric assessment. The proportion of participation was 70 %( Reference Ramos, Lopes and Barros 18 ). Between 2005 and 2008, a follow-up study was conducted which included a face-to-face interview and a second anthropometric assessment. Since sitting height data were collected only during the follow-up study, the current analysis is based only on participants who attended both evaluations. At the follow-up examination, 68 % of the cohort was re-evaluated, which results in a total of 1682 participants for the present analysis. In comparison to the remaining participants in the baseline assessment, our sample is younger and slightly more educated, but no differences were found in any of the anthropometric variables. Except for sitting height, all other variables were collected from the baseline evaluation.
Data collection and definition of variables
Age was recorded as a continuous variable. Education was recorded as completed years of schooling and later categorized into three groups: ≤4 years, 5–11 years or ≥12 years of school enrolment. Occupation was categorized as non-manual, manual or not having a paid occupation (housewives or unemployed). Participants were classified as non-smokers (never smokers), current smokers (daily or occasional) or former smokers (for at least 6 months). Regarding total physical activity, the EPIPorto Physical Activity Questionnaire, a questionnaire exploring all professional, domestic and leisure-time activities, detailing the duration and intensity for each activity during the year prior to the interview, was used, described in detail elsewhere( Reference Camoes and Lopes 19 ). This questionnaire was developed using a similar structure to the questionnaire in the European Prospective Investigation into Cancer and Nutrition (EPIC) and previous research showed that this questionnaire is a valid and reproducible instrument for the brief assessment of usual energy expenditure in Portuguese adults( Reference Camoes, Severo and Santos 20 ). To calculate energy expenditure in physical activity, participants reported the average time spent per day or week in several activities (rest, transport, work, household activities, leisure-time exercise) and energy expenditure was estimated by multiplying the related metabolic equivalent of task (MET) values by the time spent in each activity (MET-h/d). Total energy intake was estimated based on a validated semi-quantitative FFQ of the previous 12 months, comprising eighty-two food items or beverage categories, described in detail elsewhere( Reference Lopes, Aro and Azevedo 21 ).
Anthropometric measurements included body weight, height, sitting height, WC, BMI and WHtR. All measurements were collected with participants wearing light clothing and no footwear. Body weight was measured to the nearest 0·1 kg using a digital scale and height was measured to the nearest centimetre in the standing position using a wall stadiometer. Sitting height was measured with the participant sitting upright on a base plate, using the same stadiometer, and later subtracting the plate’s height. WC was measured to the nearest centimetre with a flexible and non-stretchable tape, avoiding exertion of pressure on the tissues and with the participant standing. The measure was performed midway between the lower limit of the rib cage and the iliac crest. BMI was calculated as weight (in kilograms) divided by the square of height (in metres), and further divided into the categories proposed by the WHO( 22 ). WC and height measures were used to calculate participants’ WHtR, defined as WC divided by height, both measured in centimetres. Abdominal obesity was defined as WHtR≥0·5 for men and women( Reference Browning, Hsieh and Ashwell 23 ).
Height below the first quartile of the sample distribution (<152 cm in women and <164 cm in men) was considered low and interpreted as short stature. Participants’ SHR was used as a measure of relative leg length and was calculated using the formula: SHR=(sitting height/height)×100. The lower the SHR, the relatively longer the legs are( Reference Bogin and Varela-Silva 13 ). A SHR above the third quartile of the sample distribution (≥54·05 % in women and ≥53·25 % in men) was considered high, implying these individuals had relatively short legs for their height.
Statistical analysis
Sample characteristics are presented as counts and proportions for categorical variables, mean and standard deviation for continuous variables with approximately symmetrical distributions, and median and interquartile range for continuous variables with markedly skewed distributions. We compared several sociodemographic characteristics using the χ 2 test for categorical variables and the independent-samples Student t test for continuous variables.
Height and SHR were taken as exposures (independent variables) and their association with BMI and WHtR (dependent variables) was assessed. For an initial examination of the effects of height and SHR on obesity, both variables were considered as continuous and later categorized by quartiles to formally test the existence of a linear association between exposures and outcomes (data not shown). Since distinct associations were found for specific quartiles, both for height and SHR, these analyses supported a dichotomization for height below the first quartile and for SHR above the third quartile. The association between the exposures and measures of adiposity was then quantified using logistic regression models and the results are presented as crude, age-adjusted and multivariate-adjusted odds ratios with 95 % confidence intervals. Each final model was fitted to quantify the association of stature and SHR (independent variable or exposure) with BMI and WHtR (dependent variable or outcome), adjusting for confounders, taking account of the literature review. In order to identify the confounders of the main associations, besides a significance level that was set at a level of 0·05, backward elimination and change in estimation methods were used( Reference Budtz-Jorgensen, Keiding and Grandjean 24 ): variables that did not cause changes of more than 10 % in the exposure effect estimate upon deletion (compared with the full model estimate) were removed. Within the same outcome, the Bayesian information criterion (BIC) was used to compare the fit across models, one using stature and the other using SHR as the main exposures. All analyses were stratified by sex, due to the known pathophysiological differences in adiposity.
Statistical analyses were performed using the statistical software package Stata version 11.0 and the significance level was fixed at P<0·05.
Results
A description of the study sample by the various variables is shown in Table 1. At baseline, mean age was 52·0 (sd 14·0) years for women and 52·8 (sd 14·9) years for men. Forty-one per cent of the women and 30 % of the men had four or less years of education. More than half of the individuals had non-manual occupations (53·3 and 65·8 % of women and men, respectively). A higher percentage of men were current or former smokers at baseline (70·8 %, v. 27·5 % of women). Similar levels of total physical activity were found in both sexes; however, the mean total energy intake was lower among women than men (8648 kJ/d (2067 kcal/d) v. 10 615 kJ/d (2537 kcal), P<0·001). Mean height and SHR were 155·9 (sd 6·0) cm and 53·1 (sd 1·6) % for women, and 169·0 (sd 6·9) cm and 52·3 (sd 1·5) % for men, respectively. A higher prevalence of obesity was found among women than men (25·5 v. 13·3 %), yet men were more frequently overweight (49·2 v. 36·2 %). For WHtR, a higher proportion of men presented abdominal obesity defined as WHtR≥0·5 (80·1 v. 71·1 %; Table 1).
Table 1 Characteristics of the study sample, by sex: adults aged 18–86 years (n 1682) from the EPIPorto adult cohort study, Porto, Portugal (baseline 1999–2003, follow-up 2005–2008)
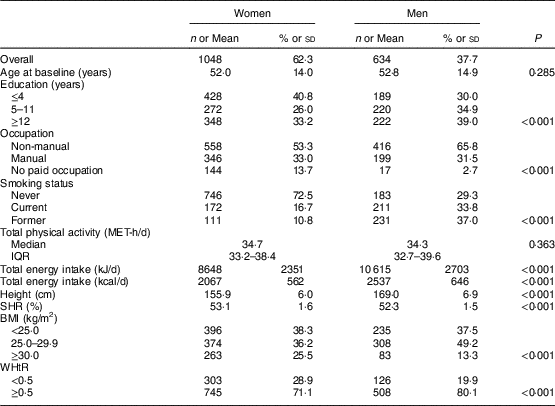
MET, metabolic equivalent of task; IQR, interquartile range; SHR, sitting height ratio; WHtR, waist-to-height ratio.
Data presented are n and % for categorial variables, mean and sd for approximately symmetrical distributions, or median and IQR for continuous variables with markedly skewed distributions. For each variable, the total may not add up to 1682 due to missing data.
Table 2 presents the association of height and sitting height with BMI and WHtR, by sex. For height, a gradual and positive association was found between short height and excessive weight among women (obesity: multivariate-adjusted OR=1·75, 95 % CI 1·17, 2·62) but not among men (obesity: multivariate-adjusted OR=0·74, 95 % CI 0·40, 1·39), independently of age, education and smoking status. Likewise, sex differences were found for the association between short stature and WHtR: women with short height showed almost a twofold increase in the odds of abdominal obesity (multivariate-adjusted OR=1·89, 95 % CI 1·24, 2·87) while no association was found among men (multivariate-adjusted OR=1·15, 95 % CI 0·66, 2·02).
Table 2 Crude and adjusted odds ratios for the association of short stature and relative short leg length (i.e. a high SHR) with overweight/obesity and abdominal obesity, by sex, among adults aged 18–86 years (n 1682) from the EPIPorto adult cohort study, Porto, Portugal (baseline 1999–2003, follow-up 2005–2008)
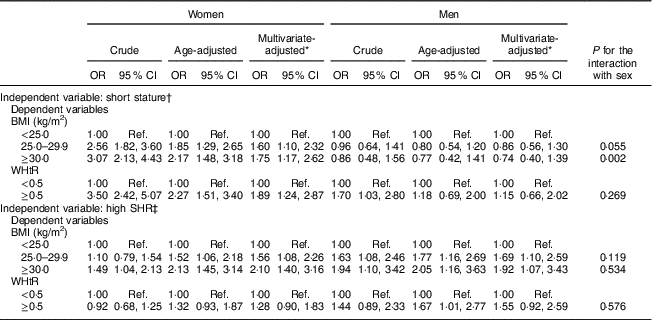
WHtR, waist-to-height ratio; SHR, sitting height ratio; Ref., reference category.
* Adjusted for age (continuous), education (4, 5–11, ≥12 years) and smoking (never, current, former).
† Short stature: <152 cm (women); <164 cm (men).
‡ High SHR: ≥54·05 % (women); ≥53·25 % (men).
For the relationship between SHR and obesity, results were similar for women and men. After adjustment for confounders, having relatively short legs (a high SHR) was associated with a higher likelihood of being overweight (women: multivariate-adjusted OR=1·56, 95 % CI 1·08, 2·26; men: multivariate-adjusted OR=1·69, 95 % CI 1·10, 2·59) and obese (women: multivariate-adjusted OR=2·10, 95 % CI 1·40, 3·16; men: multivariate-adjusted OR=1·92, 95 % CI 1·07, 3·43). Men and women showed weaker associations between SHR and WHtR (women: multivariate-adjusted OR=1·28, 95 % CI 0·90, 1·83; men: multivariate-adjusted OR=1·55, 95 % CI 0·92, 2·59; Table 2).
Taking account of BMI, a model including SHR as an indicator of stunting showed better fit than the model using stature, for both sexes (women: BIC=2011·17 v. 2048·86; men: BIC=1245·52 v. 1264·32). Regarding WHtR, a model with height was the best-fitting for women, presenting the smallest BIC (995·81 v. 1011·87), while for men a model with SHR instead of stature had better fit (BIC=581·88 v. 583·66).
Discussion
The present study shows that stunted height and stunted leg length are associated with the development of obesity during adulthood, particularly in women. Shorter women are more likely to be overweight and obese than their taller counterparts, independently of their age, education and smoking status. A similar effect was not observed in men. The SHR appears to be a more sensitive anthropometric measure to identify obesity in both sexes, as both men and women with relatively shorter legs had a higher risk of being overweight or obese. While women showed a similar effect for WHtR, this was not the case for men.
First, regarding height, our results are in accordance with previous cross-sectional studies showing that short adult stature, a marker of early and chronic undernutrition, is a risk factor for obesity among women, but not among men( Reference Bosy-Westphal, Plachta-Danielzik and Dorhofer 25 – Reference Velasquez-Melendez, Martins and Cervato 27 ). One possible explanation might be the low energy expenditure among women exposed to energy restriction during development, as shown in adolescent girls with stunting( Reference Grillol, Siqueira and Silva 28 ). However, another study conducted in Brazil found a stronger association between short stature and obesity prevalence for both sexes, although stronger in women( Reference Sichieri, Dos Santos Barbosa and Moura 29 ). Some methodological issues, such as self-reported BMI and use of the 5th percentile of the height distribution as the cut-off for early undernutrition, might explain the differences found between that study and our results. Another interesting finding in the Brazilian study is that the association with BMI at the age of 20 years was three times stronger than the association found with BMI at the time of the evaluation, suggesting that BMI is strongly influenced by an individual’s early nutrition but loses magnitude as time goes by due to other factors contributing to weight gain throughout adulthood. In our sample, the mean age at baseline was 52 years and it was not possible to have anthropometric data at earlier ages; therefore, future studies should conduct similar analyses using younger samples and evaluate them longitudinally. These studies will examine whether there is any association between short stature and obesity among men in early adulthood and also verify in which period of the life cycle the association is stronger, thus providing information about the best time to act preventively in this population. Another potential explanation for a differing association between height and BMI in men and women is related to body composition. BMI is a crude measure of adiposity, as women have more fat mass relative to weight than men, who have more lean mass for the same weight. Because of more adiposity for weight in women, the association with height might reflect the influence of poor growth on the risk of adiposity in women (who have more fat mass) compared with men (who have more lean mass).
Use of the SHR provided a more sensitive approach to examining how poor growth is reflected in an increased risk of obesity. This measure seems to be more responsive possibly because it is a measure of leg length that controls for differences in height( Reference Patel, Unwin and Bhopal 30 ). In our study, SHR was revealed to be a more sensitive measure to identify men and women with higher risk of excessive weight. The inverse relationship between measures of relative leg length and adiposity was previously reported by other authors in different populations( Reference Velasquez-Melendez, Silveira and Allencastro-Souza 4 , Reference Frisancho 31 ). Particularly in the USA, a significant relationship between BMI and SHR was found for men and women of three major ethnic-social groups, namely Whites, Blacks and Mexican Americans: adults with relatively longer legs for their total height had lower BMI( Reference Bogin and Beydoun 32 ).
The ‘sitting height’ component of the SHR measures the length of the head, neck, chest and abdomen. Accordingly, if all other aspects of body composition were equal, then for two people of equal sitting height, the BMI will be greater in the person with relatively shorter legs. According to Bogin and Beydoun( Reference Bogin and Beydoun 32 ), this fundamental biometric relationship is not enough to account for these findings. A possible explanation for this relationship comes from research in human life history and the trade-offs that occur between early development and later growth and health outcomes. Poor nutrition and health during pregnancy and during the first six years of life postpartum result in fetuses, newborns, infants and children of reduced body length, mostly due to reduced leg length( Reference Gunnell, Smith and Frankel 33 , Reference Martin, Smith and Frankel 34 ). The alterations in body proportions are likely due to competition between body segments, such as trunk v. limbs, and organs for the limited nutrients( Reference Kawachi and Subramanian 12 ).
It is hypothesized that SHR is a more sensitive factor to environmental effects than stature and that differences in relative leg/trunk proportions between individuals result from growth being affected at different periods with different intensities( Reference Bogin and Varela-Silva 14 ). Because leg length during childhood and adolescence increases very rapidly and contributes more to the variability in stature, it is more sensitive to environmental exposures than the slower growing trunk size( Reference Frisancho 31 ). This reinforces the idea that stature and SHR convey different information. The SHR controls for differences in overall size between individuals and, therefore, is likely to show a stronger association with obesity and chronic diseases than stature( Reference Bogin and Varela-Silva 14 ), as was observed in the current study.
Concerning the measures that we used to assess adiposity, we are aware that there are various ways to measure different aspects of obesity and all of them have strengths and limitations. The rationale for using BMI instead of weight to define obesity is that, if body composition is proportionally the same, taller people weigh more( Reference Harvard 35 ). Thus, modelling weight as a function of height is expected to show an inverse association which says nothing about adiposity. The same rationale applies to WC: for perfect proportionality, taller people (i.e. larger people) will have larger WC. Moreover, the use of BMI and WHtR is supported by literature that confirms that the mere presence of ratios with a common denominator on both sides of a regression equation does not make the results from the model spurious( Reference Lev and Sunder 36 ). Also, BMI is commonly used as a surrogate measure of fatness( 37 ), it is one of the most accurate surrogate markers of visceral fat and is a good indicator of insulin resistance( Reference Borruel, Molto and Alpanes 38 ). Moreover, its associations with mortality and several co-morbidities are well established( Reference Song, Jousilahti and Stehouwer 39 – Reference Visscher, Rissanen and Seidell 41 ) and their clinical meaning validated( 22 ). Regarding WHtR, this measure is significantly associated with all the risk factors for obesity and metabolic syndrome and can predict morbidity and mortality in longitudinal studies better than BMI( Reference Patel, Unwin and Bhopal 30 , Reference Ashwell, Mayhew and Richardson 42 ). Furthermore, the use of WHtR can often identify people within a moderate range of BMI who have a higher metabolic risk( Reference Hsieh, Yoshinaga and Muto 43 ) and can be even more sensitive than WC in several different populations possibly because it encompasses the adjustment to different statures( Reference Ashwell and Hsieh 17 ). Both these indices are cost-effective, easy to measure, have the same cut-offs in men and women, and allow us to provide a simple public health message to the population. For all these reasons, we believe that BMI and WHtR are important primary screening tools to assess adiposity and they should be considered in research and clinical practice.
For each one of the main associations studied, other confounders were tested based on previous knowledge, such as occupation, leisure-time physical activity, energy intake and reproductive state (for women), but no significant associations were found. Since socio-economic position early in life is also related to adult BMI( Reference Hardy, Wadsworth and Kuh 44 ), the impact of the parents’ socio-economic position (as measured by manual v. non-manual occupations) was also assessed, but no associations were found in either males or females (data not shown). One of the limitations concerning this variable is that we only analysed parental occupation; and parental education, known to be an indicator of the quality of growth environment reflecting the quality of health care and nutrition( Reference Lindeboom, Llena-Nozal and van der Klaauw 45 ), was not assessed in our study.
To better interpret the results of the present study, some methodological issues need to be considered. More than half of the individuals had non-manual occupations and more than a third had a higher level of education, suggesting that the study population might be biased towards a high socio-economic position. Since we only analysed individuals who also participated in the follow-up evaluation and individuals with higher socio-economic position are more likely to participate in follow-up evaluations( Reference Fan and Yan 46 , Reference Tin Tin, Woodward and Ameratunga 47 ), we might have experienced a selection bias. However, we evaluated almost 70 % of the total cohort and therefore believe that the possible selection bias is not sufficient to change the direction of the associations found. Also, the SHR can be overestimated in individuals with high levels of gluteofemoral fat, which contributes to a higher sitting height, therefore underestimating the relative contribution of the lower limb to total stature( Reference Bogin and Varela-Silva 13 ). Since obese people have more gluteofemoral fat, this would result in an underestimation of the association between SHR and adiposity due to a differential information bias. This should not, however, fully explain the results found, since they are in accordance with previous literature.
Our results may reflect differences in nutrition that might have existed in Portugal during the 1960s and 1970s. In contrast to many other European countries, patterns of growth and development in Portugal remained relatively unchanged for most of the 20th century since the country experienced a period of economic stagnation and a long dictatorship( Reference Cardoso 48 ). Since the 1970s changes in social, health and sanitary conditions resulted in a positive increment in height of Portuguese citizens( Reference Padez 49 ). The mean age of this sample is 52 years, which means that half of the individuals were born and grew before the 1970s. Thus, the EPIPorto sample represents a population with a wide variation in ages in transition and includes individuals who were exposed to a relatively deprived and difficult period and others exposed to a more positive environment. Further research should study these same associations in younger populations, with a lower range of ages, to examine whether the consequences of early deprivation remain the same for those born after the 1970s.
Growth deficits produced by negative environmental conditions during periods of growth and development have been extensively associated with health status, namely metabolic diseases( Reference Varela-Silva, Frisancho and Bogin 10 , Reference Asao, Kao and Baptiste-Roberts 50 , Reference Florencio, Ferreira and Cavalcante 51 ) and CHD( Reference Lawlor, Taylor and Davey Smith 6 , Reference Smith, Greenwood and Gunnell 9 , Reference Azevedo, Ramos and von Hafe 52 ). This growing body of evidence, together with our research, highlights the importance of considering a life-course approach in health, particularly in obesity. Due to its strong association with several co-morbidities( Reference Visscher, Rissanen and Seidell 41 ), a deeper knowledge of how early-life experiences are linked with this epidemic will help prevent and predict not only obesity but several other related diseases. Moreover, it is also interesting to notice that the association of height and relative leg length with BMI and WHtR shows a strong effect in the crude models, and that this strength is lost when the effect of confounders is adjusted for. This suggests that lifestyle variables and individual life trajectory also play significant roles in adult adiposity and need to be taken into account when measuring the impact of poor growth on obesity during adulthood.
Conclusion
Having relatively short legs was found to be a risk factor for overweight/obesity in Portuguese adult women and men. Short stature appears to be associated with obesity and adiposity only in women. The SHR is more sensitive to assess the effect of stunting on obesity in adulthood than stature. Environmental, behavioural and genetic factors that affect the increase of SHR and the decrease of stature should be studied in depth to provide important clues for better weight control recommendations. From a clinical perspective, findings from the present study may help health professionals to identify earlier in life those patients with a higher risk of becoming obese in adulthood and, in turn, prevent a suite of CVD.
Acknowledgements
Financial support: This study was funded by the European Regional Development Fund (FEDER) through the Operational Programme Competitiveness and Internationalization; and by national funding from the Foundation for Science and Technology (FCT) (Portuguese Ministry of Science, Technology and Higher Education) under the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (grant number POCI-01-0145-FEDER-006862; ref. UID/DTP/04750/2013). The EPIPorto study was funded by the FCT (grant numbers PraxisXXI//2/2.1/SAU/1332/95 and POCTI/ESP/35769/99). The funders had no role in the design, analysis or writing of this article. Conflict of interest: There are no conflicts of interest to disclose. Authorship: Each author participated sufficiently in the work to take public responsibility for its content and believes that the manuscript represents honest work. A.H. and V.T. collaborated in the analysis and interpretation of the data and wrote the article. H.F.V.C. collaborated in the study design, data analysis and reviewed the article with important intellectual content. A.A. designed the study, analysed and interpreted the data and reviewed the article critically. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of Hospital de São João. Written informed consent was obtained from all subjects.







